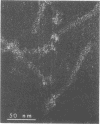
Abstract
Bacterial strain 16-12 was isolated from the root nodules of lupines and was found to be mitomycin C-inducible for the production of a bacteriophage (“16-12-1”) with a long noncontractile tail. The phage was found to attach with a fork-like terminal tail structure to the pili of strain 16-12. In addition, it was also found adsorbed to the bacterial cell poles. It is suggested that phage 16-12-1 may be pilus dependent.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradley D. E. A pilus-dependent Pseudomonas aeruginosa bacteriophage with a long noncontractile tail. Virology. 1973 Feb;51(2):489–492. doi: 10.1016/0042-6822(73)90448-0. [DOI] [PubMed] [Google Scholar]
- Bradley D. E. Basic characterization of a Pseudomonas aeruginosa pilus-dependent bacteriophage with a long noncontractile tail. J Virol. 1973 Nov;12(5):1139–1148. doi: 10.1128/jvi.12.5.1139-1148.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley D. E. Evidence for the retraction of Pseudomonas aeruginosa RNA phage pili. Biochem Biophys Res Commun. 1972 Apr 14;47(1):142–149. doi: 10.1016/s0006-291x(72)80021-4. [DOI] [PubMed] [Google Scholar]
- Bradley D. E., Pitt T. L. Pilus-dependence of four Pseudomonas aeruginosa bacteriophages with non-contractile tails. J Gen Virol. 1974 Jul;24(1):1–15. doi: 10.1099/0022-1317-24-1-1. [DOI] [PubMed] [Google Scholar]
- Bradley D. E. Shortening of Pseudomonas aeruginosa pili after RNA-phage adsorption. J Gen Microbiol. 1972 Sep;72(2):303–319. doi: 10.1099/00221287-72-2-303. [DOI] [PubMed] [Google Scholar]
- Bradley D. E. The adsorption of Pseudomonas aeruginosa pilus-dependent bacteriophages to a host mutant with nonretractile pili. Virology. 1974 Mar;58(1):149–163. doi: 10.1016/0042-6822(74)90150-0. [DOI] [PubMed] [Google Scholar]
- Bradley D. E. The adsorption of the Pseudomonas aeruginosa filamentous bacteriophage Pf to its host. Can J Microbiol. 1973 May;19(5):623–631. doi: 10.1139/m73-103. [DOI] [PubMed] [Google Scholar]
- Heumann W. Conjugation in starforming Rhizobium lupini. Mol Gen Genet. 1968;102(2):132–144. doi: 10.1007/BF01789140. [DOI] [PubMed] [Google Scholar]
- Jacobson A. Role of F pili in the penetration of bacteriophage fl. J Virol. 1972 Oct;10(4):835–843. doi: 10.1128/jvi.10.4.835-843.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotz W., Mayer F. Isolation and characterization of a bacteriophage tail-like bacteriocin from a strain of Rhizobium. J Virol. 1972 Jan;9(1):160–173. doi: 10.1128/jvi.9.1.160-173.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARX R., HEUMANN W. [On the flagellar fine structure and fimbriae in 2 Pseudomonas strains]. Arch Mikrobiol. 1962;43:245–254. [PubMed] [Google Scholar]
- Marvin D. A., Hohn B. Filamentous bacterial viruses. Bacteriol Rev. 1969 Jun;33(2):172–209. doi: 10.1128/br.33.2.172-209.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer F. Elektronenmikroskopische Untersuchung der Fimbrienkontraktion bei dem sternbildenden bodenbakterium Pseudomonas echinoides. Arch Mikrobiol. 1971;76(2):166–173. [PubMed] [Google Scholar]
- Mayer F., Schmitt R. Elektronemikrokopische, diffraktometrische und disc-elektrophoretische Untersuchungen an Fimbrien des sternbildenden Bodenbakteriums Pseudomonas echinoides und einder nicht-sternbildenden Mutante. Arch Mikrobiol. 1971;79(4):311–326. [PubMed] [Google Scholar]