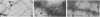Abstract
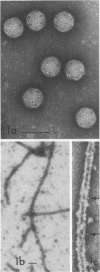
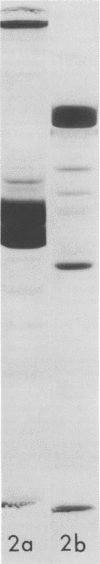
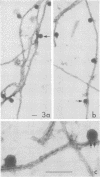
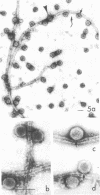
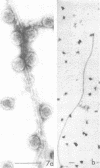
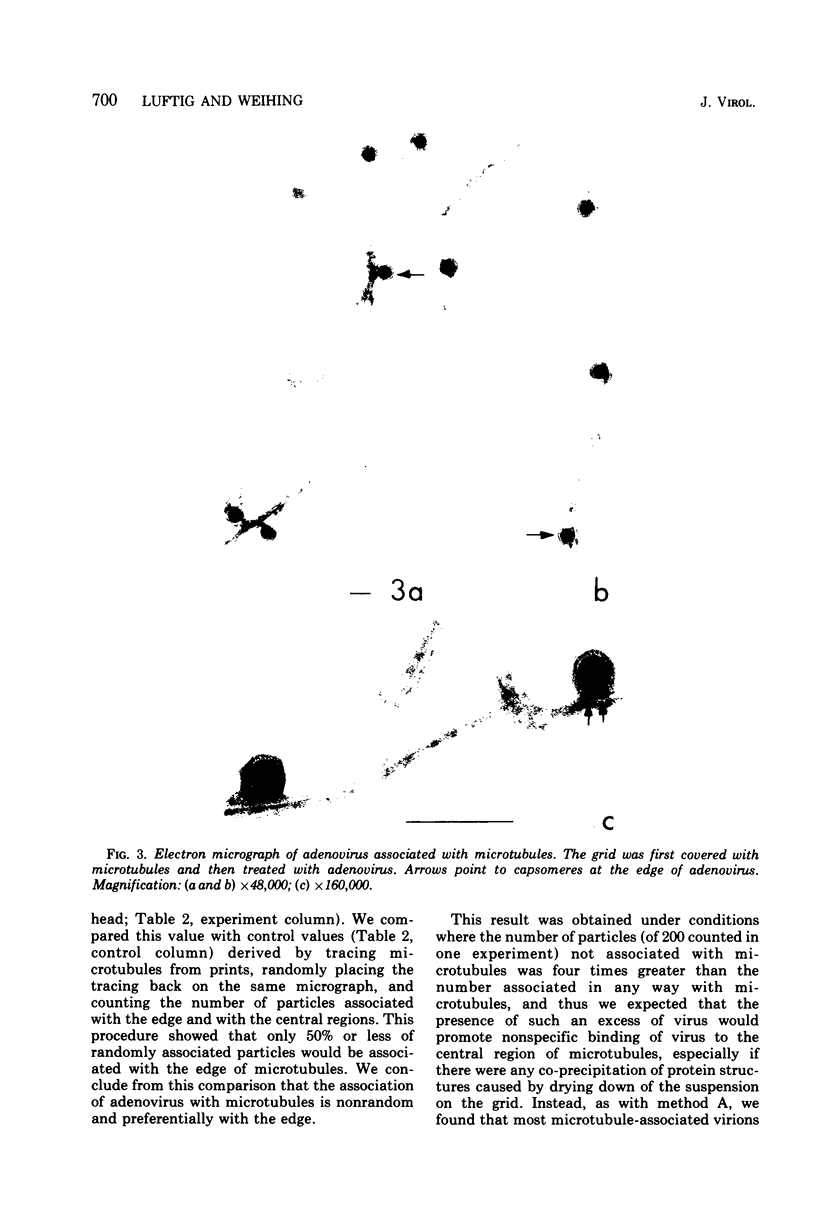
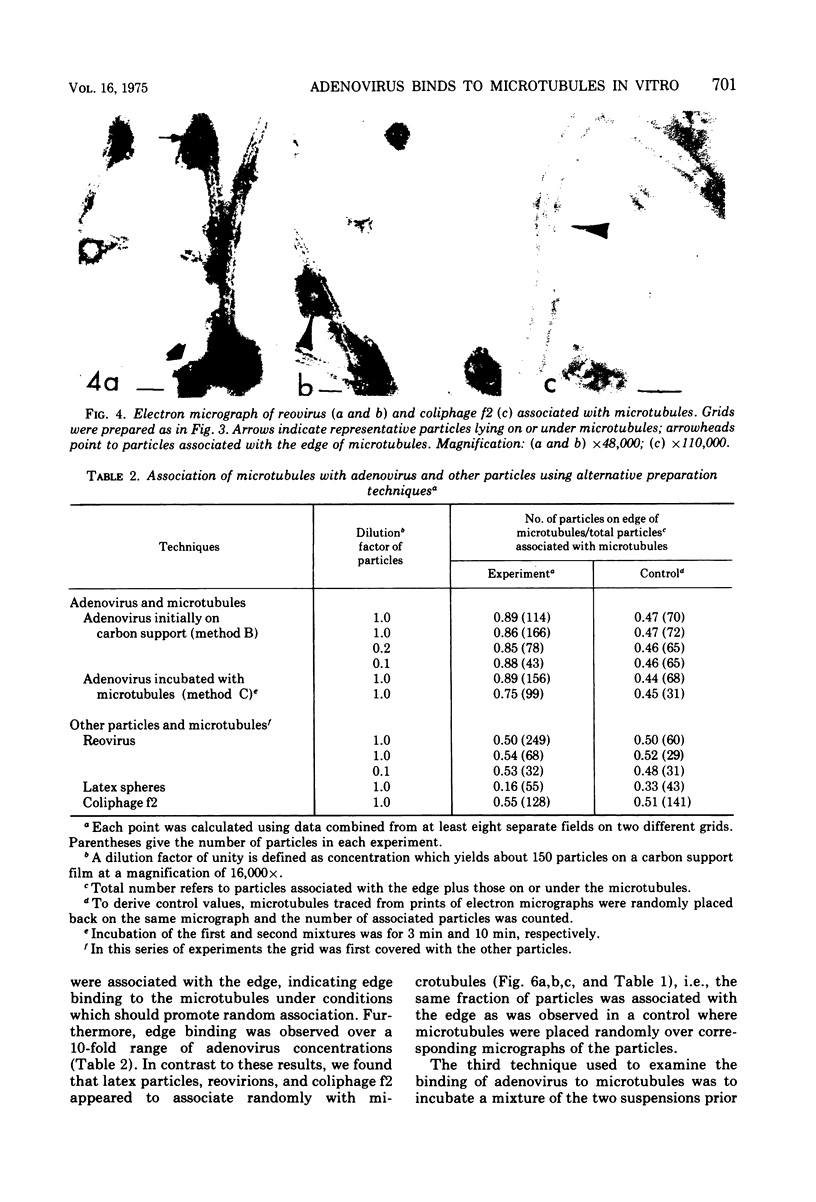
We have found by negative staining electron microscopy that when similar concentrations of adenovirus and reovirus (viruses of about the same diameter, 75 to 80 nm, and density, 1.34 to 1.36 g/cm3) were incubated with a carbon support film containing microtubules, 72% of adenovirus on the grid, but only 32% (equivalent to random association) of reovirus, were associated with microtubules. Similar concentrations of both larger and smaller particles, such as polystyrene latex spheres and coliphage f2, also exhibited a low degree of interaction, viz., 17 to 37%, with microtubules. Moreover, 90% of microtubule-associated adenovirus binds to within +/- 4 nm of the edge of microtubules, but lower fractions (again equivalent to a random association) of the other particles bind to the edge of the microtubules. The mechanism behind this phenomenon, which we denote as "edge binding," is presently obscure; however, it provides us with a second, albeit empirical, method to distinguish between the microtubular association of adenovirus and other particles. We found that edge binding of adenovirus also occurred when adenovirus was initially placed on the carbon support film and then incubated with microtubules and when adenovirus and microtubules were mixed prior to placement on the support. In contrast, reovirus or the other particles prepared by similar techniques exhibited a random amount of edge binding. The binding of adenovirus appears to involve the hexon capsomers of the virion since (i) high resolution electron micrographs showed that the edge of the virus was in contact with the edge of the microtubules, and (ii) adenovirions briefly treated with formamide to remove pentons and fibers bind as efficiently as intact virions. Core structures, which were obtained by further formamide degradation of the virion, do not associate with microtubules. These observations support the hypothesis of Dales and Chardonnet (1973) that the transport of adenovirions within infected cells is mediated by interaction with microtubules.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbanti-Brodano G., Swetly P., Koprowski H. Early events in the infection of permissive cells with simian virus 40: adsorption, penetration, and uncoating. J Virol. 1970 Jul;6(1):78–86. doi: 10.1128/jvi.6.1.78-86.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder L. I., Dentler W. L., Rosenbaum J. L. Assembly of chick brain tubulin onto flagellar microtubules from Chlamydomonas and sea urchin sperm. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1122–1126. doi: 10.1073/pnas.72.3.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borisy G. G., Olmsted J. B., Marcum J. M., Allen C. Microtubule assembly in vitro. Fed Proc. 1974 Feb;33(2):167–174. [PubMed] [Google Scholar]
- DALES S. ASSOCIATION BETWEEN THE SPINDLE APPARATUS AND REOVIRUS. Proc Natl Acad Sci U S A. 1963 Aug;50:268–275. doi: 10.1073/pnas.50.2.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DALES S. An electron microscope study of the early association between two mammalian viruses and their hosts. J Cell Biol. 1962 May;13:303–322. doi: 10.1083/jcb.13.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dales S., Chardonnet Y. Early events in the interaction of adenoviruses with HeLa cells. IV. Association with microtubules and the nuclear pore complex during vectorial movement of the inoculum. Virology. 1973 Dec;56(2):465–483. doi: 10.1016/0042-6822(73)90050-0. [DOI] [PubMed] [Google Scholar]
- Doerfler W. Nonproductive infection of baby hamster kidney cells (BHK21) with adenovirus type 12. Virology. 1969 Aug;38(4):587–606. doi: 10.1016/0042-6822(69)90179-2. [DOI] [PubMed] [Google Scholar]
- Everitt E., Sundquist B., Pettersson U., Philipson L. Structural proteins of adenoviruses. X. Isolation and topography of low molecular weight antigens from the virion of adenovirus type 2. Virology. 1973 Mar;52(1):130–147. doi: 10.1016/0042-6822(73)90404-2. [DOI] [PubMed] [Google Scholar]
- Luftig R. B., Kilham S. S. An electron microscope study of Rauscher leukemia virus. Virology. 1971 Nov;46(2):277–297. doi: 10.1016/0042-6822(71)90030-4. [DOI] [PubMed] [Google Scholar]
- Luftig R. B., Kilham S. S., Hay A. J., Zweerink H. J., Joklik W. K. An ultrastructural study of virions and cores of reovirus type 3. Virology. 1972 Apr;48(1):170–181. doi: 10.1016/0042-6822(72)90124-9. [DOI] [PubMed] [Google Scholar]
- Mayhew D. E., Carrol T. W. Barley stripe mosaic virions assoicated with spindle microtubules. Science. 1974 Sep 13;185(4155):957–958. doi: 10.1126/science.185.4155.957. [DOI] [PubMed] [Google Scholar]
- Neville D. M., Jr Molecular weight determination of protein-dodecyl sulfate complexes by gel electrophoresis in a discontinuous buffer system. J Biol Chem. 1971 Oct 25;246(20):6328–6334. [PubMed] [Google Scholar]
- Pereira H. G., Wrigley N. G. In vitro reconstitution, hexon bonding and handedness of incomplete adenovirus capsid. J Mol Biol. 1974 Jan 5;85(4):617–630. doi: 10.1016/0022-2836(74)90319-2. [DOI] [PubMed] [Google Scholar]
- Price R., Penman S. Transcription of the adenovirus genome by an -amanitine-sensitive ribonucleic acid polymerase in HeLa cells. J Virol. 1972 Apr;9(4):621–626. doi: 10.1128/jvi.9.4.621-626.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell W. J., Dentler W. L., Haimo L. T., Binder L. I., Rosenbaum J. L. Assembly of chick brain tubulin onto isolated basal bodies of Chlamydomonas reinhardi. Science. 1974 Jul 26;185(4148):357–360. doi: 10.1126/science.185.4148.357. [DOI] [PubMed] [Google Scholar]
- Stasny J. T., Neurath A. R., Rubin B. A. Effect of formamide on the capsid morphology of adenovirus types 4 and 7. J Virol. 1968 Dec;2(12):1429–1442. doi: 10.1128/jvi.2.12.1429-1442.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisenberg R. C. Microtubule formation in vitro in solutions containing low calcium concentrations. Science. 1972 Sep 22;177(4054):1104–1105. doi: 10.1126/science.177.4054.1104. [DOI] [PubMed] [Google Scholar]