Abstract
Adenosine signaling is implicated in several neuropsychiatric disorders, including alcoholism. Among its diverse functions in the brain, adenosine regulates glutamate release and has an essential role in ethanol sensitivity and preference. However, the molecular mechanisms underlying adenosine-mediated glutamate signaling in neuroglial interaction remain elusive. We have previously shown that mice lacking the ethanol-sensitive adenosine transporter, type 1 equilibrative nucleoside transporter (ENT1), drink more ethanol compared with wild-type mice and have elevated striatal glutamate levels. In addition, ENT1 inhibition or knockdown reduces glutamate transporter expression in cultured astrocytes. Here, we examined how adenosine signaling in astrocytes contributes to ethanol drinking. Inhibition or deletion of ENT1 reduced the expression of type 2 excitatory amino-acid transporter (EAAT2) and the astrocyte-specific water channel, aquaporin 4 (AQP4). EAAT2 and AQP4 colocalization was also reduced in the striatum of ENT1 null mice. Ceftriaxone, an antibiotic compound known to increase EAAT2 expression and function, elevated not only EAAT2 but also AQP4 expression in the striatum. Furthermore, ceftriaxone reduced ethanol drinking, suggesting that ENT1-mediated downregulation of EAAT2 and AQP4 expression contributes to excessive ethanol consumption in our mouse model. Overall, our findings indicate that adenosine signaling regulates EAAT2 and astrocytic AQP4 expressions, which control ethanol drinking in mice.
Keywords: ENT1, astrocytes, aquaporin 4, EAAT2, Alcoholism, ceftriaxone
INTRODUCTION
Adenosine, an inhibitory neurotransmitter and neuromodulator, attenuates neuronal activity directly and indirectly through adenosine receptors (Lovatt et al, 2012; Ruby et al, 2010). Dysregulation of adenosine signaling has been implicated in many neuropsychiatric diseases including alcohol-use disorders (Dunwiddie and Masino, 2001). In both mice and humans, type 1 equilibrative nucleoside transporter (ENT1) is responsible for the majority of adenosine transport and, therefore, is a key regulator of extracellular adenosine levels (Young et al, 2008). ENT1 null mice display elevated ethanol consumption with decreased ethanol sensitivity (Chen et al, 2010; Choi et al, 2004). One of the main contributing factors for these behaviors is increased glutamatergic signaling in the nucleus accumbens (NAc) (Nam et al, 2011). Interestingly, our recent findings suggest that ENT1 regulates astrocytic excitatory amino-acid transporter 2 (EAAT2) mRNA expression and glutamate uptake activity function in cultured astrocytes (Wu et al, 2010). As EAAT2 is responsible for the majority (∼90%) of glutamate uptake into astrocytes (Tanaka et al, 1997), this reduction of EAAT2 likely contributes to the hyperglutamatergic state in ENT1 null mice (Nam et al, 2011).
Astrocytes are the most abundant glial cell type within the central nervous system and glial pathology is common to several neuropsychiatric disorders (Miguel-Hidalgo, 2009). Importantly, astrocytes in the striatum are responsible for clearance of excessive glutamate from the synaptic cleft to prevent excitotoxicity (Danbolt, 2001; Seifert et al, 2006). Prolonged excitation of glutamate signaling has been implicated in numerous psychiatric conditions (Krystal et al, 2003; Tsai and Coyle, 1998). Specifically, elevated glutamate levels have been shown to be one of the key components of reward and motivational behaviors (Kalivas, 2009; Koob and Volkow, 2010). Whereas ENT1 is expressed in both neurons and astrocytes (Parkinson et al, 2006) and adenosine uptake activities via ENTs are equal between neurons and astrocytes, astrocytic ENT1 also appears to release adenosine, which has been shown to provide neuroprotection during oxidative stress (Tanaka et al, 2011). Therefore, characterizing the role of ENT1 in astrocytes is critical to understand the underlying mechanisms of many psychiatric disorders, including alcoholism.
Here, we examined the role of ENT1 in regulating the expression of EAAT2 and aquaporin 4 (AQP4), essential players in astrocyte function and neuroglial interactions in the striatum. Furthermore, we investigated whether ceftriaxone reduces alcohol drinking in ENT1 null mice by normalizing both EAAT2 and AQP4 expression in the striatum.
MATERIALS AND METHODS
Animals
ENT1 null mice were generated as described (Choi et al, 2004). We used F2 generation hybrid mice with a C57BL/6J × 129X1/SvJ genetic background to minimize the risk of false positives or negatives in behavioral phenotypes that could be influenced by a single genetic background (Crusio et al, 2009). We used 3- to 6-month-old null and wild-type littermates for experiments. Mice were housed in standard Plexiglas cages with food and water available ad libitum. The colony room was maintained on a 12-h light/12-h dark cycle with lights on at 0600 hours. Animal care and handling procedures were approved by the Mayo Clinic Institutional Animal Care and Use Committees in accordance with NIH guidelines.
Astrocyte Culture and Reagents
The astrocytic C8-D1A cell line was obtained from ATCC (American Type Culture Collection, VA), which was cloned from the mouse cerebellum (Alliot and Pessac, 1984). As we described previously (Wu et al, 2010), cells were maintained in Dulbecco's modified Eagle medium containing glucose (Invitrogen, Carlsbad, CA), 10% heat-inactivated fetal bovine serum (ATCC, American Type Culture Collection, VA), 1% ℒ-glutamine (Gibco, Auckland, New Zealand), and 1% Antibiotic-Antimycotic (Invitrogen). Monolayers were cultured at 37 °C in the presence of 5% CO2/95% O2 (normoxia) in a fully humidified atmosphere with medium replacement every 2-3 days. NBTI was purchased from Sigma-Aldrich (St Louis, MO).
ENT1 Knockdown in Astrocytes
The target sequence of Slc29a1-3 siRNA for mouse ENT1 is 5′-AAGATTGTGCTCATCAATTCA-3′. Slc29a1-3 siRNA or control siRNA (30 pmol) were transfected into 5 × 105 astrocytes in a six-well plate using 4 μl Lipofectamine 2000 with Plus reagent (Invitrogen). At 24 h after the transfection, total RNA was isolated using RNAeasy-Mini kit (Qiagen) and the expression levels of ENT1, EAAT2, EAAT1, and AQP4 mRNA were measured by real-time RT-PCR.
Real-time RT-PCR
Mice were anesthetized with carbon dioxide and rapidly decapitated. The caudate-putamen (CPu), NAc and cerebellum (Cbl) were isolated under a surgical microscope. To measure mRNA levels, real-time quantitative RT-PCR was performed with the iCycler IQ Real-Time PCR detection system (Bio-Rad Laboratories, Hercules, CA) using QuantiTect SYBR Green RT-PCR Kit (Qiagen, Valencia, CA) as described (Wu et al, 2010). Total RNA was isolated using the RNAeasy-Mini kit (Qiagen). Gene-specific primers for EAAT2, EAAT1, AQP4, glutamine synthetase (GS) and GAPDH were purchased (Qiagen). The following real-time RT-PCR protocol was used for all genes: reverse transcription step for 30 min at 50 °C, then denaturation at 95 °C for 15 min to activate the HotStart enzyme, followed by an additional 45 cycles of amplification and quantification (15 s at 94 °C; 10 s at 55 °C; 30 s at 72 °C), each with a single fluorescence measurement. The mRNA expressions of the genes were normalized by GAPDH as a housekeeping gene. Fold or percentage changes were calculated by subtracting GAPDH Ct values from Ct values for the gene of interest using the 2−ΔΔCt method (Livak and Schmittgen, 2001).
Western Blot
Mice were anesthetized with carbon dioxide and rapidly decapitated. Brains were quickly removed and dissected to isolate the CPu, NAc, and Cbl as previously described (Nam et al, 2011). Briefly, tissues were homogenized in an extraction buffer containing 50 mM Tris buffer (pH 7.4), 2 mM EDTA, 5 mM EGTA, 0.1% SDS, protease inhibitor cocktail (Roche), and phosphatase inhibitor cocktail type I and II (Sigma). Homogenates were centrifuged at 500 g at 4 °C for 15 min and supernatants were collected. Proteins were analyzed using Bradford protein assay (Bio-Rad). Equal amount of proteins (50 μg) were separated by 4–12% NuPAGE Bis Tris gels at 130 V for 2 h, transferred onto PVDF membranes at 30 V for 1 h (Invitrogen), and analyzed using antibodies against EAAT2 (1 : 500, Santa Cruz, 60 kDa), EAAT1 (1 : 300, Santa Cruz, 75 kDa), AQP4 (1 : 1000, Sigma, 35 kDa), GS (1 : 2000, Abcam, 42 kDa), and GAPDH (1 : 1000, Millipore, 38 kDa) as a control. Most antibodies appear to be specific to target proteins as noted by the manufacturers and as shown in representative whole immunoblots (Supplementary Figure S6). However, for EAAT1, we do not rule out the possibility of false-positive signals due to apparent non-specific bands (Supplementary Figure S6c). Therefore, we referred to EAAT1 as ‘EAAT1-like' protein levels. Blots were developed using SuperSignal West Dura Chemiluminescent Substrate (Thermo Scientific, Rockford, IL). Chemiluminescent bands were detected on a Kodak Image Station 4000R scanner (New Haven, CT) and quantified using NIH Image J software.
Immunofluorescence and Colocalization Analysis
Mice were anesthetized with pentobarbital (80 mg/kg) and transcardially perfused with 4% paraformaldehyde (Sigma-Aldrich) in PBS. Brains were removed and postfixed for 24 h in the same fixative at 4 °C. Brains were immersed in 30% sucrose for 24 h, frozen, and cut into 40-μm sections using a cryostat (Leica). Free-floating sections were incubated in 50% alcohol, followed by 10% normal donkey serum in PBS for 30 min, and then antibodies against AQP4 (1 : 250, Sigma), EAAT2 (1 : 50, Santa Cruz), and glial fibrillary acidic protein (GFAP) (1 : 100, Cell Signaling) overnight. Sections were then incubated in 2% normal donkey serum in PBS for 10 min followed by Alexa 488-conjugated secondary goat anti-rabbit (for AQP4, 1 : 1000, Cell Signaling) and goat anti-mouse (for GFAP, 1 : 1000, Cell Signaling) or Alexa 555-conjugated secondary donkey anti-goat (for EAAT2, 1 : 1000, Invitrogen), and goat anti-rabbit (for AQP4, 1 : 1000, Cell Signaling) antibodies for 2 h. Images from each brain region of interest (CPu, NAc core, NAc shell) were obtained using an LSM 510 confocal laser scanning microscope (Carl Zeiss). Areas of AQP4 expression within regions of interest (450 μm × 450 μm), were quantified using NIH Image J software. Quantitative colocalization analysis was performed using CoLocalizer Pro software (Colocalization Research Software) as described in Zinchuk and Grossenbacher-Zinchuk (2009), using a selected region-of-interest background correction method. For AQP4+EAAT2 colocalization analysis, colocalized pixels (AQP4+EAAT2, yellow pixels) were normalized by total AQP4 expressions (green+yellow pixels). In addition, for GFAP+AQP4 colocalization analysis, colocalized pixels (GFAP+AQP4, yellow pixels) were also normalized by total AQP4 expressions (red+yellow pixels).
Ceftriaxone Treatment
Mice were injected with 200 mg/kg per day of ceftriaxone (intraperitoneal (i.p.); 20 mg/ml in 0.9% NaCl, w/v) or an equal volume of saline for 5 consecutive days as previously described (Sari et al, 2010). Mice were then anesthetized with carbon dioxide and rapidly decapitated after 24 h of the last injection. Brains were quickly removed and dissected to isolate the CPu and NAc. Tissues were then frozen until real-time PCR and immunoblotting were performed.
Alcohol Self-Administration
Oral alcohol self-administration and preference were examined using a modified two-bottle choice experiment (Lee et al, 2011). Mice were given 24 h access to two bottles, one containing plain tap water and the other containing an ethanol solution. The concentration of ethanol was raised every fourth day, increasing from 3 to 6 to 10% (v/v) ethanol. To examine the effect of ceftriaxone on ethanol consumption and preference, mice were given first an equivalent volume of saline (0.9% NaCl) daily for 5 days, followed by 200 mg/kg per day ceftriaxone (i.p.) daily for 5 additional days once they were stably consuming 10% (v/v) ethanol. Ethanol consumption and preference were then measured for 5 additional days as a post-injection period. Throughout the experiments, fluid intake and body weight were measured every 2 days to calculate average daily ethanol consumption (g/kg per day) and percent preference (ethanol solution consumption/total fluid consumption × 100).
Ataxia
Ethanol-induced ataxia (motor incoordination) was evaluated using a constant velocity rotarod treadmill (UGO Basile, Verese, Italy) at a fixed speed of 20 r.p.m. as described (Chen et al, 2010). Briefly, mice were preselected by their ability to remain on the rotarod for 180 s. Mice were injected with 200 mg/kg per day ceftriaxone (i.p) or an equal volume of saline for 5 consecutive days. On the sixth day, mice were then given an ethanol injection (1.5 g/kg, i.p.) and rotarod performance was evaluated by measuring their latency to fall beginning 15 min after the injection and during sequential 15-min intervals for 1 h. Training and testing was undertaken under dim red lighting shortly after lights-out, the natural active phase for nocturnal rodents.
Statistical Analysis
All data are expressed as mean±SEM and were analyzed by unpaired two-tailed t-tests (real-time PCR, western blot analysis, and immunofluorescence for basal-level measurement), two-way ANOVA (real-time PCR and western blot analysis after ceftriaxone treatment) or two-way repeated measures ANOVA (ethanol consumption and preference). Results of comparisons were considered significantly different if the p-value was<0.05.
RESULTS
Reduced Expression of EAAT2 and AQP4 in the Striatum of ENT1 Null Mice
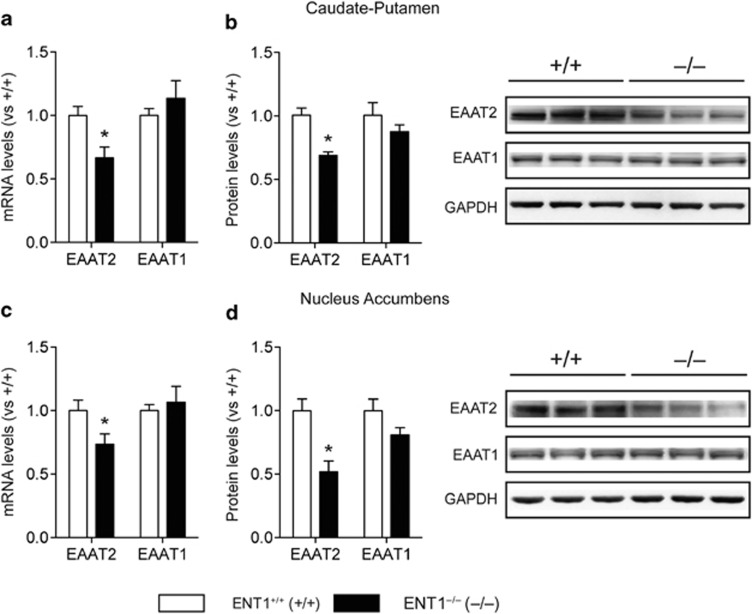
As inhibition of ENT1 downregulates EAAT2 expression in cultured astrocytes (Wu et al, 2010), we examined whether the deletion of ENT1 reduces striatal EAAT2 and astrocytic EAAT1, which are essential for motivational behavior (Reissner and Kalivas, 2010). As shown in Figure 1, in both the CPu (Figure 1a and b) and NAc (Figure 1c and d) of ENT1 null mice, EAAT2 mRNA and protein expression was downregulated, whereas EAAT1 mRNA and EAAT1-like protein levels were unchanged compared with wild-type mice. This suggests that the deletion of ENT1 downregulates EAAT2 expression in the striatum.
Figure 1.
Excitatory amino-acid transporter 2 (EAAT2) is downregulated in the striatum of equilibrative nucleoside transporter 1 (ENT1) null mice. (a, b) EAAT2, but not EAAT1, (a) mRNA and (b) protein expression levels were reduced in the caudate-putamen (CPu) of ENT1 null mice (n=10∼12). (c, d) Similarly, EAAT2, but not EAAT1, (c) mRNA and (d) protein expression levels were reduced in the nucleus accumbens (NAc) of ENT1 null mice (n=10∼12). GAPDH was used as a control. *p<0.05, compared with wild-type mice by unpaired two-tailed t-test. All data are expressed as mean±SEM.
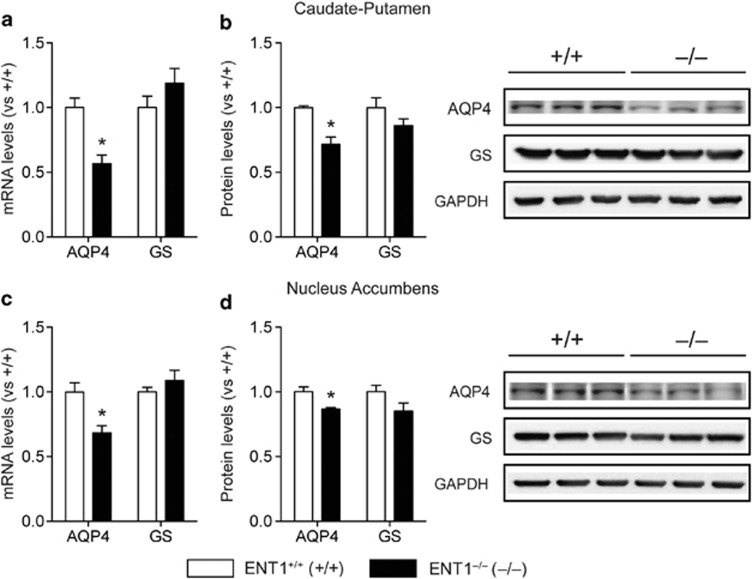
Interestingly, EAAT2 and AQP4 regulate glutamate levels through a physical interaction in astrocytes (Hinson et al, 2008). Thus, we tested whether deletion of ENT1 could alter AQP4 expression in mice. We used GS expressions as a control for astrocytic gene expression. As we expected, AQP4 mRNA and protein expression were diminished in the CPu (Figure 2a and b) and NAc (Figure 2c and d) of ENT1 null mice, whereas GS expression levels were similar between genotypes. As an additional control, we examined astrocytic gene expression in the Cbl of ENT1 null mice, where we found no changes in expression of these proteins (Supplementary Figure S1). Moreover, the protein expression pattern of astrocytic genes in the CPu, NAc, and Cbl indicated that the expression patterns of EAAT2 and AQP4 were similar among these brain regions (Supplementary Figure S2a and b). However, EAAT1-like and GS protein expressions were higher in the Cbl followed by NAc and CPu (Supplementary Figure S2c and d).
Figure 2.
Astrocytic aquaporin 4 (AQP4) gene is downregulated in the striatum of equilibrative nucleoside transporter 1 (ENT1) null mice. (a, b) AQP4 (a) mRNA and (b) protein expression levels were reduced in the caudate-putamen (CPu) of ENT1 null mice, whereas glutamine synthetase (GS) expression was unaffected (n=10∼12). (c, d) Similarly, AQP4 (c) mRNA and (d) protein expression levels were reduced in the nucleus accumbens (NAc) of ENT1 null mice, whereas GS expression was unchanged between genotypes (n=10∼12). GAPDH was used as a control. *p<0.05, compared with wild-type mice by unpaired two-tailed t-test. All data are expressed as mean±SEM.
Inhibition of ENT1 Downregulates EAAT2 and Astrocytic AQP4 Expression
As ENT1 is expressed in neurons and astrocytes, we used an astrocytic cell line, C8-D1A (Alliot et al, 1984; Wu et al, 2010), to confirm that the mRNA and protein expression changes in ENT1 null mice were specifically due to the absence of ENT1 in astrocytes. In Supplementary Figure S1, we showed that either treatment with ENT1-specific inhibitor NBTI (nitrobenzylthioinosine, 10 μM) or ENT1-specific siRNA treatment (Wu et al, 2010) downregulated EAAT2, but not EAAT1, mRNA expression in astrocytes (Figure 3a and b). These results indicate that inhibition of ENT1 function or expression is causally related to the reduction of EAAT2 expression in astrocytes.
Figure 3.
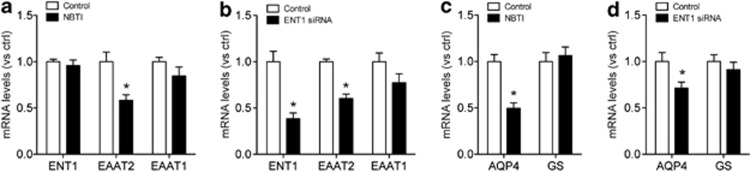
Inhibition of equilibrative nucleoside transporter 1 (ENT1) reduces excitatory amino-acid transporter 2 (EAAT2) and astrocytic aquaporin 4 (AQP4) gene expression in cultured astrocytes. (a, c) ENT1-specific inhibitor NBTI treatment (10 μM) for 24 h significantly reduced (a) EAAT2 and (c) AQP4 mRNA expression levels, but not EAAT1 or GS mRNA expression levels in cerebellar (C8-D1A) astrocytic cell line. (b, d) ENT1 siRNA treatment for 24 h significantly reduced (b) EAAT2 and (d) AQP4 mRNA expression levels, but not EAAT1 or glutamine synthetase (GS) mRNA expression levels in C8-D1A astrocytic cell line. GAPDH was used as a control. n=4∼6; *p<0.05 compared with control group by unpaired two-tailed t-test. All data are expressed as mean±SEM.
Consistent with our observations in ENT1 null mice, NBTI treatment (10 μM) for 24 h or ENT1-specific siRNA treatment diminished AQP4 mRNA levels, but not GS mRNA levels in cultured astrocytes (Figure 3c and d). These findings suggest that ENT1 regulates astrocytic EAAT2 and AQP4 mRNA levels in cultured astrocytes.
Diminished Colocalization of AQP4 and EAAT2 in the Striatum of ENT1 Null Mice
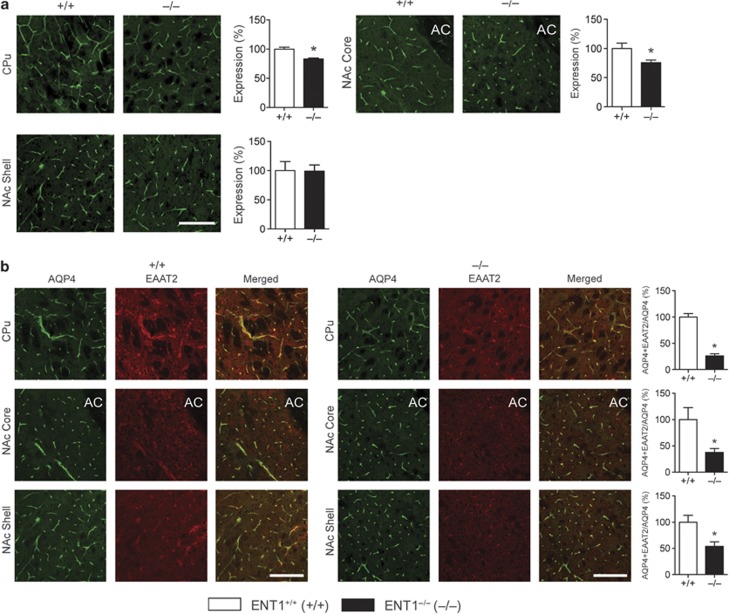
As AQP4 colocalizes with EAAT2 (Hinson et al, 2010; Hinson et al, 2008), we investigated whether this colocalization was altered in ENT1 null mice. Immunofluorescence analysis revealed that AQP4 was expressed mostly in the end-feet of astrocytes covering the vasculature (Figure 4a) and that AQP4 expression was reduced in the CPu and NAc core (Figure 4a) of ENT1 null mice compared with wild-type mice.
Figure 4.
Immunofluorescence analysis of aquaporin 4 (AQP4) in the striatum of equilibrative nucleoside transporter 1 (ENT1) null mice. (a) AQP4 expression was reduced in the caudate-putamen (CPu) and nucleus accumbens (NAc) core, but not in the NAc shell of ENT1 null mice (n=6∼12). (b) Reduced colocalization of AQP4 and excitatory amino-acid transporter 2 (EAAT2) in ENT1 null mice. ENT1 null mice show reduced colocalization between AQP4 and EAAT2 in the CPu, NAc core, and shell (n=3∼6). Expression of colocalization was normalized with expression of AQP4 (AQP4+EAAT2/AQP4). Scale bar=100 μm. *p<0.05, compared with wild-type mice by unpaired two-tailed t-test. All data are expressed as mean±SEM.
Next, we quantified the colocalized areas (AQP4+EAAT2, yellow), which were normalized by total AQP4 levels (green and yellow areas), to verify that an observed decrease in colocalization was not just because levels of both AQP4 and EAAT2 are reduced in ENT1 null mice. Our results showed that colocalization of AQP4 and EAAT2 was diminished in striatal astrocytes of ENT1 null mice compared with wild-type littermates (Figure 4b), indicating a possible loss of functional interaction between AQP4 and EAAT2 in the striatum of ENT1 null mice. Similarly, we have investigated whether the deletion of ENT1 can alter colocalization between AQP4 and GFAP. As indicated in Supplementary Figure S3, AQP4 was expressed mostly in the end-feet of astrocytes covering the vasculature, whereas GFAP was expressed mainly in the star-shaped astrocyte soma with some expression also observed in the end-feet. However, we did not notice any significant alteration of colocalization between AQP4 and GFAP in the striatum of ENT1 null mice compared with wild-type littermates (Supplementary Figure S3). Taken together, these data suggest that ENT1 is required for both the expression and colocalization of AQP4 with EAAT2 in striatal astrocytes.
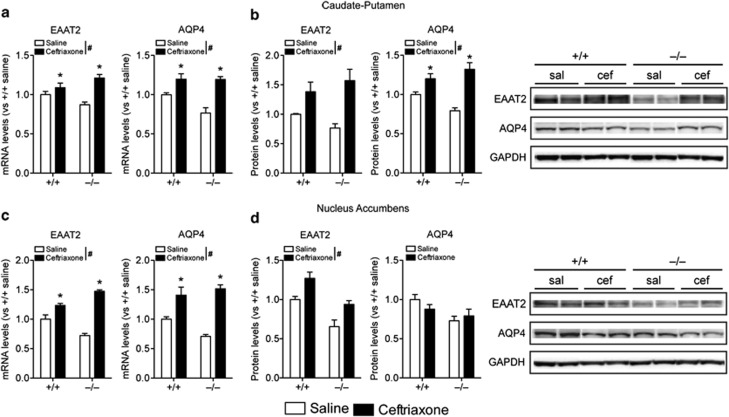
Ceftriaxone Normalizes Striatal EAAT2 and AQP4 Expression in ENT1 Null Mice
As ENT1 null mice exhibit downregulated EAAT2 in the striatum, we explored whether ceftriaxone, a β-lactam antibiotic that is well known for its ability to upregulate EAAT2 expression and increase glutamate uptake activity (Kim et al, 2011; Sari et al, 2010), can also upregulate other astrocytic genes that are downregulated in ENT1 null mice. In the CPu, daily treatment of ceftriaxone (200 mg/kg, i.p.) for 5 days upregulated EAAT2 and AQP4 mRNA expression in both ENT1 null and wild-type mice (Figure 5a). Furthermore, western blot analysis demonstrated that EAAT2 and AQP4 protein expression were increased after ceftriaxone treatment in the CPu of ENT1 null mice (Figure 5b). Therefore, ceftriaxone increases EAAT2 and AQP4 expression levels both transcriptionally and translationally. We did not observe any significant changes in EAAT1 or GS mRNA levels as well as EAAT1-like or GS protein levels after ceftriaxone treatment (Supplementary Figure S4a and b).
Figure 5.
Ceftriaxone rescues downregulation of excitatory amino-acid transporter 2 (EAAT2) and astrocytic aquaporin 4 (AQP4) in equilibrative nucleoside transporter 1 (ENT1) null mice. (a, b) In the caudate-putamen (CPu), both (a) mRNA and (b) protein levels of EAAT2 and AQP4 were upregulated after ceftriaxone treatment (200 mg/kg, intraperitoneal) in ENT1 null and wild-type mice (n=4∼7). (c) However, in the nucleus accumbens (NAc), EAAT2, and AQP4 mRNA levels were upregulated, (d) whereas only EAAT2 protein levels were increased after the treatment (n=4∼7). GAPDH was used as a control. sal=saline; cef=ceftriaxone. *p<0.05 compared with the saline treatment groups (Tukey post-hoc test), and #p<0.05 for the main effect of treatment. All data are expressed as mean±SEM.
In the NAc, we observed similar changes as in the CPu. Ceftriaxone treatment upregulated EAAT2 and AQP4 mRNA expressions in both ENT1 null and wild-type mice (Figure 5c). Western blot analysis showed that EAAT2, but not AQP4, protein level was increased in response to ceftriaxone treatment (Figure 4d). We did not observe any ceftriaxone-induced changes in EAAT1 or GS mRNA levels as well as EAAT1-like or GS protein levels in the NAc (Supplementary Figure S4c and d). Taken together, these results suggest that ceftriaxone can rescue the downregulation of EAAT2 and astrocyte-specific AQP4 in the striatum of ENT1 null mice.
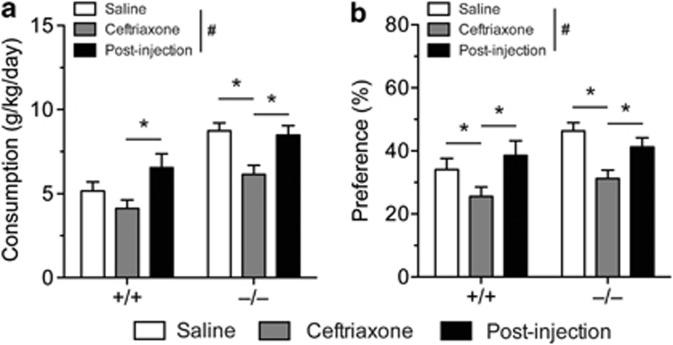
Ceftriaxone Reduces Alcohol Drinking in ENT1 Null Mice
ENT1 null mice exhibit excessive alcohol consumption and preference compared with wild-type mice (Nam et al, 2011) and pharmacological treatment alleviating the hyperglutamatergic state reduces their alcohol drinking (Lee et al, 2011; Nam et al, 2011). As ceftriaxone has been shown to reduce alcohol drinking in alcohol-preferring rats by upregulating EAAT2 (Sari et al, 2011), we investigated whether ceftriaxone-induced upregulation of EAAT2 and AQP4 expression in the striatum could also alleviate the excessive alcohol-drinking phenotype in ENT1 null mice. We measured ethanol drinking using a modified two-bottle choice experiment (Lee et al, 2011), and showed that ceftriaxone treatment (200 mg/kg per day, 5 days, i.p.) reduced ethanol consumption compared with the saline-treated groups (Figure 6a). Two-way repeated measures ANOVA showed effects of genotype (F(1, 76)=11.586, p=0.002), treatment (F(2, 76)=29.475, p<0.001) and an interaction between genotype and treatment (F(2, 76)=4.068, p=0.021). Post-hoc analysis revealed that ceftriaxone treatment reduced ethanol consumption in ENT1 null mice, but not in wild-type mice compared with the saline-treated group. Ceftriaxone treatment reduced ethanol preference as well (Figure 6b). Two-way repeated measures ANOVA showed effects of treatment (F(2, 70)=25.959, p<0.001) and an interaction between genotype and treatment (F(2, 70)=3.131, p=0.050), but no effect of genotype (F(1, 70)=1.593, p=0.215). Post-hoc analysis demonstrated that ceftriaxone treatment reduced ethanol preference in both ENT1 null and wild-type mice compared with the saline-treated group. We also examined whether ceftriaxone could alter the ataxic effect of ethanol. We did not observe any changes in ethanol-induced ataxia of either ENT1 null or wild-type mice (Supplementary Figure S5a and b). Therefore, our results suggest that ceftriaxone can reduce ethanol drinking without altering ethanol sensitivity in mice.
Figure 6.
Ceftriaxone reduces alcohol drinking in equilibrative nucleoside transporter 1 (ENT1) null and wild-type mice. Ceftriaxone (200 mg/kg per day, 5 days, intraperitoneal) reduces (a) ethanol consumption and (b) preference during the 5-day injection period (n=19∼21). *p<0.05 compared with the saline-treated or post-injection period (Tukey post-hoc test), and #p<0.05 for the main effect of treatment. All data are expressed as mean±SEM.
DISCUSSION
Our present study demonstrates a novel mechanism implicating the main adenosine transporter, ENT1, in the regulation of astrocyte-specific gene expression. Astrocytes have an essential role in regulating neurotransmitter levels in the synaptic cleft (Halassa et al, 2007; Seifert et al, 2006). The removal of glutamate via astrocytic EAAT2 is especially critical to neuronal activity and viability. Here, we observed the reduction of AQP4 and EAAT2, two astrocytic proteins that interact to control glutamate signaling, as a consequence of deletion or inhibition of ENT1 both in vivo and in vitro. These observations suggest that adenosine homeostasis is critical for astrocytic cellular function. Because astrocyte dysfunction has been implicated in addictive and psychiatric disorders (Halassa et al, 2007), including alcoholism (Miguel-Hidalgo et al, 2002), the present results on ENT1 regulation of astrocyte-enriched genes may lead to important advances in the understanding of psychiatric illnesses and the development of novel therapeutics for alcohol-use disorders.
Hyperglutamatergic signaling in corticostriatal circuitry is common to several neuropsychiatric diseases, including addictive disorders. As EAAT2 is responsible for 90% of glutamate uptake activity in glial cells in the striatum (Tanaka et al, 1997), diminished EAAT2 expression contributes in large part to the hyperglutamatergic state observed in the NAc of ENT1 null mice and associated alcohol-related behaviors (Nam et al, 2011). The murine EAAT2 (Slc1a2) gene is located in a central part of chromosome 2 (E2) (Kirschner et al, 1994), near quantitative trait loci that modulate neuroexcitability and seizure frequency in mouse models of alcohol withdrawal and epilepsy (Crabbe and Belknap, 1993). EAAT2 is also implicated in preclinical models of morphine, methamphetamine, and cocaine addiction (Abulseoud et al, 2012; Sari et al, 2009). Moreover, it is noteworthy that genetic polymorphisms of EAAT2 have been linked to alcoholism in humans (Sander et al, 2000). As excessive glutamate levels and prolonged glutamate-driven excitation are toxic to neurons (Danbolt, 2001; Seifert et al, 2006), it is possible that ENT1 null mice may show some degree of neuronal damage, although we have not observed any neurodegeneration in the 3- to 6-month-old mice we used in our experiments. We speculate that aged ENT1 null mice may display phenotypes related to other neurological disorders (Danbolt, 2001; Seifert et al, 2006).
Disruption of the levels or function of AQP4 has been implicated in brain injury resulting from acute and binge ethanol exposure (Katada et al, 2012; Sripathirathan et al, 2009) as well as other neurological disorders, including ischemia (Taniguchi et al, 2000), edema (Vizuete et al, 1999), and neuromyelitis optica (Hinson et al, 2008), all of which involve disrupted brain water homeostasis and neuronal damage. Our results demonstrate that ENT1 deletion or inhibition in the striatum reduces AQP4 and EAAT2 expression. As AQP4 and EAAT2 are known to form macromolecular complexes (Hinson et al, 2008) and glutamate regulates AQP4 water permeability through group I metabotropic glutamate receptors (Gunnarson et al, 2008), reduced expression of EAAT2 may further diminish AQP4 function. Therefore, the observed reduction in both proteins by ENT1 deletion or inhibition in the striatum may further compromise glutamate-related neuroglial interactions. A number of reports have suggested that expression of EAAT2 and AQP4 is regulated by PKC isoforms. PKC activators, such as PMA, TPA, and PDBu, which decrease cell surface expression and transporting activity of EAAT2 (Gonzalez-Gonzalez et al, 2008; Kalandadze et al, 2002) also downregulate AQP4 expression and reduce water permeability (Yamamoto et al, 2001), whereas GS expression is unaffected (Brodie et al, 1998). Interestingly, ENT1 null mice show reduced NMDA receptor-dependent PKCγ activity in the NAc (Nam et al, 2011), implying that reduced striatal EAAT2 and AQP4 expression in ENT1 null mice may involve altered PKC signaling.
Interestingly, we found that the expression of GS was similar between genotypes (Figure 2 and Supplementary Figure S2), and that ENT1 did not appear to control GS expression. This indicates that ENT1 is associated with EAAT2 and AQP4 in a specific manner, and that reduced astrocyte viability itself is unlikely to result from ENT1 deletion or inhibition. It also rules out the possibility that disruption in the conversion of glutamate to glutamine in astrocytes contributes to the hyperglutamatergic state and drinking behavior of ENT1 null mice.
Ceftriaxone is known to upregulate EAAT2 expression and increase glutamate uptake activity via the NF-κB pathway, providing neuroprotection from glutamate neurotoxicity (Kim et al, 2011; Sari et al, 2010). Of particular relevance to the present study is the ability of ceftriaxone to reduce alcohol intake in rodent models (Sari et al, 2011). Although this effect has been attributed to the upregulation of EAAT2 and reductions in brain glutamate levels, our data suggest that AQP4 upregulation may have a role in reducing drinking behavior as well. Like EAAT2, AQP4 may be regulated via the same NF-κB pathway possibly downstream of adenosine A1 receptor in astrocytes. As expected, ceftriaxone treatment suppressed alcohol drinking in our study. Surprisingly, ceftriaxone did not alter sensitivity to an intoxicating dose of ethanol, unlike other medications that target the glutamatergic pathway (Nam et al, 2010), in which ethanol sensitivity is inversely correlated with ethanol consumption (Naassila et al, 2002; Nam et al, 2011). It is important to note that ceftriaxone was able to upregulate EAAT2 and AQP4 in the presence or absence of ENT1. This finding is consistent with our observation that ceftriaxone was able to reduce alcohol preference in both ENT1 null and wild-type mice. Therefore, ameliorating astrocytic dysregulation via upregulation of EAAT2 may be a unique therapeutic strategy to reduce alcohol drinking in a wide range of alcohol abuse phenotypes and could help prevent the development of alcohol dependence in vulnerable populations.
In summary, we demonstrated that ENT1 regulates the expression of several genes that are essential for maintaining astrocytic cell functions in the striatum. We also showed that dysregulation of astrocytes by the inhibition or deletion of ENT1 appears to be central to alcohol drinking. We showed that augmenting astrocytic functions with ceftriaxone reduced alcohol drinking in both ENT1 null and wild-type mice. Finally, we revealed that upregulation of AQP4 in addition to EAAT2 may be central to the beneficial effects of ceftriaxone. Taken together, our study provides evidence that astrocyte dysfunction may have a central role in the pathophysiology of alcoholism and that astrocytes represent a promising therapeutic target for alcohol-use disorders.
Acknowledgments
We thank D Frederixon for preparing the manuscript, and Drs O Abulseoud and X Li for their helpful discussions. This project was funded by the Samuel Johnson Foundation for Genomics of Addiction Program at Mayo Clinic and in parts by grants from the National Institutes of Health (NIH) (AA015164, AA018779).
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Abulseoud OA, Miller JD, Wu J, Choi DS, Holschneider DP. Ceftriaxone upregulates the glutamate transporter in medial prefrontal cortex and blocks reinstatement of methamphetamine seeking in a condition place preference paradigm. Brain Res. 2012;1456:14–21. doi: 10.1016/j.brainres.2012.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alliot F, Pessac B. Astrocytic cell clones derived from established cultures of 8-day postnatal mouse cerebella. Brain Res. 1984;306:283–291. doi: 10.1016/0006-8993(84)90377-9. [DOI] [PubMed] [Google Scholar]
- Brodie C, Kuperstein I, Acs P, Blumberg PM. Differential role of specific PKC isoforms in the proliferation of glial cells and the expression of the astrocytic markers GFAP and glutamine synthetase. Brain Res Mol Brain Res. 1998;56:108–117. doi: 10.1016/s0169-328x(98)00035-7. [DOI] [PubMed] [Google Scholar]
- Chen J, Nam HW, Lee MR, Hinton DJ, Choi S, Kim T, et al. Altered glutamatergic neurotransmission in the striatum regulates ethanol sensitivity and intake in mice lacking ENT1. Behav Brain Res. 2010;208:636–642. doi: 10.1016/j.bbr.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DS, Cascini MG, Mailliard W, Young H, Paredes P, McMahon T, et al. The type 1 equilibrative nucleoside transporter regulates ethanol intoxication and preference. Nat Neurosci. 2004;7:855–861. doi: 10.1038/nn1288. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Belknap JK. Behavior genetic analyses of drug withdrawal. Alcohol Alcohol Suppl. 1993;2:477–482. [PubMed] [Google Scholar]
- Crusio WE, Goldowitz D, Holmes A, Wolfer D. Standards for the publication of mouse mutant studies. Genes Brain Behav. 2009;8:1–4. doi: 10.1111/j.1601-183X.2008.00438.x. [DOI] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Dunwiddie TV, Masino SA. The role and regulation of adenosine in the central nervous system. Annu Rev Neurosci. 2001;24:31–55. doi: 10.1146/annurev.neuro.24.1.31. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Gonzalez IM, Garcia-Tardon N, Gimenez C, Zafra F. PKC-dependent endocytosis of the GLT1 glutamate transporter depends on ubiquitylation of lysines located in a C-terminal cluster. Glia. 2008;56:963–974. doi: 10.1002/glia.20670. [DOI] [PubMed] [Google Scholar]
- Gunnarson E, Zelenina M, Axehult G, Song Y, Bondar A, Krieger P, et al. Identification of a molecular target for glutamate regulation of astrocyte water permeability. Glia. 2008;56:587–596. doi: 10.1002/glia.20627. [DOI] [PubMed] [Google Scholar]
- Halassa MM, Fellin T, Haydon PG. The tripartite synapse: roles for gliotransmission in health and disease. Trends Mol Med. 2007;13:54–63. doi: 10.1016/j.molmed.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Hinson SR, McKeon A, Lennon VA. Neurological autoimmunity targeting aquaporin-4. Neuroscience. 2010;168:1009–1018. doi: 10.1016/j.neuroscience.2009.08.032. [DOI] [PubMed] [Google Scholar]
- Hinson SR, Roemer SF, Lucchinetti CF, Fryer JP, Kryzer TJ, Chamberlain JL, et al. Aquaporin-4-binding autoantibodies in patients with neuromyelitis optica impair glutamate transport by down-regulating EAAT2. J Exp Med. 2008;205:2473–2481. doi: 10.1084/jem.20081241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalandadze A, Wu Y, Robinson MB. Protein kinase C activation decreases cell surface expression of the GLT-1 subtype of glutamate transporter. Requirement of a carboxyl-terminal domain and partial dependence on serine 486. J Biol Chem. 2002;277:45741–45750. doi: 10.1074/jbc.M203771200. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10:561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- Katada R, Nishitani Y, Honmou O, Mizuo K, Okazaki S, Tateda K, et al. Expression of aquaporin-4 augments cytotoxic brain edema after traumatic brain injury during acute ethanol exposure. Am J Pathol. 2012;180:17–23. doi: 10.1016/j.ajpath.2011.09.011. [DOI] [PubMed] [Google Scholar]
- Kim K, Lee SG, Kegelman TP, Su ZZ, Das SK, Dash R, et al. Role of excitatory amino acid transporter-2 (EAAT2) and glutamate in neurodegeneration: opportunities for developing novel therapeutics. J Cell Physiol. 2011;226:2484–2493. doi: 10.1002/jcp.22609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschner MA, Copeland NG, Gilbert DJ, Jenkins NA, Amara SG. Mouse excitatory amino acid transporter EAAT2: isolation, characterization, and proximity to neuroexcitability loci on mouse chromosome 2. Genomics. 1994;24:218–224. doi: 10.1006/geno.1994.1609. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal JH, Petrakis IL, Mason G, Trevisan L, D'Souza DC. N-methyl-D-aspartate glutamate receptors and alcoholism: reward, dependence, treatment, and vulnerability. Pharmacol Ther. 2003;99:79–94. doi: 10.1016/s0163-7258(03)00054-8. [DOI] [PubMed] [Google Scholar]
- Lee MR, Hinton DJ, Wu J, Mishra PK, Port JD, Macura SI, et al. Acamprosate reduces ethanol drinking behaviors and alters the metabolite profile in mice lacking ENT1. Neurosci Lett. 2011;490:90–95. doi: 10.1016/j.neulet.2010.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lovatt D, Xu Q, Liu W, Takano T, Smith NA, Schnermann J, et al. Neuronal adenosine release, and not astrocytic ATP release, mediates feedback inhibition of excitatory activity. Proc Natl Acad Sci USA. 2012;109:6265–6270. doi: 10.1073/pnas.1120997109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ. The role of glial cells in drug abuse. Curr Drug Abuse Rev. 2009;2:76–82. doi: 10.2174/1874473710902010076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ, Wei J, Andrew M, Overholser JC, Jurjus G, Stockmeier CA, et al. Glia pathology in the prefrontal cortex in alcohol dependence with and without depressive symptoms. Biol Psychiatry. 2002;52:1121–1133. doi: 10.1016/s0006-3223(02)01439-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naassila M, Ledent C, Daoust M. Low ethanol sensitivity and increased ethanol consumption in mice lacking adenosine A2A receptors. J Neurosci. 2002;22:10487–10493. doi: 10.1523/JNEUROSCI.22-23-10487.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam HW, Lee MR, Hinton DJ, Choi DS. Reduced effect of NMDA glutamate receptor antagonist on ethanol-induced ataxia and striatal glutamate levels in mice lacking ENT1. Neurosci Lett. 2010;479:277–281. doi: 10.1016/j.neulet.2010.05.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam HW, Lee MR, Zhu Y, Wu J, Hinton DJ, Choi S, et al. Type 1 equilibrative nucleoside transporter regulates ethanol drinking through accumbal N-methyl-D-aspartate receptor signaling. Biol Psychiatry. 2011;69:1043–1051. doi: 10.1016/j.biopsych.2011.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson FE, Ferguson J, Zamzow CR, Xiong W. Gene expression for enzymes and transporters involved in regulating adenosine and inosine levels in rat forebrain neurons, astrocytes and C6 glioma cells. J Neurosci Res. 2006;84:801–808. doi: 10.1002/jnr.20988. [DOI] [PubMed] [Google Scholar]
- Reissner KJ, Kalivas PW. Using glutamate homeostasis as a target for treating addictive disorders. Behav Pharmacol. 2010;21:514–522. doi: 10.1097/FBP.0b013e32833d41b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby CL, Adams C, Knight EJ, Nam HW, Choi DS. An essential role for adenosine signaling in alcohol abuse. Curr Drug Abuse Rev. 2010;3:163–174. doi: 10.2174/1874473711003030163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander T, Ostapowicz A, Samochowiec J, Smolka M, Winterer G, Schmidt LG. Genetic variation of the glutamate transporter EAAT2 gene and vulnerability to alcohol dependence. Psychiatr Genet. 2000;10:103–107. doi: 10.1097/00041444-200010030-00001. [DOI] [PubMed] [Google Scholar]
- Sari Y, Prieto AL, Barton SJ, Miller BR, Rebec GV. Ceftriaxone-induced up-regulation of cortical and striatal GLT1 in the R6/2 model of Huntington's disease. J Biomed Sci. 2010;17:62. doi: 10.1186/1423-0127-17-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sari Y, Sakai M, Weedman JM, Rebec GV, Bell RL. Ceftriaxone, a beta-lactam antibiotic, reduces ethanol consumption in alcohol-preferring rats. Alcohol Alcohol. 2011;46:239–246. doi: 10.1093/alcalc/agr023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sari Y, Smith KD, Ali PK, Rebec GV. Upregulation of GLT1 attenuates cue-induced reinstatement of cocaine-seeking behavior in rats. J Neurosci. 2009;29:9239–9243. doi: 10.1523/JNEUROSCI.1746-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert G, Schilling K, Steinhauser C. Astrocyte dysfunction in neurological disorders: a molecular perspective. Nat Rev Neurosci. 2006;7:194–206. doi: 10.1038/nrn1870. [DOI] [PubMed] [Google Scholar]
- Sripathirathan K, Brown J, Neafsey EJ, Collins MA. Linking binge alcohol-induced neurodamage to brain edema and potential aquaporin-4 upregulation: evidence in rat organotypic brain slice cultures and in vivo. J Neurotrauma. 2009;26:261–273. doi: 10.1089/neu.2008.0682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka A, Nishida K, Okuda H, Nishiura T, Higashi Y, Fujimoto S, et al. Peroxynitrite treatment reduces adenosine uptake via the equilibrative nucleoside transporter in rat astrocytes. Neurosci Lett. 2011;498:52–56. doi: 10.1016/j.neulet.2011.04.060. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Watase K, Manabe T, Yamada K, Watanabe M, Takahashi K, et al. Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science. 1997;276:1699–1702. doi: 10.1126/science.276.5319.1699. [DOI] [PubMed] [Google Scholar]
- Taniguchi M, Yamashita T, Kumura E, Tamatani M, Kobayashi A, Yokawa T, et al. Induction of aquaporin-4 water channel mRNA after focal cerebral ischemia in rat. Brain Res Mol Brain Res. 2000;78:131–137. doi: 10.1016/s0169-328x(00)00084-x. [DOI] [PubMed] [Google Scholar]
- Tsai G, Coyle JT. The role of glutamatergic neurotransmission in the pathophysiology of alcoholism. Annu Rev Med. 1998;49:173–184. doi: 10.1146/annurev.med.49.1.173. [DOI] [PubMed] [Google Scholar]
- Vizuete ML, Venero JL, Vargas C, Ilundain AA, Echevarria M, Machado A, et al. Differential upregulation of aquaporin-4 mRNA expression in reactive astrocytes after brain injury: potential role in brain edema. Neurobiol Dis. 1999;6:245–258. doi: 10.1006/nbdi.1999.0246. [DOI] [PubMed] [Google Scholar]
- Wu J, Lee MR, Choi S, Kim T, Choi D-S. ENT1 regulates ethanol-sensitive EAAT2 expression and function in astrocytes. Alcohol Clin Exp Res. 2010;34:1110–1117. doi: 10.1111/j.1530-0277.2010.01187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto N, Sobue K, Miyachi T, Inagaki M, Miura Y, Katsuya H, et al. Differential regulation of aquaporin expression in astrocytes by protein kinase C. Brain Res Mol Brain Res. 2001;95:110–116. doi: 10.1016/s0169-328x(01)00254-6. [DOI] [PubMed] [Google Scholar]
- Young JD, Yao SY, Sun L, Cass CE, Baldwin SA. Human equilibrative nucleoside transporter (ENT) family of nucleoside and nucleobase transporter proteins. Xenobiotica. 2008;38:995–1021. doi: 10.1080/00498250801927427. [DOI] [PubMed] [Google Scholar]
- Zinchuk V, Grossenbacher-Zinchuk O. Recent advances in quantitative colocalization analysis: focus on neuroscience. Prog Histochem Cytochem. 2009;44:125–172. doi: 10.1016/j.proghi.2009.03.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.