Abstract
Disrupted-in-schizophrenia-1 (DISC1) is associated with mental disorders, including major depression. We previously showed that DISC1-Q31L mutant mice have depression-like behaviors and can therefore be used to study neurobiological mechanisms of depression and antidepressant (AD) medication action. First, we found reduced levels of dopamine, serotonin and norepinephrine in the nucleus accumbens (NAC) of DISC1-Q31L mutants. Next, we assessed social-conditioned place preference as a reward-dependent task and the capacity of distinct ADs to correct impaired social behavior in DISC1-Q31L mice. Bupropion, but not fluoxetine or desipramine, was able to correct deficient social facilitation, social reward, and social novelty in DISC1-Q31L mutants, whereas all three ADs were able to improve social motivation and behavioral despair in DISC1-Q31L mutants. Furthermore, we sought to correlate social anhedonia with molecular and cellular features including dendritic spine density, β-arrestin-1,2, and cAMP-response-element-binding protein (CREB) in the NAC as biomarkers related to depression and the DISC1 pathway. DISC1-Q31L mutants showed reduced levels of β-arrestin-1,2, CREB, and spine density in the NAC, further supporting the construct validity of the genetic model. Bupropion induced the greatest effect on CREB in DISC1-Q31L mutants, whereas all studied ADs corrected the reduced levels of β-arrestin-1,2 and modestly ameliorated deficient spine density in this brain region. Overall, we find neurobiological changes accompanying social anhedonia in the NAC of DISC1-Q31L mutant mice, consistent with a role for DISC1 in regulating social reward as an endophenotype of depression.
Keywords: DISC1, nucleus accumbens, dopamine, CREB, social anhedonia, depression
INTRODUCTION
Depression is a common public health problem with a 16% lifetime prevalence and it is one of the leading causes of disability and morbidity worldwide (Treadway and Zald, 2011). Depression, like most other mental disorders, is currently diagnosed using clinical criteria, without reference to etiology or diagnostic biological markers, and at least one third of patients with depression do not improve with existing antidepressant (AD) medication treatments (Rush et al, 2006). Our understanding of the pathophysiology and genetics of depression remain limited (Cryan and Holmes, 2005). Hence, new preclinical animal models to link genetic susceptibility factors with the pathophysiology of depression could help to identify promising molecular targets for new treatments.
Several criteria are used to assess the utility of an animal model, including etiological validity (having the same genetic and environmental factors that cause depression in humans), face validity (the mouse behaviors must be related to symptoms of depression), construct validity (similarity of neurobiological mechanisms as in humans), and predictive validity (ADs should correct abnormal behaviors seen in an animal model) (Cryan and Holmes, 2005). The advent of various techniques for creating mutant mice provides an opportunity to investigate the function of candidate gene and signaling pathways in the pathogenesis of depression. Nearly 80 different mutant mouse strains with phenotypes relevant to depression have been developed (Cryan and Holmes, 2005). However, ‘none of these discoveries has so far been translated into a new bona fide treatment for depression' (Berton and Nestler, 2006).
Disrupted-in-schizophrenia-1 (DISC1) has attracted much attention in the last decade because of its causal role in several psychiatric disorders, including major depression (Bradshaw and Porteous, 2011). Moreover, several genes encoding DISC1 interacting proteins are also independent risk factors for major depression, including dysbindin, phosphodiesterase-4B (PDE4B), and pericentrin (Bradshaw and Porteous, 2011). DISC1 acts as a hub protein, interacting with many proteins (Camargo et al, 2007) and linking together divergent signaling pathways, such as dopamine (DA), PDE4B/cAMP, Akt/mTOR, and GSK-3/β-catenin (Bradshaw and Porteous, 2011), all of which are involved in the neurobiology of mood disorders (Zhang, 2009; Beaulieu, 2011).
Several DISC1 mouse models have been created (Papaleo et al, 2012) that all show behavioral phenotypes related to human psychiatric disorders, including hyperactivity, deficient sensorimotor gating, poor working memory, reduced sociability, and behavioral despair. DISC1-Q31L mutant mice have a point mutation in the second exon (A127T) that leads to a glutamine to leucine substitution at amino acid 31 in the DISC1 protein (Q31L). The DISC1-Q31L mice are unique among other DISC1 genetic mouse models in showing a differential response to ADs vs antipsychotics in correcting abnormal behavior (Clapcote et al, 2007). The Q31L point mutation is located within the binding site for both PDE4B and GSK-3 based on peptide mapping (Mao et al, 2009; Murdoch et al, 2007). Biochemical analysis revealed that the DISC1-Q31L mutation leads to decreased PDE4B activity (Clapcote et al, 2007), reduced interaction with GSK-3β and almost no binding to GSK-3α. As a result, auto-phosphorylation of GSK-3 at Tyr279/216 sites is increased and DISC1-Q31L mutant mice have increased sensitivity to TDZD-8, a GSK-3 blocker (Lipina et al, 2011). Given the roles of PDE4B (Zhang, 2009) and GSK-3 (Jope and Roh, 2006; Beaulieu, 2011) in such mood disorders as bipolar disorder and depression, we sought to further investigate pathophysiological correlates of depression in the DISC1-Q31L mouse model.
The monoamine neurotransmitters (DA, serotonin, and noradrenaline) have been implicated in depression (Charney, 1998), so we measured their levels in several brain regions implicated in depression in DISC1-Q31L and wild-type (WT) mice. Given that consistent reduction of DA, serotonin and norepinephrine (NE) were found in the nucleus accumbens (NAC), a key brain area of reward (Shirayama and Chaki, 2006) and that anhedonia, the inability to experience pleasure, has been proposed as translational endophenotype of depression (Cryan and Holmes, 2005), we also assessed reward-related behavior in DISC1-Q31L mutants. Social behavior involves reward processes in different contexts, such as monogamous pair-bonding, aggression, maternal–infant attachment, or social play among juveniles (Panksepp and Lahvis, 2007). As mice prefer familiar conspecifics and form strong social attachments with familiar partners (Krames and Shaw, 1973), we hypothesized that pair-bonding among familiar cagemates would be preferable and rewarding in adult mice. Therefore, we assessed social-conditioned place preference (SCPP) in adult DISC1-Q31L and WT mice, and the efficacy of distinct ADs (bupropion, fluoxetine, and desipramine) to correct diminished social behavior. Finally, we sought to correlate ADs effects with molecular and cellular changes in the NAC. We found that all studied ADs were able to improve social motivation and behavioral despair in DISC1-Q31L mutants, but only the DA drug (bupropion) completely restored deficient social discrimination, facilitation, and reward. In parallel, we found reduced levels of β-arrestin-1,2, cAMP-response-element-binding protein (CREB), and spine density in the NAC of DISC1-Q31L mutant mice. Bupropion, but not fluoxetine or desipramine, induced the greatest effect on CREB expression in DISC1-Q31L mutants, whereas all three ADs corrected the reduced levels of β-arrestin-1,2 and modestly ameliorated deficient spine density in the NAC. Our findings support for DISC1 as a genetic risk for depression and demonstrate the usefulness of DISC1-Q31L mutant mice as an animal model of depression that may help to develop new treatments.
MATERIALS AND METHODS
Animals
The DISC1-Q31L mutant line was backcrossed for 8–14 generations to C57BL/6J mice obtained from The Jackson Laboratory (Bar Habor, ME). DISC1-Q31L heterozygous mice were intercrossed to generate homozygous, heterozygous, and WT animals. All experiments were performed with experimentally naïve 10–16-week-old male DISC1-Q31L homozygous mice (DISC1-Q31L) and their WT littermates. Groups of three to five same-sex littermates were housed in filtered polycarbonate cages under controlled temperature (21±1 °C), lighting (lights on: 7 am–7 pm) and humidity (50–60%). The animals were given sterile food (Purina mouse chow) and water ad libitum. All animal procedures were approved by the Animal Care and Use Committee of the Toronto Centre of Phenogenomics and were conducted in accordance with the requirements of the Province of Ontario Animals for Research Act 1971 and the Canadian Council on Animal Care.
Analysis of Brain Monoamines
Samples were obtained for each brain region, and tissue pellets were retained for later determination of protein content. Levels of DA, 3,4-dihydroxyphenylacetic acid (DOPAC), homovanillic acid (HVA); serotonin (5-HT) and NE were quantified using high-performance liquid chromatography with electrochemical detection. Analyses were performed on a system consisting of a Thermo Separation Products (TSP) P4000 pump (TSP, Piscataway, NJ), a TSP AS3000 autosampler with cooling unit, an ESA Coulochem II electrochemical detector (ESA 50ll Analytical Cell and 5020 Guard Cell; ESA Biosciences, Chelmsford, MA) and a Spectra Physics 4290 Integrator (Spectra Physics, Irvine, CA) connected to a computer running TSP WOW chromatography software. The mobile phase, an aqueous mixture of 0.098 M glacial acetic acid, 0.09 M sodium acetate (pH 3.7), 0.118 mM ethylenediaminetetraacetic acid, 7% methanol, and 0.8 mM octane sulfonate was delivered at a flow rate of 0.8 ml/min. Separation of the 100 ml samples was performed on an ACE 250 × 4.6 mm column with Ace C18, 5 mm stationary phase. Peak heights recorded at E2 were detected using electrode potentials as follows (Guard cell +450 mV: analytical cell E1+100 mV; E2—400 mV). Quantification of monoamines was performed on 0.1 N perchloric acid extracts in a procedure involving two 30 min runs per sample. An appropriately diluted sample was run followed by a second run consisting of one-half sample and one-half standard cocktail (pure monoamines in concentrations of 10 pg/ml). Monoamine levels in pg/mg tissue wet weight were then calculated.
Behavioral Tests
SCPP was conducted as described previously (Panksepp and Lahvis, 2007) with adjustments to allow for testing of adult mice. DISC1-Q31L mice and WT littermates were weaned at post-natal day 21 (PD 21), separated by genotype and left until 10-12 weeks of age. On the day prior to social conditioning, mice were socially isolated from14:00–16:00 h in individual clean cages containing corncob bedding and nesting material. Social conditioning took place over the next 3 days, which included two conditioning sessions per day (conditioning sessions occurred at 10:00–12:00 h and 16:00–18:00 h each day). During a conditioning session, mice of each genotype were either reunited or socially isolated for 20 min in one of two distinct environments (washcloth was used as an isolation-associated surface and an incontinence (absorbent) pad oriented by soft surface as material associated with social interactions) situated within each peripheral compartment of a three-chambered box (51 cm long × 25.5 cm wide × 23 cm tall). During the social conditioning sessions, each pair of mice was kept the same through the experiment, ie, each mouse was reunited with the same, familiar cagemate. These manipulations allowed us to assess the reward value of pair-bonding with a familiar partner, not just social interactions in general. Unconditioned mice from the control groups of both genotypes were socially isolated during conditioning sessions. To ensure that mice in the control groups received an equivalent amount of experience with the unconditioned stimulus (ie, social interaction), they were reunited with their respective social group once per day for 20 min in a novel, standard mouse cage (38 cm long × 20 cm wide × 15.5 cm tall) that was lined with corncob bedding and situated within a procedure room distinct from where the conditioning sessions were conducted. All variables associated with the conditioning procedure were randomized and counter-balanced across the unconditioned and conditioned groups of mice. Habituation: On the final day of conditioning, mice were allowed to freely explore the entire three-compartment testing arena without dividing walls for a 15 min with no conditioning cues present. On the test day, an individual mouse was placed in the central compartment of the testing arena and its behavior throughout the arena was videotaped and scored for 20 min (Observer 5.0, Noldus Information Technology, The Netherlands). The time spent in each compartment (peripheral compartments contained the socially-paired and isolation-paired materials, respectively) and the number of transitions made between each compartment during the testing session were analyzed. During the conditioning sessions, the time spent exploring the compartment (sniffing, biting, going underneath the washcloth or absorbent pad, rearing, walking) was analyzed as an average of three sessions.
Social Affiliation and Novelty
The social behavior in this task was measured as previously described (Clapcote et al, 2007; Lipina et al, 2011), using a three-chambered box. The test mouse is placed in the center chamber with access to the two side chambers. These side chambers contain mesh cylinders that are either empty or have another mouse inside. At the beginning of each experimental session, the test mouse was placed in the central chamber and was allowed to freely explore for 5 min. Next, an unfamiliar mouse (male C57BL/6J, ‘stranger 1') was placed inside a cylinder in one of the outer chambers. Time spent by the test mouse in each outer chamber was recorded over a 10 min period, to estimate social motivation (session 1). Another unfamiliar mouse (male C57BL/6 J, ‘stranger 2') was then placed inside an identical cylinder in the opposite outer chamber, and the activity of the test mouse was likewise recorded for a further 10 min, to evaluate social novelty (session 2). The amount of time spent near each cylinder and the number of entries into each chamber were scored using event-recording software (The Observer 5.0, Noldus Information Technology). The apparatus was cleaned with 70% ethanol after each session.
Social Interaction in a Neutral Arena
Each mouse was placed in unfamiliar neutral cage (30 cm long × 17 cm wide × 12 cm tall) filled with bedding under standard illumination and agonistic interactions were scored when the mouse encountered a weight- and age-matched unfamiliar C57BL/6J male mouse (the standard opponent). Standard opponents were used only once, and neutral cages were changed for each tested pair. The observation period started with the first interaction of the mice and lasted for 10 min. During this period, the following behaviors of the experimental animal were recorded: sniffing of the partner, following the opponent, self-grooming, freezing (sitting on one place >5 s with slight movements of head), walking, and digging of the bedding. Behavior was video-recorded and scored using event-recording software (The Observer 5.0, Noldus Information Technology).
Drug Treatments
Bupropion (4 mg/kg; Sigma), fluoxetine (5 mg/kg; Tocris) and desipramine (10 mg/kg; Sigma) were dissolved in saline (0.9% NaCl) on the day of administration and given in a volume of 10 ml/kg intraperitoneally twice per day (9 am and 6 pm) for seven days. The social conditioning sessions of SCPP occurred between injections on days 5–7. Antidepressant or saline-treated mice were tested in the SCPP, social affiliation/novelty and the forced swim test 20 h after the last injection. The doses of drugs were chosen based on the literature (Clapcote et al, 2007; Lipina and Roder, 2010) and preliminary experiments. For the biochemical and cellular experiments, brains were collected 20 h after the last injection following either cervical dislocation or intracardial perfusion, respectively.
Western Blots
Protein levels of β-arrestin-1,2, CREB and phosphorylation of CREB at Ser129/133
Immunoblotting was performed as previously described (Lipina et al, 2011). Tissue extracts from the NAC were prepared by homogenization in RIPA lysis buffer (Santa Cruz Biotechnology) containing Tris-buffered saline, 1% Nonider P-40, 0.5% sodium deoxycholate, 0.1% SDS, 0.004% sodium azide, PMSF, sodium-orthovanadate, protease inhibitor cocktail and phosphatase inhibitor cocktail (Sigma), followed by centrifugation at 10 000 r.p.m. for 10 min at 4 °C. Protein concentration was determined by Bradford assay (Protein Assay, Bio-Rad). The supernatants were stored at −80 °C until analysis. Equal amounts of protein extracts (20–40 μg) were boiled with Laemmli sample buffer and were separated on 10% or 15% (to distinguish β-arrestin-1 and β-arrestin-2) SDS PAGE and transferred to PVDF membrane (Life Sciences). Blots were immunostained overnight at 4 °C with the following antibodies: anti-phospho-CREB-Ser129/133 (1 : 1000, Invitrogen); anti-CREB (1 : 1000, Cell Signaling Technology); anti-β-arrestin-1,2 (1 : 5000, Cell Signaling Technology), and anti-β-actin (1 : 2000, Santa Cruz Biotechnology). Immune complexes were detected using appropriate peroxidase-conjugated secondary antibodies along with a chemiluminescent reagent (ThermoScientific).
Golgi-Cox Staining
Mice were anesthetized with xylazine/ketamine (10 ml/kg) and intracardially perfused with 0.9% saline. Brains were removed and immersed in Golgi-Cox solution in the dark for 14 days before transferring to 30% sucrose solution for 5 days. Sections of 200 μm were sliced using a microtome (Leica VT1000S, Germany) and placed on 2% gelatinized microscope slides. The slides were stored in a humidified chamber for 3 days prior to further staining and fixation. Dendritic spine density was measured with Golgi-stained images captured at × 100 magnification under bright-field illumination with a Nikon Eclipse E600 microscope. 75–100 neurons from 3–5 mice per treatment group were selected for spine analysis. Spines were counted only on the dendrites of medium spiny neurons in the NAC, as demarcated in the Golgi Atlas Of The Postnatal Mouse Brain (Valverde, 1998). Acquisition parameters were kept the same for all images and were blinded prior to analysis. Spine density was expressed as the number of spines per dendritic length (μm).
Statistical Analysis
Behavioral, biochemical, and spine density data were analyzed using two-way ANOVA with repeated-measures using the appropriate between-subject and within-subject factors followed by Tukey's honestly significant difference (HSD) post-hoc tests. High-HPLC results were analyzed in each brain region by unpaired t-tests. Statistical analyses were performed using Statistica for Windows (Statsoft 5.5, Tulsa, OK). Data are expressed as mean±SD. A significance level of p<0.05 was used for all analyses.
RESULTS
Post-Mortem Monoamine and Metabolite Levels
To examine whether the DISC1-Q31L genetic mutation might affect brain monoamines, we assessed levels of serotonin (5-HT), DA, NE, and their metabolites in the frontal cortex, striatum, hippocampus, and the NAC in DISC1-Q31L and WT mice (Table 1). There were consistent reductions of DA (p<0.05), NE (p<0.05), and 5-HT (p<0.05) in the NAC of DISC1-Q31L mutants as compared with WT mice. In addition, levels of 3,4-dihydroxyphenylalanine (DOPA) (p<0.05) and DOPAC (p<0.01) were increased in the same brain area in DISC1-Q31L mice. HPLC analysis also detected increase of HVA in the frontal cortex (p<0.05), decrease of HVA in the hippocampus (p<0.01), increase of 5-hydroxyindoleacetic acid (HIAA) in the striatum (p<0.01) and the hippocampus (p<0.001) in DISC1-Q31L mutants compared with WT mice.
Table 1. Content of DA, NE, Serotonin, and their Metabolites (ng/mg of tissue) in the Frontal Cortex, Striatum, NAC, and Hippocampus of WT (n=7) and DISC1-Q31L mice (n=7).
| DOPA | DA | DOPAC | HVA | NE | NM | 5-HT | 5-HIAA | |
|---|---|---|---|---|---|---|---|---|
| Frontal cortex | ||||||||
| WT | 0.001±0.001 | 0.049±0.003 | 1.054±0.183 | 0.114±0.008 | 0.596±0.029 | 0.102±0.012 | 0.315±0.013 | 0.255±0.030 |
| DISC1-Q31L | 0.009±0.005 | 0.062±0.008 | 0.927±0.045 | 0.144±0.008 * | 0.603±0.026 | 0.108±0.017 | 0.319±0.022 | 0.326±0.019 |
| Striatum | ||||||||
| WT | 0.005±0.002 | 12.815± 0.284 | 2.103±0.037 | 1.439±0.043 | 0.159±0.009 | 0 | 0.545±0.016 | 0.410±0.017 |
| DISC1-Q31L | 0.034±0.028 | 13.453± 0.296 | 2.180±0.074 | 1.652±0.099 | 0.149±0.006 | 0 | 0.543±0.007 | 0.512±0.013** |
| Nucleus accumbens | ||||||||
| WT | 0.017±0.007 | 9.939±0.844 | 3.463±0.064 | 1.715±0.459 | 0.295±0.026 | 0.043±0.002 | 0.196±0.023 | 0.163±0.016 |
| DISC1-Q31L | 0.043±0.009* | 7.802±0.372* | 4.493±0.204** | 1.359±0.076 | 0.222±0.008* | 0.039±0.002 | 0.141±0.009* | 0.149±0.012 |
| Hippocampus | ||||||||
| WT | 0.001±0.001 | 0.039±0.003 | 1.054±0.183 | 0.114±0.008 | 0.696±0.029 | 0.102±0.011 | 0.415±0.013 | 0.255±0.030 |
| DISC1-Q31L | 0.003±0.003 | 0.022±0.002 | 1.171±0.369 | 0.046±0.005** | 0.718±0.032 | 0.067±0.004 | 0.526±0.014 | 0.509±0.019*** |
Abbreviations: DA, dopamine; DISC1, disrupted-in-schizophrenia-1; DOPA, 3,4-dihydroxyphenylalanine; DOPAC, 3,4-dihydroxy-phenylacetic acid, HVA, homo-vanillic acid; NAC, nucleus accumbens; NE, norepinephrine, NM, normetanephrine; WT, wild type; 5-HT, 5-hidroxitryptamine; 5-HIAA, 5-hydroxyindoleacetic acid.
*p<0.05; **p<0.01; ***p<0.001 in comparison with WT mice.
Social-Conditioned Place Preference
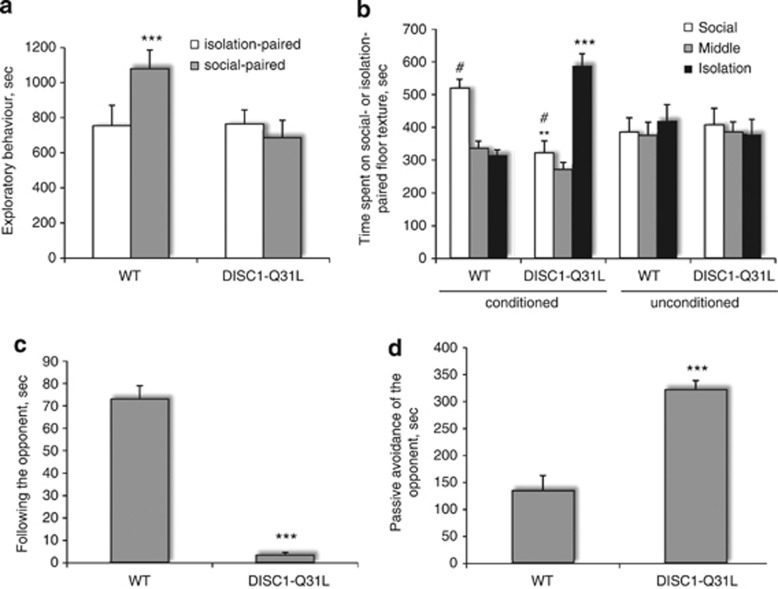
Interacting with conspecifics can be a powerful, natural reward that has been demonstrated in many species and in different conditions (Trezza et al, 2010). We assessed performance of DISC1-Q31L and WT mice in the SCPP task (Figure 1a and b). Analysis of exploratory behavior during the conditioning sessions revealed an effect of social facilitation to increase activity in WT mice but not in DISC1-Q31L mutants (Figure 1a). ANOVA with repeated measures revealed a main effect of genotype (F1,22=164.8; p<0.001), social-paired conditioning (F1,22=76.9; p<0.001), and their interactions (F1,22=81.0; p<0.001). During the test session, socially-conditioned WT mice had a strong preference for the location where social interactions took place, whereas DISC1-Q31L mutants spent more time in the compartment where they were isolated during the conditioning sessions (Figure 1b). ANOVA with repeated measures found a significant effect of genotype (F1,43=16.7, p<0.001), side of the social box (F2,86=189.7, p<0.001), genotype × social conditioning (F1,42=25.9; p<0.001), genotype × side of the social box (F2,86=73.3; p<0.001), social conditioning × side of the social box (F2,86=28.0; p<0.001), and genotype × social conditioning × side of the social box (F2,86=96.4; p<0.001) interactions. Control (unconditioned) mice of both genotypes without social- or isolation-conditioning spent an equal amount of time in each compartment in the SCPP task. Performance in the SCPP test was not affected by motor activity as the number of transitions between the compartments was comparable among genotypes (72.8±14.7 and 62.4±16.5 in the conditioned WT and DISC1-Q31L, and 75.7±10.1 and 70.9±15.6 in unconditioned WT and DISC1-Q31L groups, respectively).
Figure 1.
(a–d) Social anhedonia in DISC1-Q31L mutant mice. (a) No social facilitation was observed in DISC1-Q31L mutants (n=10) during the social-conditioning sessions of the social conditioning place preference (SCPP) test. DISC1-Q31L mutants do not increase their exploration during the socially paired sessions in contrast to their WT littermates (n=14 mice); ***p<0.001—in comparison with isolation-paired WT mice. (b) DISC1-Q31L mutants do not value socializing with familiar cagemates during the test session in SCPP. DISC1-Q31L mutants spent more time in the compartment where they were isolated during the conditioning sessions, whereas socially-conditioned WT mice had a strong preference for locations where social interactions took place. The number of transitions between the compartments is comparable between the genotypes. **p<0.01; ***p<0.001—in comparison with WT; #p<0.001—in comparison with isolation-paired compartment within each genotype; (c–d) DISC1-Q31L mutant mice showed social avoidance of the standard opponent assessed by Neutral Arena task. (c) Amount of time following the opponent was significantly reduced in DISC1-Q31L mutants (n=6) and (d) duration of passive avoidance of the opponent was increased in DISC1-Q31L mice in comparison with WT mice (n=6). ***p<0.001—in comparison with WT mice.
Social Interactions in a Neutral Arena
In this task, sociability is assessed in a neutral arena as the response of the tested mouse to a weight- and age-matched unfamiliar C57BL/6J male mouse (the standard opponent). DISC1-Q31L mice did not follow the opponent as much as WT animals (Figure 1c; F1,10=32.4; p<0.001) and passively avoided social contacts with the opponent (Figure 1d; F1,10=18.6; p<0.001), which is in agreement with the lack of social facilitation and deficient social reward seen in DISC1-Q31L mutants in the SCPP task.
Effects of Buproprion, Fluoxetine, and Desipramine on SCPP
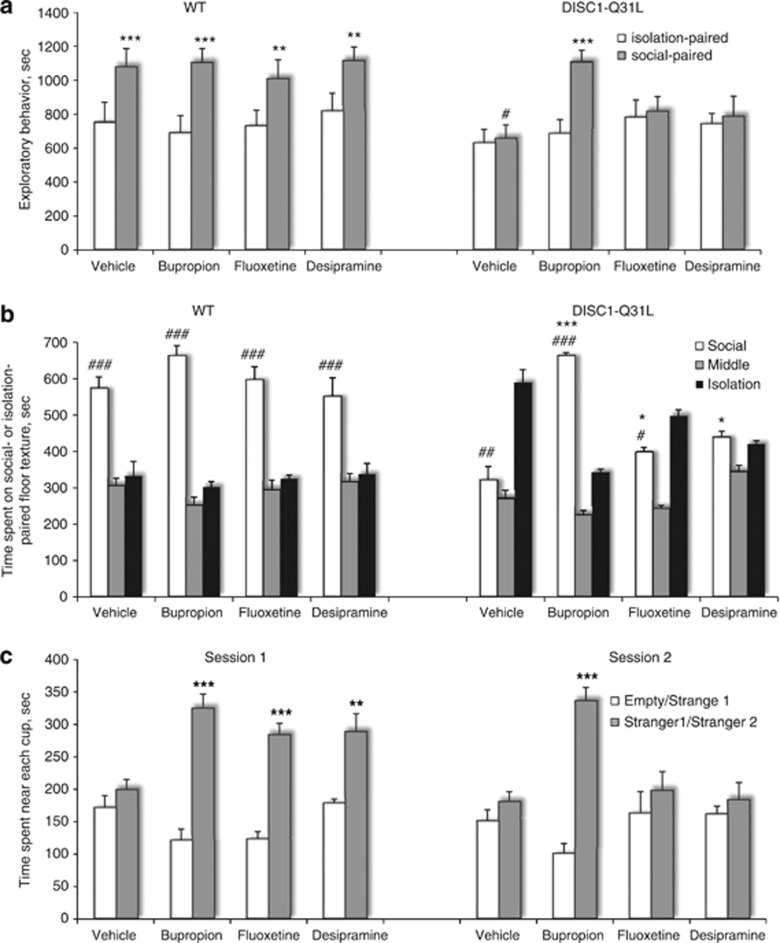
Based on our finding of reduced monoamine levels in the NAC of DISC1-Q31L mutants (Table 1), we examined the ability of three different ADs that preferentially act on DA (bupropion), 5-HT (fluoxetine), or NE (desipramine) neurotransmitter systems to reverse deficient social facilitation and the reduced SCPP in DISC1-Q31L mutants (Figure 2a and b). Analysis of ADs-treated DISC1-Q31L mice during the social-conditioning sessions found that only bupropion induced social facilitation (Figure 2a). ANOVA with repeated measures detected a main effect of the drug (F3,49=12.8; p<0.001), genotype (F1,49=115.6; p<0.001), social-paired conditioning (F1,49; p<0.001), and their interactions. We found that bupropion also had the most pronounced effect on DISC1-Q31L mutants in the SCPP task (Figure 2b). ANOVA found a main effect of the drug (F3,49=3.05; p<0.05), genotype (F1,49=4.3, p<0.05), side of the social box (F2,98=214.8, p<0.001), and their interactions. Bupropion-treated DISC1-Q31L mice switched their preference and spent a significantly longer time on the social-paired floor texture (p<0.001) than vehicle-treated DISC1-Q31L mice. Both fluoxetine and desipramine modestly increased the time spent in the compartment associated with social interactions and slightly reduced duration of time spent on isolation-paired floor texture, but as a result, both these drugs did not correct deficient SCPP in DISC1-Q31L mutants. There was no effect of ADs on number of transitions in each experimental group (Supplementary Table S1). None of the studied ADs affected behavior of WT mice in the SCPP task (Figure 2a and b).
Figure 2.
(a–c) Effects of bupropion, fluoxetine, and desipramine on deficient social behavior in DISC1-Q31L mice. (a)Bupropion-treated DISC1-Q31L mice significantly increased their exploratory behavior during the social-conditioning session, whereas fluoxetine- and desipramine-treated DISC1-Q31L mutants did not show social facilitation. N=6–8 mice per group; #p<0.001—in comparison with social-paired WT mice; **p<0.01, ***p<0.001—in comparison with isolation-paired mice within each experimental group. (b) Only bupropion-treated DISC1-Q31L mutant mice preferred to spend time on social-paired floor texture over the isolation-associated compartment during the test session in the SCPP task, whereas fluoxetine- and desipramine-treated DISC1-Q31L mice spent equal amount of time on both compartments of the SCPP chamber. N=6–8 per each group. #p<0.05; ##p<0.01; ###p<0.001—in comparison with isolation-paired context within each experimental group; *p<0.05; ***p<0.01—in comparison with vehicle-treated DISC1-Q31L mice. (c) All three antidepressant drugs significantly improved social motivation in DISC1-Q31L mice (N=7–8 mice per group) assessed in the ‘session 1' (social affiliation), whereas only bupropion, but not fluoxetine or desipramine, improved deficient social recognition in DISC1-Q31L mutants assessed in the ‘session 2' (social novelty). Antidepressants had no effect on motor activity of mice of both genotypes (Supplementary Table S2) and did not affect performance of WT mice (Supplementary Figure S1) (N=6–8 mice per group); **p<0.01; ***p<0.001—in comparison with either time spent near the ‘empty' cup in the ‘session 1' or near the cup containing ‘stranger 1' in the ‘session 2' within each drug treatment.
Effects of Buproprion, Fluoxetine, and Desipramine on Social Affiliation/Novelty
DISC1-Q31L mutant mice consistently expressed abnormal social behavior assessed by SCPP, neutral arena, and social affiliation/novelty (Clapcote et al, 2007) tests. Although specific aspects of social behavior can be rewarding, it is unknown whether social proximity and/or social discrimination also impart a reward value. In this respect we compared pharmacological effects of ADs in the social affiliation/novelty task (Figure 2c). ANOVA detected a main effect of the genotype (F1,51=17.2; p<0.001), ‘stranger 1' (F1,51=143.2, p<0.001), gene × ‘stranger 1' (F1,51= 43.8, p<0.001), drug × ‘stranger 1' (F3,51=12.1; p<0.001), and gene × drug × ‘stranger 1' interactions (F3,51=33.8; p<0.001). In ‘session 1' vehicle-treated DISC1-Q31L mutant mice did not prefer the chamber containing an unfamiliar conspecific mouse (‘stranger 1') over the chamber containing an empty cup (Figure 2c) unlike their WT littermates (Supplementary Figure S1). Bupropion-, fluoxetine-, and desipramine-treated DISC1-Q31L mutants significantly improved their social motivation assessed in ‘session 1' without any effect on number of entries (Supplementary Table S2).
ANOVA found a significant effect of genotype (F1,51=18.3; p<0.001), ‘stranger 2' (F1,51=145.7; p<0.001), gene × drug (F3,51=9.3; p<0.01), gene × ‘stranger 2' (F1,51=25.6; p<0.001), and gene × drug × ‘stranger 2' interactions (F3,51=8.5; p<0.01). In ‘session 2', vehicle-treated DISC1-Q31L mutants did not discriminate familiar ‘stranger 1' from new one ‘stranger 2' (Figure 2c), whereas WT mice switched their social preference and spent more time in a chamber containing a new unfamiliar mouse (‘stranger 2') (Supplementary Figure S1). Notably, only bupropion-treated DISC1-Q31L mice significantly improved their social recognition, increasing duration of time spent near ‘stranger 2' (p<0.001), but fluoxetine and desipramine had no effect on social recognition (Figure 2c). ADs had no effect on social behavior of WT mice assessed in both the sessions (Supplementary Figure S1) and on number of entries in both the genotypes (Supplementary Table S2).
Spine Density in the NAC of DISC1-Q31L Mutants and Effects of ADs
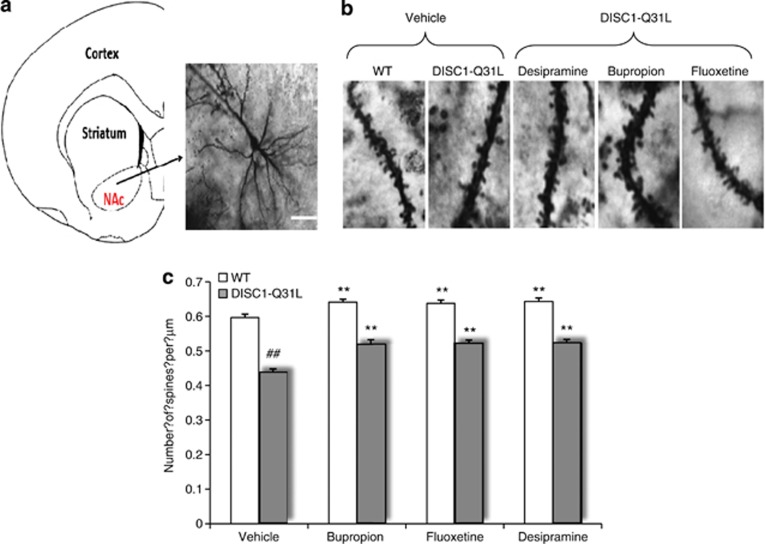
An emerging hypothesis suggests that the pathogenesis and treatment of depression is likely to involve altered neuronal plasticity in the hippocampus in which dendritic spines have a critical role (Calabrese et al, 2006). Although the NAC has not been studied as much, medium spiny neurons present in the NAC are critical for signaling by DA, serotonin, and NE (Shirayama and Chaki, 2006). Hence, disruption of synaptic connections within these neurons could result in dysfunction of reward pathways. Therefore, we examined effects of DISC1-Q31L mutation, as well as ADs treatments on dendritic spines on medium spiny neurons within the NAC (Figure 3a and c). ANOVA found a significant main effect of genotype (F1,173=317.81, p<0.01) and drug treatment (F3,173=20.75, p<0.01) on spine density in the NAC. There were fewer dendritic spines in the NAC in vehicle-treated DISC1-Q31L (p<0.01) compared with WT. Chronic treatments with ADs resulted in modest increases in spine density in both WT and DISC1-Q31L mice, suggesting that different types of ADs have a common effect on spine density within the NAC.
Figure 3.
(a–c) The reduced spine density in the NAC of DISC1-Q31L mice was modestly meliorated by bupropion, fluoxetine, and desipramine. (a) Coronal section of mouse brain with a higher magnification image of neuron in the NAC. Golgi-stained images of spine protrusions at × 100 magnification in wild-type (WT) and DISC1-Q31L mutant mice. (b) High magnification ( × 100) of Golgi-stained images of spine protrusions on apical dendrites. (c) Quantitative analysis of spine density in the NAC in ADs-treated DISC1-Q31L and WT mice. ##p<0.01—in comparison with WT mice; **p<0.01—in comparison with vehicle-treated mice within each experimental group. N=75–100 neurons from 3–5 mice per each treatment group.
Reduced Expression of β-arrestin-1,2 and Total CREB in the NAC, but not in the Hippocampus of DISC1-Q31L Mutant Mice.
Next, we focused our efforts on β-arrestin-1,2 and the transcription factor CREB as molecular biomarkers relevant to depression (Carleton et al, 2005; Schreiber et al, 2009; Uzbek and Hughes, 2011; Ran et al, 2011) and DISC1 pathways (Bolger et al, 2003; Borg et al, 2010; Morris et al, 2003; Malagasy et al, 2012). Both β-arresting and CREB have been implicated in signaling pathways relevant for the pathogenesis of depression and the action of ADs (reviewed in Schreiber et al, 2009). The β-arresting form a complex with PDE4D (Bolger et al, 2003) and PKB (Akt) (Bjørgo et al, (2010)), upstream of GSK-3, and these are DISC1-interacting proteins (Bradshaw and Porteous, 2011). ATF4, a molecular regulator of CREB, also directly interacts with DISC1 (Morris et al, 2003), which can regulate ATF4 activity (Malavasi et al, 2012). Hence, we aimed to probe biochemical changes induced by the DISC1-Q31L mutation in the NAC, as well as in the hippocampus, in order to detect whether biochemical alterations would be specific to the NAC.
First, we performed control experiments and probed β-arrestin-1,2 in brain lysates of β-arrestin-1 and β-arrestin-2 knockout mice as negative controls. As can be seen in Supplementary Figure S3, there was a clear absence of β-arrestin-1 or β-arrestin-2 bands probed by β-arrestin-1,2 antibodies in the corresponding knockout mouse lines, verifying the specificity of the antibodies used.
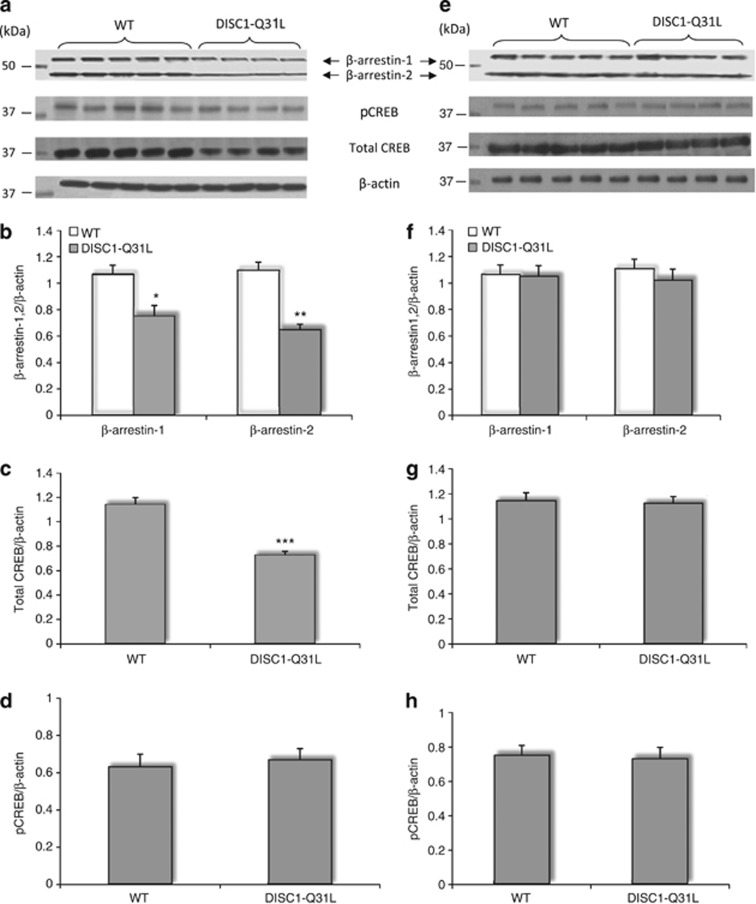
ANOVA detected a main effect of genotype on β-arrestin-1 (F1,20=18.3; p<0.05) and on β-arrestin-2 (F1,20=31.5; p<0.01) in the NAC (Figure 4a and b), but not in the hippocampus (Figure 4e and f). The protein levels of both β-arrestin-1 and β-arrestin-2 were significantly reduced in the NAC of DISC1-Q31L mutant mice in comparison with WT. A similar decrease in β-arrestin-1 was detected in mononuclear leukocytes of patients with depression (Schreiber et al, 2009), supporting our findings.
Figure 4.
(a–h) The reduced protein levels of β-arrestin-1,2 and total CREB in the NAC but not in the hippocampus of DISC1-Q31L mice. (a) Representative immunoblots are shown, probed with antibodies against β-arrestin-1,2, total CREB, pCREB at Ser129/133, as well as β-actin as a loading control in the NAC of WT and DISC1-Q31L mice. Quantification of immunoblots data for β-arrestin-1, β-arrestin-2 as normalized to the total amount of β-actin (b); for pCREB at Ser129/133 as normalized to the total amount of β-actin (c), and for the total CREB as normalized to the total amount of β-actin (d). (e) Representative immunoblots are shown, probed with antibodies against β-arrestin-1,2, total CREB, pCREB at Ser129/133, as well as β-actin as a loading control in the hippocampus of WT and DISC1-Q31L mice. Quantification of immunoblots data for β-arrestin-1, β-arrestin-2 as normalized to the total amount of β-actin (f); for pCREB at Ser129/133 as normalized to the total amount of β-actin (g), and for the total CREB as normalized to the total amount of β-actin (h). N=8–12 animals per genotype; *p<0.05; **p<0.01; ***p<0.001—in comparison with WT.
Next, we examined the phopshorylation of CREB at Ser129/133 and total expression of CREB in lysates from NAC and the hippocampus of DISC1-Q31L and WT mice. We found significantly reduced level of total CREB (F1,20=109.6; p<0.001), but not the p-Ser129/133-CREB (F1,20=1.3; p>0.05) in the NAC of DISC1-Q31L mutants compared with WT littermates (Figure 4a, c and d). In contrast, there were no changes in total CREB, as well as p-Ser129/133-CREB in the hippocampus of DISC1-Q31L mice (Figure 4e, f and h). All together, our findings highlighting specific alterations of β-arrestin-1,2 and CREB in the NAC.
Effects of Bupropion, Fluoxetine and Desipramine on β-arrestin-1,2, Total CREB and Phosphorylation of CREB at Ser 129/133 in the NAC of DISC1 Mice
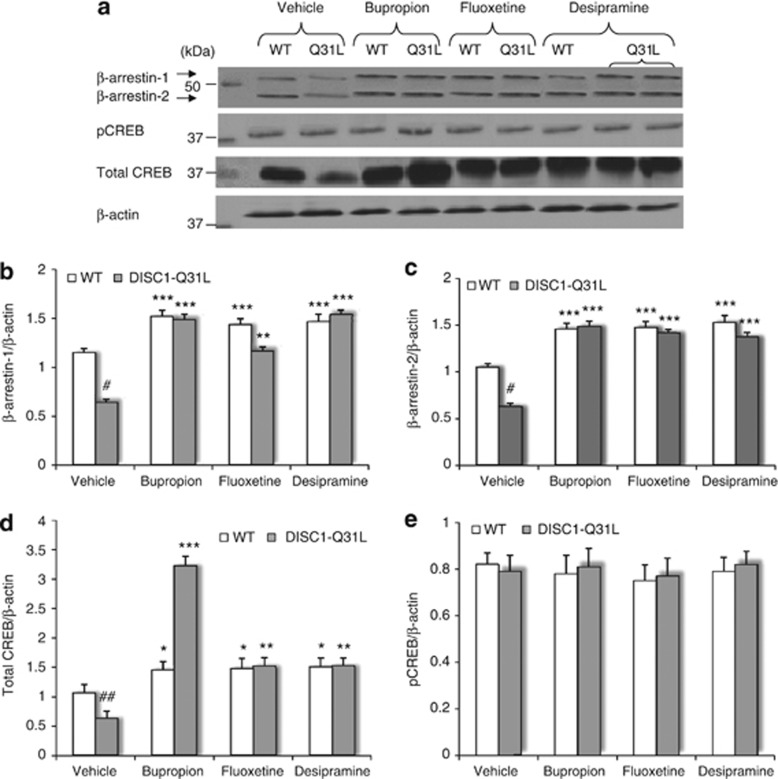
Next, we sought to correlate the pharmacological efficts of ADs on social behavior with molecular alterations in the NAC of DISC1 mice. ANOVA found a main effect of genotype (F3,39=33.5; p<0.001), drug treatment, (F3,39=48.2; p<0.001) and gene × drug interactions (F3,39=18.3; p<0.001) on β-arrestin-1, as well as a main effect of drug (F3,39=51.8; p<0.001) and gene × drug (F3,39=20.8; p<0.001) on β-arrestin-2 protein levels (Figure 5a–c). All three ADs increased β-arrestin-1,2 levels in both genotypes. Normalization of β-arrestin-1,2 protein levels in the NAC of DISC1-Q31L mice by bupropion, fluoxetine and desipramine is consistent with clinical findings (Schreiber et al, 2009), where β-arrestin-1 levels in leukocytes of depressive patients have been suggested as a biomarker for treatment response.
Figure 5.
(a–e) Effects of bupropion, fluoxetine, and desipramine on protein amount of β-arrestin-1,2, total CREB, and phosphorylation of CREB at Ser 129/133 in the NAC of DISC1-Q31L mice. (a) Representative immunoblots are shown, probed with antibodies against β-arrestin-1,2, total CREB, pCREB at Ser 129/133, as well as β-actin as a loading control in antidepressants-treated DISC1-Q31L (Q31L) and WT mice. Quantification of immunoblots data for β-arrestin-1 and β-arrestin-2 as normalized to the total amount of β-actin (b–c); (d) for the total CREB as normalized to the total amount of β-actin and (e) for pCREB at Ser129/133 as normalized to the total amount of β-actin. N=6–7 mice per each group. #p<0.05; ##p<0.01; ###p<0.001—in comparison with vehicle-treated WT mice; *p<0.05; **p<0.01; ***p<0.001—in comparison with vehicle-treated mice within each genotype.
ANOVA detected significant main effect of genotype (F1,39=40.2; p<0.001), drug treatment (F3,39=124.4; p<0.001), and their interactions (F3,39=80.4; p<0.001) on the total amount of CREB in the NAC of DISC1 mice (Figure 5a and d), with no effect on pCREB at Ser129/133 (F1,39=2.3; p>0.05—genotype; F3,39=0.89; p>0.05—drug treatment) (Figure 5a and e) in WT and DISC1-Q31L mice, consistent with previously reported data (Pascual-Brazo et al, 2012). Notably, bupropion elicited the most pronounced effect on CREB (p<0.001) in DISC1-Q31L mutants (Figure 5a and d).
DISCUSSION
DISC1-Q31L as a Genetic Mouse Model of Depression
The neurobiology of depression is still poorly understood and new, more effective treatments are highly desirable. In the current study we examined the DISC1-Q31L genetic mouse model of depression for etiological, face, construct, and predictive validity (Cryan and Holmes, 2005). First, we identified that the NAC is a key brain area associated with social anhedonia as depression-like behavioral endophenotype in DISC1-Q31L mutants, where revealed alterations in neurochemical (reduced content of DA, serotonin, and NE), molecular (reduced levels of β-arrestin-1,2 and total CREB), and cellular (reduced spine density) function that are similar to biological endophenotypes of depression in humans (Carlezon et al, 2005; Schreiber et al, 2009; Hasler et al, 2004). This provides further support for construct validity of the DISC1-Q31L model. Given the importance of the NAC for reward-related behavior (Shirayama and Chaki, 2006) and depression (Treadway and Zald, 2011), the DISC1-Q31L genetic mouse offers a model to study neurobiological mechanisms of anhedonia, common to rodents and humans (Cryan and Holmes, 2005).
In the current study we found deficient social reward in DISC1-Q31L mutants that was corrected by bupropion, suggesting that social anhedonia in the SCPP task might be a novel endophenotype of depression. Of course, further validations in other animal models of depression are needed to confirm this notion. Despite the ability of bupropion to correct social anhedonia and associated changes in CREB, we cannot exclude the possibility that other neurotransmitter systems and pathways are also involved in modulating depression-related behaviors in DISC1-Q31L mice. There are neurochemical changes in other brain regions (Table 1), as well as reduced spine density in the hippocampus and frontal cortex (Lee et al, 2011). All studied ADs we examined were able to ameliorate social motivation and behavioral despair (Figure 2c, Supplementary Figure S2). Hence, it is important to investigate neurobiological mechanisms in other brain areas (eg, hippocampus) underlying particular behavioral endophenotypes of depression in DISC1 mutants.
Social Reward
Humans are social and the natural tendency to attend to the social world is present early in development and remains throughout life. Social rewards can cross species, as shown by chimpanzees learning a discrimination task to gain the reward of grooming the experimenter (Falk, 1958). Physical contact with another member of the same species can itself be rewarding too (Angermeir, 1960). In our study we demonstrated the reward value of interacting with a familiar partner in adult WT mice in the SCPP task. Notably, during the social-conditioning sessions, socially-paired WT mice increased their exploratory activity, demonstrating an effect of social facilitation, ie, the effect of the presence of another individual. Social facilitation influences feeding, locomotion, emotional, exploratory, and operant behavior in rodents (Wills et al, 1983), and occurs more frequently among familiar individuals (Krames and Shaw, 1973). In contrast, no social facilitation was observed in the conditioning sessions in DISC1-Q31L mutants and these mice did not develop SCPP. These data suggest a loss of social reward in DISC1-Q31L mutants, and that social facilitation significantly contributed to SCPP in WT mice.
ADs can influence neuronal substrates important for social behavior in depression (Novick, 2011), but are not effective in children with autism (McPheeters et al, 2011), another disorder associated with altered social behavior. Interestingly, there is some overlap between neural areas mediating social behavioral therapy with those affected by ADs medications (Novick, 2011). We compared here the effects of three ADs on distinct aspects of social behavior in the DISC1-Q31L mouse model of depression. Of these ADs only bupropion corrected the deficient social reward in SCPP in DISC1-Q31L mutants, despite the fact that all three ADs rescued high immobility in the forced swim test (Supplementary Figure S2) and ameliorated deficient social motivation (Figure 2c) in DISC1-Q31L mutants. Interestingly, bupropion specifically rescued deficient social recognition (Figure 2c), which in turn induced social facilitation and led to the development of SCPP in DISC1-Q31L mice.
Such distinct efficacies of ADs on DISC1-Q31L mutants highlight the role of the mesolimbic DA system in social discrimination and social reward. Indeed, a selective blockade of DA D3 receptors enhances social novelty discrimination in rats (Watson et al, 2011), whereas D2 antagonists induced the opposite effect, suggesting that mutated DISC1-Q31L might affect the function of D2/D3 receptors, which are implicated in reward-seeking activity (Beaulieu and Gainetdinov, 2011) and the action of bupropion (Kitamura et al, 2010). Nevertheless, given that only one dose was used for each drug, perhaps higher concentrations or/and longer periods of treatments might be needed for fluoxetine and desipramine to normalize social anhedonia in DISC1-Q31L mice.
Dopamine and Depression
Anhedonia and loss of motivation are core features of depression (Kapur and Mann, 1996) and altered DA neurotransmission in the mesolimbic system could be involved in depression (Nestler and Carlezon, 2006). Approximately 40% of patients diagnosed with major depression suffer from clinically significant anhedonia (Pelizza and Ferrari, 2009) and it is a difficult symptom to treat (Nutt et al, 2007; Price et al, 2009). However, neuroimaging, pharmacological and genetic studies strongly indicate impaired DA function in some patients with depression (Nestler and Carlezon, 2006; Dunlop and Nemeroff, 2007; Yadid and Friedman, 2008).
Indeed, analysis of brain monoamines and their metabolites in DISC1-Q31L mice revealed lower levels of DA, as well as serotonin and NE in the NAC. These findings are consistent with the idea of hypofunction of monoamine neurotransmitter systems in depression (Charney, 1998). However, decreases in DA in combination with increased levels of DOPA and the DA metabolite—DOPAC could reflect higher DA turnover in the NAC of DISC1-Q31L mutant mice. The increased amount of DA metabolite could be related to increased enzymatic activity of monoamine oxidase (MAO), which has been implicated into the pathology of depression (Lung et al, 2011). MAO inhibitors are used as a last line of treatment when other AD drugs are not effective (Pae et al, 2009). The possible upregulation of DA mesolimbic system in DISC1-Q31L mutant mice is consistent with the earlier demonstration of an increased firing of DA neurons ex vivo in the ventral tegmental area, which project to the NAC, in susceptible but not resilient mice chronically exposed to social defeat (Krishnan et al, 2007). Moreover, increased firing rates and bursting events negatively correlated with social avoidance and were reversed by fluoxetine (Cao et al, 2010).
Synaptic Plasticity and Depression
Dendritic spine plasticity is a critical factor of reorganization of the brain and impaired adaptive changes in spines are suggested to underlie such neuropsychiatric disorders as anxiety, depression, and drug addiction (Berton and Nestler 2006). Neuronal plasticity in the hippocampus has been extensively studied with regard to spine density and depression, with less attention paid to the NAC. However, medium spiny neurons present in the NAC are critical connections for various neurotransmitters including DA, serotonin, and NE. Hence, reduced spine density in the NAC of DISC1-Q31L mutants impairs synaptic connectivity within these neurons and could result in dysfunction of monoamine systems. Our results are consistent with previously reported reductions in dendritic spine density in the hippocampus and frontal cortex of DISC1-Q31L mutants, (Lee et al, 2011) and further support a role for DISC1 in modulating synaptic transmission (Bradshaw and Porteous, 2011).
However, it raises the question of the specific role of deficient spine density in the NAC for depression-related phenotypes in DISC1-Q31L mutant mice. DISC1 regulates spine formation through neurodevelopment (Bradshaw and Porteous, 2011), and likely results in a global spine deficiency in DISC1-Q31L mutants. Indeed, the inability of ADs to fully normalize the reduced spine density in adult DISC1-Q31L mice suggest that ADs might need to be given at earlier stage of development or for a longer period to fully correct spine density. The incomplete normalization of spine density by ADs treatments contrasts with complete correction of CREB, β-arrestins and deficient social behavior in DISC1 mutants. This suggests that other structural changes must underlie the behavioral effects of ADs in the NAC, or that structural changes in other brain regions such as the hippocampus should be investigated. Indeed, the hippocampus has different neurochemical and biochemical alterations than the NAC (Table 1, Figure 4a–h). Notably, the levels of DA metabolite—HVA and 5-HT metabolite—5-HIAA (both products of MAO), were significantly changed in the hippocampus of DISC1-Q31L mutants, suggesting impaired MAO function in this brain region. Given the role of MAO in depression (Lung et al, 2011), and that a MAO inhibitor significantly increased dendritic branching of CA3 hippocampal pyramidal neurons in primate brain (Lakshmana et al, 1998), it would be worth exploring the effects of ADs on spine density and biochemical cascades in the hippocampus in DISC1-Q31L mutants.
The reduced spine density in DISC1-Q31L mice would likely affect their adaptive synaptic plasticity in response to stressful events and hence, might be a critical component predisposing to the development of depression. Indeed, a range of stress procedures significantly influence the neuronal morphology of a variety of cell types, including neurons of the medial prefrontal cortex (Radley et al, 2006), medium spiny neurons of the NAC (Campioni et al, 2009; Vialou et al, 2010; Christoffel et al, 2011) or hippocampal pyramidal neurons (Magariños and McEwen, 1995; Sousa et al, 2000; Chen et al, 2010). In contrast to our findings, stress elicited different effects on spine density in distinct brain areas: induces more stubby spine structures, its functional correlate, mEPSCs (Campioni et al, 2009; Vialou et al, 2010; Christoffel et al, 2011), or increases AMPA/NMDA ratios (Campioni et al, 2009) in the NAC, but decreases spine density in the hippocampus (Magariños and McEwen, 1995; Sousa et al, 2000; Chen et al, 2010).
DISC1 Pathway and Depression
DISC1 and its network is comprised of 158 protein–protein interactions (Camargo et al, 2007) that regulate inter- and intra-cellular components, so point mutations such as DISC1-Q31L could affect many other proteins. Indeed, in the current study we detected effects of the DISC1-Q31L point mutation on β-arrestin-1,2, CREB, dendritic spine density, monoamine levels, and social behaviors. The β-arrestins act as scaffolds and adapters in receptor endocytosis, signal transduction via different pathways and also can translocate into the nucleus and associate with such transcription factors as p300 and CREB (Ma and Pei, 2007). β-arrestin-1,2 have been implicated in the action of AD medications through modulating DA, serotonin and noradrenaline signaling in brain (Small et al, 2006; Beaulieu and Gainetdinov, 2011; Schreiber et al, 2009). Notably, β-arrestin-1,2 directly interacts with PDE4D (Bolger et al, 2003) and PKB (Akt) (Bjørgo et al, 2010), upstream regulators of GSK-3 activity (Jope and Roh, 2006). GSK-3 signaling can be regulated by a β-arrestin-2 complex (Beaulieu et al, 2005; Beaulieu et al, 2007). DISC1 forms and regulates a multi-enzymatic complex with PDE4B (Millar et al, 2005) and GSK-3 (Mao et al, 2009), which act synergistically (Lipina et al, 2011). The reduction in β-arrestin-1,2 in DISC1-Q31L mutants is consistent with clinical findings (Avissar et al, 2004; Matuzany-Ruban et al, 2005) and suggests that DISC1 may tune the function of β-arrestin-1,2 via PDE4 and GSK-3. The β-arrestins directly regulate the function of DA receptors, DA neurotransmission and DA-related behaviors (Beaulieu and Gainetdinov, 2011), as well as modulate the signaling of 5-HT (Bohn and Schmid, 2010) and adrenergic receptors (Shenoy, 2011). Thus, the reduced β-arrestin-1,2 level specifically within the NAC, but not in the hippocampus, of DISC1-Q31L mutants could contribute to the monoaminergic imbalance in this brain region.
The transcription factor CREB, localized within the nucleus, mediates the expression of several genes (eg, TH, BDNF, Dynorphin, CRF, GluR1), which ultimately change the function of neuronal circuits (Carlezon et al, 2005). We found reduced total CREB expression in DISC1-Q31L mutants within the NAC. Given that the DISC1-Q31L mutation decreases PDE4B enzymatic activity (Clapcote et al, 2007), we suggest that accumulating cAMP levels in the cell could influence CREB function. The reduced level of β-arrestin-1,2 found in DISC1-Q31L mutants may also contribute to reduced CREB expression as β-arrestins can directly promote CREB transcription (Ma and Pei, 2007).
ATF4 was originally described as a transcriptional repressor, negatively regulating transcription via the CRE of the human enkephalin promoter (Karpinski et al, 1992) that could act as a repressor of long-term memory storage (Bartsch et al, 1995; Chen et al, 2003). A recent study (Malavasi et al, 2012) revealed an inhibitory effect of DISC1 on ATF4 luciferase activity, an effect that was weakened by DISC1 variants 37W and 607F, suggesting that DISC1-Q31L mutation could increase ATF activity that in turn could down-regulate CREB expression. Hence, it would be interesting to further study the functional role of the DISC1-ATF4/CREB complex in mesolimbic brain areas in regards to depression-related behaviors in the DISC1-Q31L mouse. One approach could be to manipulate by protein–protein interactions and ATF4/CREB gene expression in the NAC and compare effects of such manipulations within ventral tegmental area, as another key brain region that modulates NAC function (Krishnan et al, 2007). Interestingly, ATF4 over-expression in the NAC of C57BL/6J inbred mice decreased their emotional reactivity and increased depression-like behavior (Green et al, 2008). The down-regulation of CREB expression, induced by the DISC1-Q31L mutation is similar to the effect of various stressors used to model depression (Alfonso et al, 2006; Nibuya et al, 1996), or to the CREB-deficient mice that expressed depression-like behaviors (Zubenko and Hughes, 2011). Moreover, the decreased expression of CREB has been detected in temporal cortex (Dowlatshahi et al, 1999) and in neutrophils (Ren et al, 2011) of drug-free depressed patients.
Conclusions
Collectively, our findings lead us to propose DISC1 as a genetic modulator of depression at several levels of organization of the nervous system. We have shown reduced levels of monoamines, deficient dendritic spine density, accompanied by reduced levels of β-arrestin-1,2 and CREB within the NAC induced by DISC1-Q31L mutation. Thus DISC1 can help to integrate the classical monoaminergic theory of depression (Charney, 1998) and the new neuroplasticity hypothesis (Berton and Nestler, 2006). Moreover, we demonstrated here that DISC1 has an important role in social behavior and that social anhedonia could be a useful phenotype for modeling depression in rodents. In summary, the DISC1-Q31L genetic mouse model offers an opportunity to further study pathological mechanisms of depression at multiple levels of analysis, including genetic, cellular, biochemical, electrophysiological, or networks of brain pathways, which could ultimately lead to more effective treatments for depression.
Acknowledgments
We gratefully acknowledge Dr John Chambers for performing HPLC-EC, Drs JR Woodgett and O Kaidanovich-Beilin for pCREB-Ser129/133 and total CREB antibodies and Professor MG Caron and Caroline Ray for providing brains of β-arrestin-1-KO and β-arrestin-2-KO mice. TVL was generously supported by Venture Women Sinai Award. AHCW was supported by a CIHR Clinician-Scientist Fellowship and is a NARSAD Independent Investigator.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Alfonso J, Frick LR, Silberman DM, Palumbo ML, Genaro AM, Frasch AC. Regulation of hippocampal gene expression is conserved in two species subjected to different stressors and antidepressant treatments. Biol Psychiatry. 2006;59:244–251. doi: 10.1016/j.biopsych.2005.06.036. [DOI] [PubMed] [Google Scholar]
- Angermeir WR. Some basic aspects of social reinforcement in albaino rats. J Comp Physiol Psychol. 1960;58:364–367. [Google Scholar]
- Avissar S, Matuzany-Ruban A, Tzukert K, Schreiber G. Beta-Arrestin-1 levels: Reduced in leukocytes of patients with depression and elevated by antidepressants in rat brain. Am J Psychiatry. 2004;161:2066–2072. doi: 10.1176/appi.ajp.161.11.2066. [DOI] [PubMed] [Google Scholar]
- Bartsch D, Ghirardi M, Skehel PA, Karl KA, Herder SP, Chen M, et al. Aplysia CREB2 represses long-term facilitation: relief of repression converts transient facilitation into long-term functional and structural change. Cell. 1995;83:979–992. doi: 10.1016/0092-8674(95)90213-9. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Sotnikova TD, Marion S, Lefkowitz RJ, Gainetdinov RR, Caron MG. An Akt/beta-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell. 2005;122:261–273. doi: 10.1016/j.cell.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Gainetdinov RR, Caron MG. The Akt-GSK-3 signaling cascade in the actions of dopamine. Trends Pharmacol Sci. 2007;28:166–172. doi: 10.1016/j.tips.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev. 2011;63:182–217. doi: 10.1124/pr.110.002642. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM. A role for Akt and glycogen synthase kinase-3 as integrators of dopamine and serotonin neurotransmission in mental health. J Psychiatry Neurosci. 2011;37:7–16. doi: 10.1503/jpn.110011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berton O, Nestler EJ. New approaches to antidepressant drug discovery: beyond monoamines. Nat Rev Neurosci. 2006;7:137–151. doi: 10.1038/nrn1846. [DOI] [PubMed] [Google Scholar]
- Bjørgo E, Solheim SA, Abrahamsen H, Baillie GS, Brown KM, Berge T, et al. Cross talk between phosphatidylinositol 3-kinase and cyclic AMP (cAMP)-protein kinase a signaling pathways at the level of a protein kinase B/beta-arrestin/cAMP phosphodiesterase 4 complex. Mol Cell Biol. 2010;7:1660–1672. doi: 10.1128/MCB.00696-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn LM, Schmid CL. Serotonin receptor signaling and regulation via β-arrestins. Crit Rev Biochem Mol Biol. 2010;45:555–566. doi: 10.3109/10409238.2010.516741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger GB, McCahill A, Huston E, Cheung YF, McSorley T, Baillie GS, et al. The unique amino-terminal region of the PDE4D5 cAMP phosphodiesterase isoform confers preferential interaction with beta-arrestins. J Biol Chem. 2003;278:49230–49238. doi: 10.1074/jbc.M303772200. [DOI] [PubMed] [Google Scholar]
- Bradshaw NJ, Porteous DJ. DISC1-binding proteins in neural development, signalling and schizophrenia. Neuropharmacology. 2011;62:1230–1241. doi: 10.1016/j.neuropharm.2010.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese B, Wilson MS, Halpain S. Development and regulation of dendritic spine synapses. Physiology (Bethesda) 2006;21:38–47. doi: 10.1152/physiol.00042.2005. [DOI] [PubMed] [Google Scholar]
- Camargo LM, Collura V, Rain JC, Mizuguchi K, Hermjakob H, Kerrien S, et al. Disrupted-In-Schizophrenia-1 interactome: evidence for the close connectivity of risk genes and a potential synaptic basis for schizophrenia. Mol Psychiatry. 2007;12:74–86. doi: 10.1038/sj.mp.4001880. [DOI] [PubMed] [Google Scholar]
- Campioni MR, Xu M, McGehee DS. Stress-induced changes in nucleus accumbens glutamate synaptic plasticity. J Neurophysiol. 2009;101:3192–3198. doi: 10.1152/jn.91111.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao JL, Covington HE, Friedman AK, Wilkinson MB, Walsh JJ, Cooper DC, et al. Mesolimbic dopamine neurons in the brain reward circuit mediate susceptibility to social defeat and antidepressant action. J Neurosci. 2010;30:16453–16458. doi: 10.1523/JNEUROSCI.3177-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA, Duman RS, Nestler EJ. The many faces of CREB. Trends Neurosci. 2005;28:436–445. doi: 10.1016/j.tins.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Charney DS. Monoamine dysfunction and the pathophysiology and treatment of depression. J Clin Psychiatry. 1998;59:11–14. [PubMed] [Google Scholar]
- Chen A, Muzzio IA, Malleret G, Bartsch D, Verbitsky M, Pavlidis P, et al. Inducible enhancement of memory storage and synaptic plasticity in transgenic mice expressing an inhibitor of ATF4 (CREB-2) and C/EBP proteins. Neuron. 2003;39:655–669. doi: 10.1016/s0896-6273(03)00501-4. [DOI] [PubMed] [Google Scholar]
- Chen Y, Rex CS, Rice CJ, Dubé CM, Gall CM, Lynch G, et al. Correlated memory defects and hippocampal dendritic spine loss after acute stress involve corticotropin-releasing hormone signaling. Proc Natl Acad Sci USA. 2010;107:13123–13128. doi: 10.1073/pnas.1003825107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoffel DJ, Golden SA, Dumitriu D, Robison AJ, Janssen WG, Ahn HF, et al. IκB kinase regulates social defeat stress-induced synaptic and behavioral plasticity. J Neurosci. 2011;31:314–321. doi: 10.1523/JNEUROSCI.4763-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapcote SJ, Lipina TV, Millar JK, Mackie S, Christie S, Ogawa F, et al. Behavioral phenotypes of Disc1 missense mutations in mice. Neuron. 2007;54:387–402. doi: 10.1016/j.neuron.2007.04.015. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Holmes A. The ascent of mouse: advances in modelling human depression and anxiety. Nat Rev Drug Discov. 2005;4:775–790. doi: 10.1038/nrd1825. [DOI] [PubMed] [Google Scholar]
- Dowlatshahi D, MacQueen GM, Wang JF, Reiach JS, Young LT. G Protein-coupled cyclic AMP signaling in postmortem brain of subjects with mood disorders: effects of diagnosis, suicide, and treatment at the time of death. J Neurochem. 1999;73:1121–1126. doi: 10.1046/j.1471-4159.1999.0731121.x. [DOI] [PubMed] [Google Scholar]
- Dunlop BW, Nemeroff CB. The role of dopamine in the pathophysiology of depression. Arch Gen Psychiatry. 2007;64:327–337. doi: 10.1001/archpsyc.64.3.327. [DOI] [PubMed] [Google Scholar]
- Falk JL. The grooming behaviour of the chimpanzee as a reinforcer. J Exp Animal Behav. 1958;1:83–85. doi: 10.1901/jeab.1958.1-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green TA, Alibhai IN, Unterberg S, Neve RL, Ghose S, Tamminga CA, et al. Induction of activating transcription factors (ATFs) ATF2, ATF3, and ATF4 in the nucleus accumbens and their regulation of emotional behavior. J Neurosci. 2008;28:2025–2032. doi: 10.1523/JNEUROSCI.5273-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler G, Drevets WC, Manji HK, Charney DS. Discovering endophenotypes for major depression. Neuropsychopharmacology. 2004;29:1765–1781. doi: 10.1038/sj.npp.1300506. [DOI] [PubMed] [Google Scholar]
- Jope RS, Roh M-S. Glycogen synthase kinase-3 (GSK-3) in psychiatric diseases and therapeutic interventions. Curr Drug Targets. 2006;7:1421–1434. doi: 10.2174/1389450110607011421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur S, Mann JJ.1996Role of the dopaminergic system in depression Eur J Pharmacol 29827–30.8867915 [Google Scholar]
- Karpinski BA, Morle GD, Huggenvik J, Uhler MD, Leiden JM. Molecular cloning of human CREB-2: an ATF/CREB transcription factor that can negatively regulate transcription from the cAMP response element. Proc Natl Acad Sci USA. 1992;89:4820–4824. doi: 10.1073/pnas.89.11.4820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura Y, Yagi T, Kitagawa K, Shinomiya K, Kawasaki H, Asanuma M, et al. Effects ofbupropion on the forced swim test and release of dopamine in the nucleus accumbens in ACTH-treated rats. Naunyn Schmiedebergs Arch Pharmacol. 2010;382:151–158. doi: 10.1007/s00210-010-0521-x. [DOI] [PubMed] [Google Scholar]
- Krames B, Shaw L. Role of previous experience in the male rat's reaction to odors from roup and alien conspecifics. J Comp Physiolo Psychol. 1973;82:444–448. doi: 10.1037/h0034126. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, et al. Molecular adaptations underlying susceptibility and resistance to socialdefeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Lakshmana MK, Rao BS, Dhingra NK, Ravikumar R, Govindaiah, Ramachandra, et al. Chronic (-) deprenyl administration increases dendritic arborization in CA3 neurons of hippocampus and AChE activity in specific regions of the primate brain. Brain Res. 1998;796:38–44. doi: 10.1016/s0006-8993(98)00312-6. [DOI] [PubMed] [Google Scholar]
- Lee FH, Fadel MP, Preston-Maher K, Cordes SP, Clapcote SJ, Price DJ, et al. Disc1 point mutations in mice affect development of the cerebral cortex. J Neurosci. 2011;31:3197–3206. doi: 10.1523/JNEUROSCI.4219-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipina TV, Roder JC. A new model of the disrupted latent inhibition in C57BL/6J mice after bupropion treatment. Psychopharmacology. 2010;208:487–498. doi: 10.1007/s00213-009-1749-3. [DOI] [PubMed] [Google Scholar]
- Lipina TV, Wang M, Liu F, Roder JC. Synergistic interactions between PDE4B and GSK-3: DISC1 mutant mice. Neuropharmacology. 2011;62:1252–1262. doi: 10.1016/j.neuropharm.2011.02.020. [DOI] [PubMed] [Google Scholar]
- Lung FW, Tzeng DS, Huang MF, Lee MB. Association of the MAOA promoter uVNTR polymorphism with suicide attempts in patients with major depressive disorder. BMC Med Genet. 2011;12:74. doi: 10.1186/1471-2350-12-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Pei G. Beta-arrestin signaling and regulation of transcription. J Cell Sci. 2007;120:213–218. doi: 10.1242/jcs.03338. [DOI] [PubMed] [Google Scholar]
- Mao Y, Ge X, Frank CL, Madison JM, Koehler AN, Doud MK, et al. Disrupted-in-schizophrenia-1 regulates neuronal progenitor proliferation via modulation of GSK3beta/beta-catenin signaling. Cell. 2009;136:1017–1031. doi: 10.1016/j.cell.2008.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magariños AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: involvement of glucocorticoid secretion and excitatory amino acid receptors. Neuroscience. 1995;69:89–98. doi: 10.1016/0306-4522(95)00259-l. [DOI] [PubMed] [Google Scholar]
- Malavasi EL, Ogawa F, Porteous DJ, Millar JK. DISC1 variants 37W and 607F disrupt its nuclear targeting and regulatory role in ATF4-mediated transcription. Hum Mol Genet. 2012;21:2779–2792. doi: 10.1093/hmg/dds106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matuzany-Ruban A, Avissar S, Schreiber G. Dynamics of beta-arrestin-1 protein and mRNA levels elevation by antidepressants in mononuclear leukocytes of patients with depression. J Affect Disorder. 2005;88:307–312. doi: 10.1016/j.jad.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Millar JK, Pickard BS, Mackie S, James R, Christie S, Buchanan SR, et al. DISC1 and PDE4B are interacting genetic factors in schizophrenia that regulate cAMP signaling. Science. 2005;310:1187–1191. doi: 10.1126/science.1112915. [DOI] [PubMed] [Google Scholar]
- McPheeters ML, Warren Z, Sathe N, Bruzek JL, Krishnaswami S, Jerome RN, et al. A systematic review of medical treatments for children with autism spectrum disorders. Pediatrics. 2011;127:e1312–e1321. doi: 10.1542/peds.2011-0427. [DOI] [PubMed] [Google Scholar]
- Morris JA, Kandpal G, Ma L, Austin CP. DISC1 (Disrupted-In-Schizophrenia 1) is a centrosome-associated protein that interacts with MAP1A, MIPT3, ATF4/5 and NUDEL: regulation and loss of interaction with mutation. Hum Mol Genet. 2003;12:1591–1608. doi: 10.1093/hmg/ddg162. [DOI] [PubMed] [Google Scholar]
- Murdoch H, Mackie S, Collins DM, Hill EV, Bolger GB, Klussmann E, et al. Isoform-selective susceptibility of DISC1/phosphodiester complexes to dissociation by elevated intracellular cAMP levels. J Neurosci. 2007;27:9513–9524. doi: 10.1523/JNEUROSCI.1493-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Carlezon WA. The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Nibuya M, Nestler EJ, Duman RS. Chronic antidepressant administration increases the expression of cAMP response element binding protein (CREB) in rat hippocampus. J Neurosci. 1996;16:2365–2372. doi: 10.1523/JNEUROSCI.16-07-02365.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick AM. Antidepressant psychopharmacology and the social brain. Psychiatry. 2011;74:72–86. doi: 10.1521/psyc.2011.74.1.72. [DOI] [PubMed] [Google Scholar]
- Nutt D, Demyttenaere K, Janka Z, Aarre T, Bourin M, Canonico PL, et al. The other face of depression, reduced positive affect: the role of catecholamines in causation and cure. J Psychopharmacol. 2007;21:461–467. doi: 10.1177/0269881106069938. [DOI] [PubMed] [Google Scholar]
- Pae CU, Tharwani H, Marks DM, Masand PS, Patkar AA. Atypical depression: a comprehensive review. CNS Drugs. 2009;23:1023–1037. doi: 10.2165/11310990-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Panksepp JB, Lahvis GP. Social reward among juvenile mice. Genes Brain Behav. 2007;7:661–671. doi: 10.1111/j.1601-183X.2006.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papaleo F, Lipska BK, Weinberger DR. Mouse models of genetic effects on cognition: Relevance to schizophrenia. Neuropharmacology. 2012;62:1204–1220. doi: 10.1016/j.neuropharm.2011.04.025. [DOI] [PubMed] [Google Scholar]
- Pascual-Brazo J, Castro E, Díaz A, Valdizán EM, Pilar-Cuéllar F, Vidal R, et al. Modulation of neuroplasticity pathways and antidepressant-like behavioural responses following the short-term (3 and 7 days) administration of the 5-HT4 receptor agonist RS67333. Int J Neuropsychopharmacol. 2012;15:631–643. doi: 10.1017/S1461145711000782. [DOI] [PubMed] [Google Scholar]
- Pelizza L, Ferrari A. Anhedonia in schizophrenia and major depression: state or trait. Ann Gen Psychiatry. 2009;8:22–31. doi: 10.1186/1744-859X-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price J, Cole V, Goodwin GM. Emotional side-effects of selective serotonin reuptake inhibitors: qualitative study. Br J Psychiatry. 2009;195:211–217. doi: 10.1192/bjp.bp.108.051110. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Miller M, Janssen WG, Liston C, Hof PR, et al. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cereb Cortex. 2006;16:313–320. doi: 10.1093/cercor/bhi104. [DOI] [PubMed] [Google Scholar]
- Ren X, Dwivedi Y, Mondal AC, Pandey GN. Cyclic-AMP response element binding protein (CREB) in the neutrophils of depressed patients. Psychiatry Res. 2011;185:108–112. doi: 10.1016/j.psychres.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163:1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- Shenoy SK. β-arrestin-biased signaling by the β-adrenergic receptors. Curr Top Membr. 2011;67:51–78. doi: 10.1016/B978-0-12-384921-2.00003-3. [DOI] [PubMed] [Google Scholar]
- Shirayama Y, Chaki S. Neurochemistry of the nucleus accumbens and its relevance to depression and antidepressant action in rodents. Curr Neuropharmacol. 2006;4:277–291. doi: 10.2174/157015906778520773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber G, Golan M, Avissar S. Beta-arrestin signaling complex as a target for antidepressants and as a depression marker. Drug News Perspect. 2009;22:467–480. [PubMed] [Google Scholar]
- Small KM, Schwarb MR, Glinka C, Theiss CT, Brown KM, Seman CA. Alpha2A- and alpha2C-adrenergic receptors form homo- and heterodimers: the heterodimeric state impairs agonist-promoted GRK phosphorylation and beta-arrestin recruitment. Biochemistry. 2006;45:4760–4767. doi: 10.1021/bi052074z. [DOI] [PubMed] [Google Scholar]
- Sousa N, Lukoyanov NV, Madeira MD, Almeida OF, Paula-Barbosa MM. Reorganization of the morphology of hippocampal neurites and synapses after stress-induced damage correlates with behavioral improvement. Neuroscience. 2000;97:253–266. doi: 10.1016/s0306-4522(00)00050-6. [DOI] [PubMed] [Google Scholar]
- Treadway MT, Zald DH. Reconsidering anhedonia in depression: Lessons from translational neuroscience. Neurosci Biobehav Rev. 2011;35:537–555. doi: 10.1016/j.neubiorev.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trezza V, Baarendse PJ, Vanderschuren LJ. The pleasures of play: pharmacological insights into social reward mechanisms. Trends Pharmacol Sci. 2010;31:463–469. doi: 10.1016/j.tips.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valverde F. Golgi Atlas of the Postnatal Mouse Brain. Springer: Austria; Wien, New York; 1998. [Google Scholar]
- Vialou V, Robison AJ, Laplant QC, Covington HE, Dietz DM, Ohnishi YN, et al. DeltaFosB in brain reward circuits mediates resilience to stress and antidepressant responses. Nat Neurosci. 2010;13:745–752. doi: 10.1038/nn.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills GD, Wesley AL, Moore FR, Sisemore DA. Social interactions among rodent conspecifics: a review of experimental paradigms. Neurosci Biobehav Rev. 1983;7:315–323. doi: 10.1016/0149-7634(83)90035-0. [DOI] [PubMed] [Google Scholar]
- Watson DJ, Marsden CA, Millan MJ, Fone KC. Blockade of dopamine D3 but not D2 receptors reverses the novel object discrimination impairment produced by post-weaning social isolation: implications for schizophrenia and its treatment. Int J Neuropharmacol. 2011;18:1–14. doi: 10.1017/S1461145711000435. [DOI] [PubMed] [Google Scholar]
- Yadid G, Friedman A. Dynamics of the dopaminergic system as a key component to the understanding of depression. Prog Brain Res. 2008;172:265–286. doi: 10.1016/S0079-6123(08)00913-8. [DOI] [PubMed] [Google Scholar]
- Zhang HT. Cyclic AMP-specific phosphodiesterase-4 as a target for the development of antidepressant drugs. Curr Pharm. 2009;15:1688–1698. doi: 10.2174/138161209788168092. [DOI] [PubMed] [Google Scholar]
- Zubenko GS, Hughes HB. Replacement of homologous mouse DNA sequence with pathogenic 6-base human CREB1 promoter sequence creates murine model of major depressive disorder. Am J Med Genet B Neuropsychiatr Genet. 2011;156:517–531. doi: 10.1002/ajmg.b.31197. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.