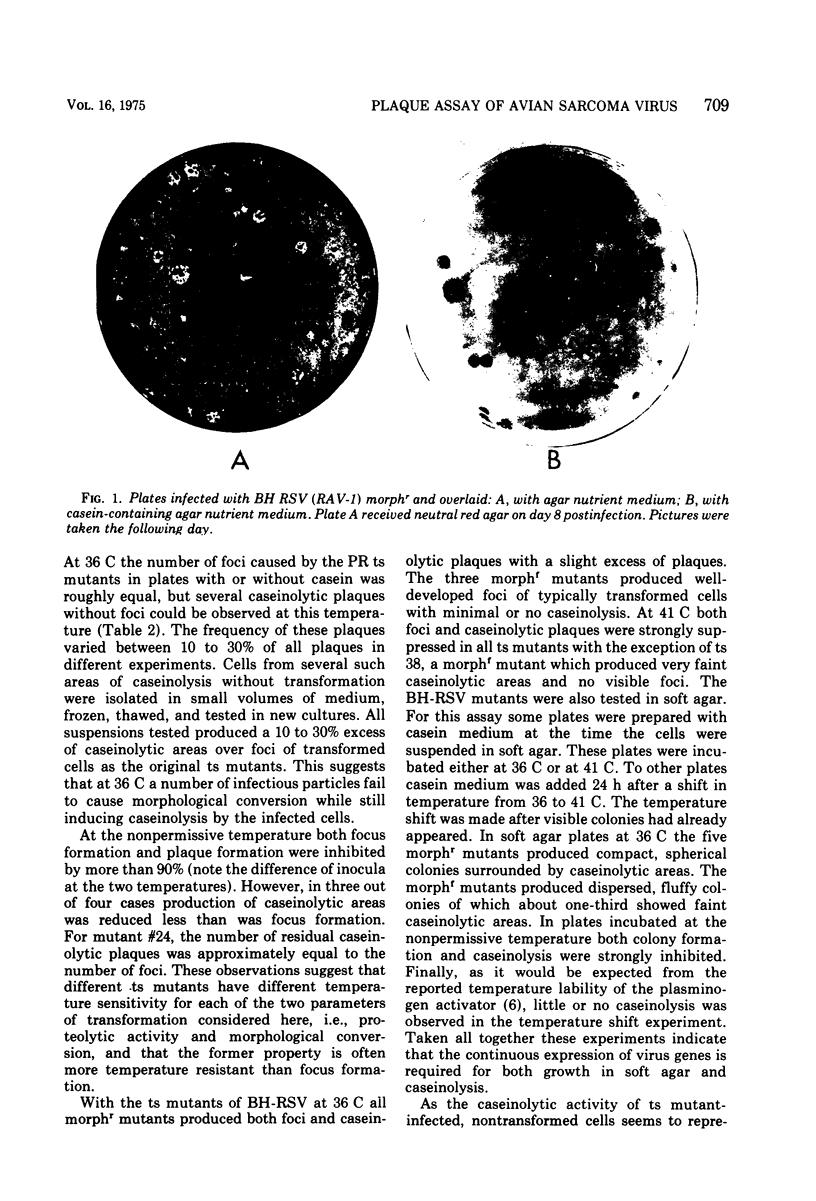
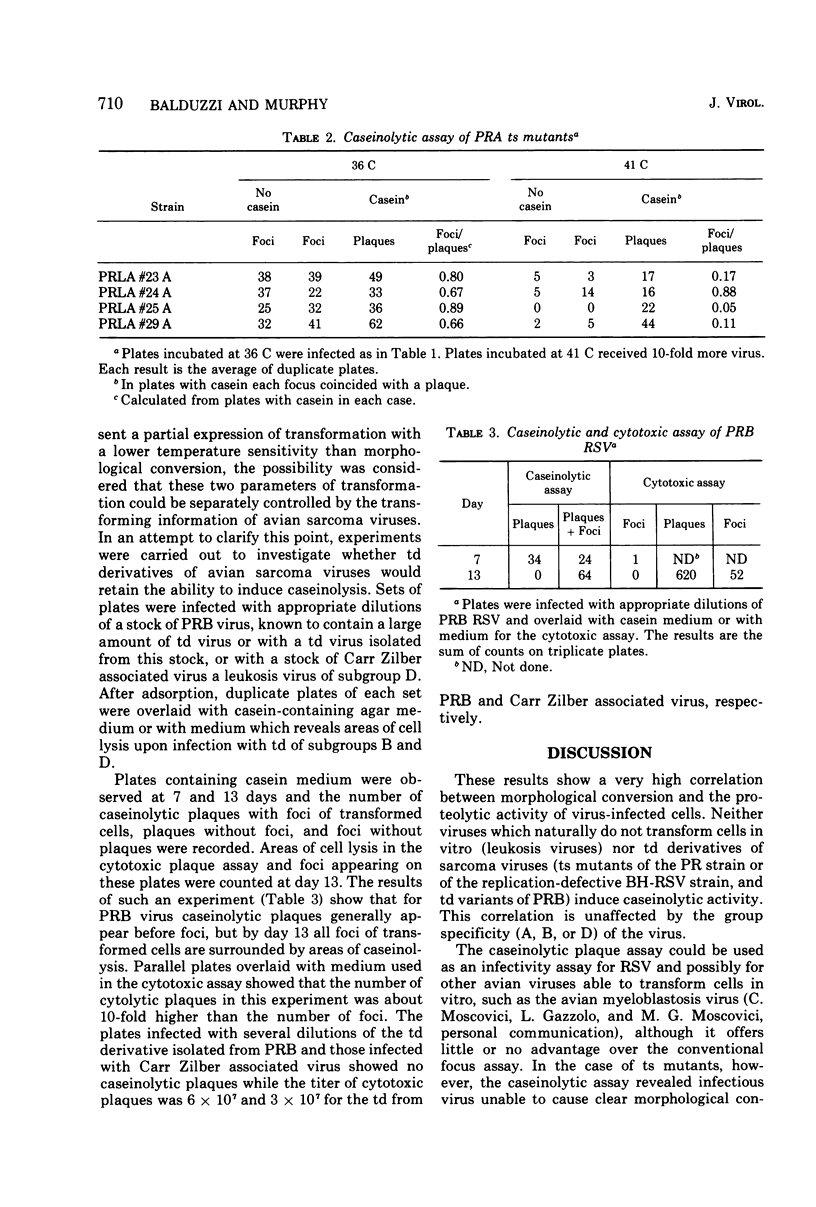
Abstract
The caseinolytic activity of several strains of Rous sarcoma virus (RSV), conditional and nonconditional mutants of RSV, and nontransforming avian leukosis viruses was investigated. Only those viruses capable of transforming chick fibroblasts in vitro induced lysis of casein incorporated into an agar overlay. Lysis produced distinct clear areas in the turbid casein-agar gel which allowed a quantitative "plaque" assay of cell transformation. Casein plaque formation could not be separated from morphological conversion in cultures infected by wild-type RSV strains. In plates infected by mutants temperature sensitive for transformation, the caseinolytic activity appeared to be affected by temperature to a lesser extent than morphological conversion.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balduzzi P., Morgan H. R. Mechanism of oncogenic transformation by Rous sarcoma virus. I. Intracellular inactivation of cell-transforming ability of Rous sarcoma virus by 5-bromodeoxyuridine and light. J Virol. 1970 Apr;5(4):470–477. doi: 10.1128/jvi.5.4.470-477.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg A. R. Increased protease levels in transformed cells: a casein overlay assay for the detection of plasminogen activator production. Cell. 1974 Jun;2(2):95–102. doi: 10.1016/0092-8674(74)90097-x. [DOI] [PubMed] [Google Scholar]
- Graf T. A plaque assay for avian RNA tumor viruses. Virology. 1972 Nov;50(2):567–578. doi: 10.1016/0042-6822(72)90408-4. [DOI] [PubMed] [Google Scholar]
- HAM R. G. CLONAL GROWTH OF MAMMALIAN CELLS IN A CHEMICALLY DEFINED, SYNTHETIC MEDIUM. Proc Natl Acad Sci U S A. 1965 Feb;53:288–293. doi: 10.1073/pnas.53.2.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TEMIN H. M. The control of cellular morphology in embryonic cells infected with rous sarcoma virus in vitro. Virology. 1960 Feb;10:182–197. doi: 10.1016/0042-6822(60)90038-6. [DOI] [PubMed] [Google Scholar]
- Unkeless J. C., Tobia A., Ossowski L., Quigley J. P., Rifkin D. B., Reich E. An enzymatic function associated with transformation of fibroblasts by oncogenic viruses. I. Chick embryo fibroblast cultures transformed by avian RNA tumor viruses. J Exp Med. 1973 Jan 1;137(1):85–111. doi: 10.1084/jem.137.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt P. K. Envelope classification of avian RNA tumor viruses. Bibl Haematol. 1970;(36):153–167. doi: 10.1159/000391704. [DOI] [PubMed] [Google Scholar]
- Wyke J. A., Linial M. Temperature-sensitive avian sarcoma viruses: a physiological comparison of twenty mutants. Virology. 1973 May;53(1):152–161. doi: 10.1016/0042-6822(73)90474-1. [DOI] [PubMed] [Google Scholar]