Abstract
A large injection of a retrograde tracer into the inferior colliculus of guinea pigs labeled two bands of cells in the ipsilateral auditory cortex: a dense band of cells in layer V and a second band of cells in layer VI. On the contralateral side, labeled cells were restricted to layer V. The ipsilateral layer VI cells were distributed throughout temporal cortex, suggesting projections from multiple auditory areas. The layer VI cells included pyramidal cells as well as several varieties of non-pyramidal cells. Small tracer injections restricted to the dorsal cortex or external cortex of the inferior colliculus consistently labeled cells in layer VI. Injections restricted to the central nucleus of the inferior colliculus labeled layer VI cells only rarely. Overall, 10% of the cells in temporal cortex that project to the ipsilateral inferior colliculus were located in layer VI, suggesting that layer VI cells make a significant contribution to the corticocollicular pathway.
Keywords: auditory system, efferent, descending pathways, corticofugal, pyramidal cell, non-pyramidal cell
A projection from cerebral cortex to the inferior colliculus (IC) has been known for many years, but interest in this pathway has grown as recent physiological studies have revealed a remarkably wide range of effects associated with it (e.g. Popelár et al., 2003; Suga and Ma, 2003; Yan et al., 2005; Sun et al., 2007; Wu and Yan, 2007; Nakamoto et al., 2008; Suga, 2008). Most of these studies have recorded the responses of IC cells to acoustic stimuli before, during and after various manipulations of the cortical inputs (e.g. stimulation or inactivation of auditory cortex). The results show that auditory cortex can affect the selectivity of IC cells for the frequency or intensity of a sound, the relative timing of two sounds, or binaural cues associated with the location of a sound.
Almost all of the studies cited above have recorded from cells in the central nucleus of the inferior colliculus (ICc). While there is evidence in some species for a direct projection from auditory cortex to the ICc (e.g. guinea pig: Feliciano and Potashner, 1995; rat: Saldaña et al., 1996), this pathway may not be present across species and, even when present, can be dwarfed by a much denser projection from auditory cortex to the dorsal cortex of the inferior colliculus (ICd) and external cortex of the inferior colliculus (ICx). These latter subdivisions project to the ICc, and could provide a disynaptic route through which cortical projections affect the ICc cells (Jen et al., 2001). Thus there may be several pathways by which cortex could affect ICc cells. The situation is further complicated by the discovery that auditory cortex also projects directly to brainstem auditory nuclei below the IC (Feliciano et al., 1995). Recent studies suggest that these cortical projections could directly affect the ascending inputs to the IC (Schofield and Coomes, 2005; Peterson and Schofield, 2007). It seems likely that numerous corticofugal pathways could play an important role in IC function; understanding these roles will require more detailed understanding of the circuitry, including the origins and termination patterns of the direct pathway from cortex to the IC.
A hallmark of projections from auditory cortex, like those from other neocortical areas, is the discrete laminar organization of their cells of origin (reviewed by Winer, 1992). Projections to other cortical areas arise from all the cellular layers, but projections to subcortical targets originate from layers V and VI. Two of the largest projections, in terms of the number of cortical cells of origin, are to the IC and to the thalamus. Kelly and Wong (1981) demonstrated clear differences in the laminar distributions of auditory cortical projection cells, with corticocollicular cells located in a band in layer V and corticothalamic cells distributed in two bands, including a wide band in layer VI and a narrower band in layer V. The layers differ in their inputs and in the morphology and physiology of their cells, suggesting that projections from the two layers would serve distinct functions (Winer, 1992). This hypothesis has been explored in greatest detail for the corticothalamic projection. A bi-laminar origin of corticothalamic cells has been demonstrated in many cortical areas across many systems. Most importantly, the projections from the two layers have been distinguished not only anatomically but also physiologically; they have different effects on thalamic cells and are considered to serve different functions (e.g. Ojima, 1994; see reviews by Sherman and Guillery, 2002; Sherman, 2007).
The study by Kelly and Wong (1981) was done in cats and suggested that corticocollicular cells originate exclusively from layer V. Other studies have demonstrated a projection from layer V cells to the IC in many species (reviewed in Winer, 1992). However, there are also reports that a portion of the corticocollicular pathway arises from layer VI cells. Injection of a retrograde tracer into the IC has labeled layer VI cells in rats (Games and Winer, 1988; Doucet et al., 2003), hedgehog tenrecs (Künzle, 1995) and gerbils (Bajo and Moore, 2005). We described the presence of layer VI corticocollicular cells in guinea pigs (Haas et al., 2003; Coomes et al., 2005), but these results conflicted with earlier reports (Druga et al., 1988).
The purpose of the present study was to address several questions about the layer VI corticocollicular cells in guinea pigs. How many layer VI corticocollicular cells are there (in comparison to layer V cells)? What cortical areas contain the layer VI cells? Previous studies describe the layer V corticocollicular projection as originating from a wide area of temporal cortex including primary and secondary (or core and belt) auditory areas; however, the distribution of the layer VI cells has not been described in any detail. What is the morphology of the layer VI cells? Layer V corticocollicular cells are described as medium and large pyramids (Winer and Prieto, 2001) with tufted or non-tufted apical dendrites (Bajo and Moore, 2005). Layer VI contains a variety of cell types. Both pyramidal and non-pyramidal layer VI cells project to the thalamus (Kelly and Wong, 1981). The types of layer VI cells that project to the IC are unknown. Finally, which IC subdivisions are targets of layer VI cells? The corticocollicular projection in guinea pigs terminates densely in the ICd and ICx (Druga et al., 1988); a less dense projection to the ICc has been documented with both anatomical and physiological techniques (Feliciano and Potashner, 1995; Lim and Anderson, 2007). Identifying the subdivisions that are targets of layer VI cells will be essential for understanding the functional role of the layer VI projections.
EXPERIMENTAL PROCEDURES
Experiments were performed on adult guinea pigs (13 albino animals obtained from Charles River Laboratories, Wilmington, MA, USA and two pigmented animals obtained from Elm Hill Breeding Laboratories, Chelmsford, MA, USA). All procedures were approved by the Institutional Animal Care and Use Committee and administered following the National Institutes of Health guidelines for the care and use of laboratory animals. In accordance with these guidelines, all efforts were made to minimize the number of animals used and their suffering.
Surgery and perfusion
Prior to surgery, each guinea pig was anesthetized with halothane (3.5% for induction, 2.5–2.75% for maintenance) in oxygen and nitrous oxide or with isoflurane (4–5% for induction, 1.75–3% for maintenance) in oxygen. The guinea pig was given atropine sulfate (0.08 mg/kg, i.m.) to reduce bronchial secretions. The eyes were kept moist with a coating of antibiotic ointment (Neosporin Ophthalmic) or moisturizer. The animal was placed in a stereotaxic frame on a feedback-controlled heating pad to maintain body temperature.
During surgery, an incision was made in the scalp and the margins of the incision were injected with a long-lasting local anesthetic (0.25% bupivacaine; Sensorcaine; Astra USA, Inc., Westborough, MA, USA). Stereotaxic coordinates were used to guide all injections. A dental drill was used to open the skull at appropriate locations.
Four different fluorescent retrograde tracers were used: Fast Blue (FB, 5% aqueous solution; EMS-Chemie GmbH, Gross-Umstadt, Germany), red and green fluorescent microspheres (“RetroBeads,” both from Lumafluor, Naples, FL, USA) and FluoroRuby (FR, 10% solution in saline; tetramethylrhodamine dextran, 10,000 molecular weight, Invitrogen). Large injections were made with a 10 µl Hamilton microsyringe. The tracer was injected at multiple sites (two to six) in one IC. Each site received an injection of 0.1–0.2 µl. Each tracer was injected with a microsyringe used only for that tracer. Small injections were made with a micropipette (25–28 µm inside diameter) attached to a Nanoliter Injector (World Precision Instruments, Sarasota, FL, USA). Up to 138 nl was injected at a single site by injecting smaller amounts (9.2 nl, 13.8 nl or 23.0 nl) at 1-min intervals until the desired total was reached. In some animals, different tracers were injected at different locations within a single IC (Table 1). Data from some animals were used in a previous study identifying cortical cells that project bilaterally to the IC (Coomes et al., 2005). In two animals, cholera toxin B subunit (“CTB,” 1% in saline; List Biological Laboratories, Campbell, CA, USA) was injected with a 1 µl Hamilton microsyringe (Table 1).
Table 1.
Tracer injection summary
| Case | Tracer | Left ICa | Right ICa | Angle | Injection tool | # Of sites injected | Volume injected/site |
|---|---|---|---|---|---|---|---|
| 225 | FR | c x | 0 | 10 µl | 6 | 0.15 µl | |
| 226 | FB | c d x | 0 | ″ | 6 | 0.15 µl | |
| 229 | GB | c d x | 0 | ″ | 6 | 0.15 µl | |
| 308 | GB | c d x | 40 | ″ | 2 | 0.15 µl | |
| 310 | GB | c d x | 40 | ″ | 2 | 0.15 µl | |
| RB | c d x | 50 | ″ | 2 | 0.15 µl | ||
| 322 | FB | x | 0 | ″ | 1 | 0.25 µl | |
| 359 | FB | c x | 0 | ″ | 1 | 0.15 µl | |
| GB | c x | 0 | ″ | 1 | 0.2 µl | ||
| 361 | FB | c d x | 0 | nano | 1 | 138 nl | |
| RB | c x | 0 | 10 µl | 1 | 0.1 µl | ||
| GB | c x | 0 | 10 µl | 1 | 0.1 µl | ||
| 364 | RB | c x | 0 | nano | 1 | 92 nl | |
| GB | c x | 0 | nano | 1 | 115 nl | ||
| FB | x | 0 | 10 µl | 1 | 0.2 µl | ||
| 370 | RB | x | 0 | nano | 1 | 55.2 nl | |
| GB | d | 0 | nano | 1 | 138 nl | ||
| FB | c | 0 | nano | 1 | 69 nl | ||
| 374 | FB | c | 90 | nano | 1 | 36.8 nl | |
| RB | x | 90 | nano | 1 | 36.8 nl | ||
| 379 | GB | d | 50 | nano | 1 | 36.8 nl | |
| 386 | GB | c x | 50 | nano | 1 | 55.2 nl | |
| 531 | CTB | c d x | 0 | 1 µl | 2 | 0.2 µl | |
| 534 | CTB | c d | 0 | 1 µl | 1 | 0.3 µl |
Abbreviations: 1 µl, µl microsyringe; 10 µl, 10 µl microsyringe; CTB, cholera toxin B; FR, FluoroRuby; nano, nanoliter injector.
Subdivisions of the IC included in the injection: c, central nucleus; d, dorsal cortex; x, external cortex.
In most cases, the microsyringe or micropipette (“needle”) was inserted into the IC using a vertical approach. In these cases, the needle traversed the visual cortex on the way to the IC (Spatz et al., 1991). This approach sometimes left a small amount of tracer in visual cortex along the insertion track. To make certain that this deposit was not responsible for the label in temporal cortex, we also made injections using two other approaches (Table 1). For two injections (in GP374), the needle was mounted in a horizontal plane and entered the IC from the caudal surface. For this approach, the needle traversed the cerebellum. In a third approach, the needle was mounted at an angle, rotated in the parasagittal plane caudally 40 or 50° from vertical (Table 1). This angle approximates the angle of the caudal surface of the IC as well as the border between the caudal part of ICd and the ICc. This approach was the most effective for producing injections confined to the caudal ICd. This approach also traversed the cerebellum, albeit a different part than was traversed by horizontally-oriented needles.
Following the injections, the exposed brain was covered with Gelfoam and the scalp was sutured. Ketoprofen (3 mg/kg, i.p.) was injected to provide post-operative analgesia. After surgery the animal was placed in a clean cage and monitored until it regained the ability to stand. At that point, it was transported in its cage to the animal facility.
After an appropriate interval for the transport of the injected tracers (4–12 days for fluorescent tracers; 7–8 days for CTB), the animal was sacrificed with CO2 gas (inhalation, 10 min) or with an overdose of pentobarbital (440 mg/kg; i.p.) and the animal was perfused through the aorta with approximately 100 ml of Tyrode’s solution (pH 7.4), 350 ml of 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4 (PB), and 350 ml of 4% paraformaldehyde with 10% sucrose in PB. The brain was then removed and stored at 4 °C in 4% paraformaldehyde with 25–30% sucrose in PB. The following day, the brain was cut on a sliding microtome into 40, 50, or 80 µm thick sections. In some cases, the entire brain was cut in the transverse plane. In other cases, the cortex was separated from the brainstem and thalamus. The cortex was frozen and cut in the transverse plane on a sliding microtome. The brainstem and thalamus were split at the midline and each piece was frozen and cut in the parasagittal plane. Sections were collected in six series. For cases with fluorescent tracers, at least four series of sections were mounted on gelatin-coated slides and allowed to dry. For cases with CTB injections, at least two series of sections were stained for CTB. These sections were treated with 3% normal rabbit serum with 0.2% Triton-X 100 followed by goat anti-CTB (diluted 1:12,000; List Biological), biotinylated rabbit–anti-goat secondary antibody (Vector) and avidin– biotin–peroxidase (ABC Elite kit, Vector Laboratories). The label was visualized with nickel-enhanced diaminobenzidine.
In all cases, at least one series of sections was stained with thionin for cytoarchitecture. In most cases with small injections, one or two series of sections were stained with one of several procedures to help distinguish IC subdivisions. We used a histochemical stain for nicotinamide-adenine dinucleotide phosphate-diaphorase (NADPH-d; Druga and Syka, 1993) or an immunohistochemical stain for neuronal nitric oxide synthase (nNOS). For this stain, we used antinNOS made in mouse (Sigma; # N2280, diluted 1:1000), followed by a biotinylated goat–anti-mouse secondary antibody (1:200 dilution; Vector Laboratories) and avidin– biotin–peroxidase (ABC Elite kit, Vector Laboratories) staining with nickel-enhanced diaminobenzidine. Stained sections were mounted on slides and dried overnight. All sections were then coverslipped with DPX (Aldrich Chemical Company, Inc., Milwaukee, WI, USA).
Data analysis
For analysis of injections into the IC, the IC subdivisions—the ICc, ICd, and ICx—were identified using adjacent sections stained with thionin and NADPH-d or anti-nNOS and criteria described previously (Faye-Lund and Osen, 1985; Druga and Syka, 1993, 2001; Malmierca et al., 1995; Paloff and Hinova-Palova, 1998; Coote and Rees, 2008).
Retrogradely-labeled cortical cells were examined with 20× or 40× objectives and plotted in a series of sections (spaced 300 or 600 µm apart) through temporal cortex using a Neurolucida plotting system (MicroBrightField, Williston, VT, USA) connected to a Zeiss Axioplan II fluorescence microscope. The borders of cortical laminae were added by comparison with adjacent thionin-stained sections or by removing the coverslips from the plotted sections, re-hydrating them, staining them with thionin, and re-applying coverslips. The layers were identified according to criteria described previously (Schofield et al., 2006). In cases with CTB injections, well-labeled cells were drawn with a camera lucida attached to a Zeiss Axioskop. The cells were drawn with a 40× objective, with locator drawings made with a 2.5× objective. The initial drawings were inked, scanned into Adobe Photoshop using a flatbed scanner (Canon LiDE 60) and transferred to Adobe Illustrator for labeling.
Photomicrographs were taken with an Optronics Magnafire camera attached to a Zeiss Axioskop microscope or a Zeiss Axiocam HRm camera attached to a Zeiss AxioImager Z1 microscope. For the latter cases, initial image processing (e.g. contrast adjustment) was done with Axiovision software (Zeiss). For all images, Adobe Photoshop was used to make final adjustments of levels and color balance, to arrange and label photographs, and to erase “background” outside the sections.
RESULTS
The results are based on a series of experiments in which different tracers were injected into the IC (Table 1). The results were similar with pigmented or albino animals. Large injections were made by depositing tracer at up to six sites within the IC. We use the results of these experiments to describe the distribution of labeled cortical cells and the morphology of the layer VI cells. Small, single deposits were made in other animals in attempts to make injections confined to individual subdivisions of the IC. We use these results to describe the projections of cortical cells in layers V and VI to different parts of the IC. Lastly, we present quantitative results comparing the relative numbers of cortical cells in layers V and VI that project to the IC.
Distribution of corticocollicular cells
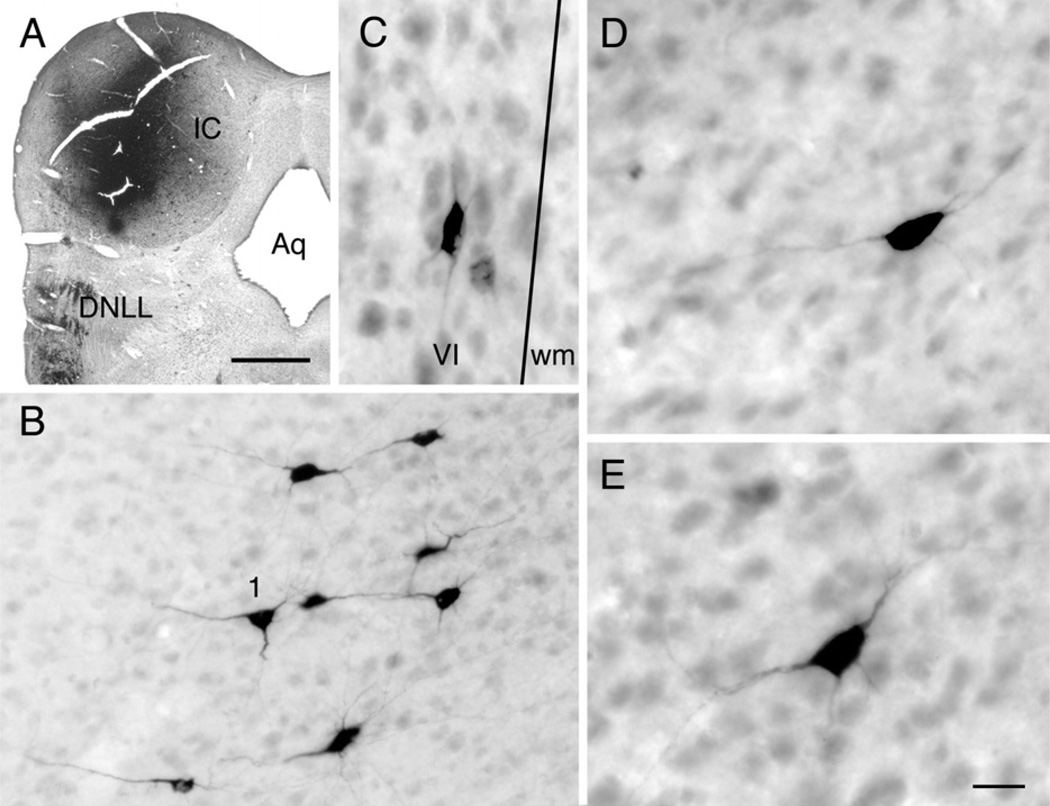
Fig. 1 shows a large injection of FB into one IC. Multiple deposits of tracer were made, and multiple foci can be observed within the IC. The injection was confined completely to the right IC, except for a slight encroachment into the caudal tip of the superficial layer of the superior colliculus (Fig. 1A). The injection site included all subdivisions of the IC. This was consistent with the presence of labeled cells in brainstem auditory nuclei, including the medial superior olivary nucleus (indicating inclusion of ICc) and labeled cells in dorsal column nuclei (indicating inclusion of ICx).
Fig. 1.
Photomicrographs showing results of a large injection of FB into the right IC. (A) Parasagittal section through the center of one of the deposit sites (“1”) in experiment GP226. The margins of a second deposit site, rostral and lateral to the first, is also visible (“2”). ICc, ICd and ICx — central nucleus, dorsal cortex and external cortex of the IC; SC, superior colliculus. Rostral is right, dorsal is up. Scale bar=1 mm. (B) Low magnification image showing FB-labeled cells in ipsilateral temporal cortex. White lines indicate the border between layers V and VI; the border of layer VI with white matter is at the bottom edge of the image. Scale bar=50 µm. Transverse section; cortical surface (lateral) is up. Labeled boxes are shown at higher magnification in C and D. (C, D) High magnification images showing FB-labeled cells in layers V (C) and VI (D). Scale bar=10 µm for both panels.
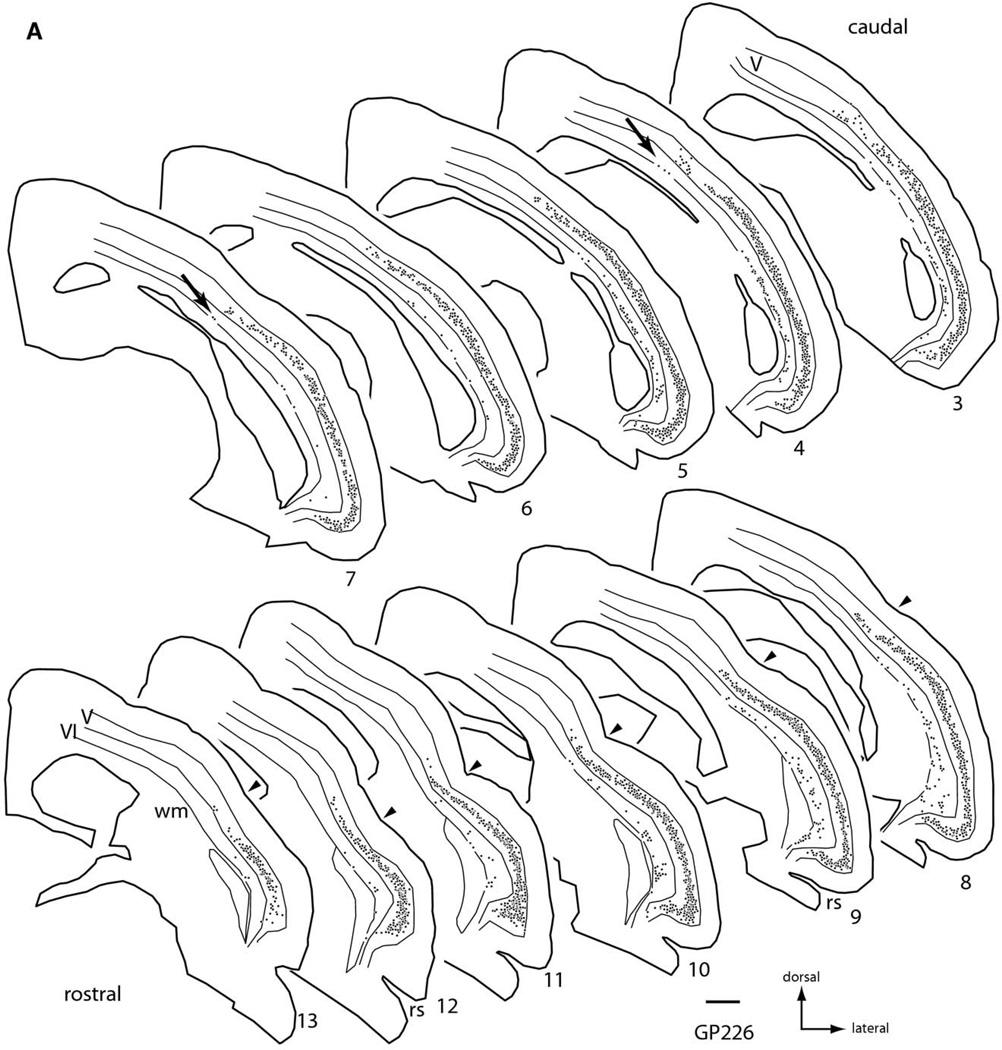
There were many cells labeled in both ipsilateral and contralateral temporal cortex (Fig. 2). On the ipsilateral side, labeled cells were located in layers V and VI (Fig. 2A). In layer V, labeled cells could be found at any depth, but the vast majority were concentrated in the center of the layer. The cells in layer V formed a dense, nearly continuous sheet of cells that extended for a long distance across cortex. In the dorso-ventral dimension, the sheet extended ventrally to the rhinal sulcus and dorsally to or beyond the pseudosylvian sulcus (arrowheads in Fig. 2). In the rostro-caudal dimension, the sheet extended from the rostral pole of the temporal lobe (Fig. 2, section 13) caudally for over 6 mm (section 3). In layer VI, the majority of the labeled cells were found in the deeper half of the layer, and often hugged the border with the white matter. The number of cells was considerably smaller than that in layer V (discussed in more detail below), but the distribution of layer VI cells across cortex was fairly broad. Ventrally, layer VI cells were present near the rhinal sulcus (e.g. sections 8, 9, Fig. 2A). Dorsally, layer VI cells usually did not extend as far as the labeled layer V cells (although see arrows in sections 4, 7, Fig. 2A). In the rostral sections where the pseudosylvian sulcus is apparent, the layer VI cells extended dorsally to but rarely beyond the sulcus.
Fig. 2.
Plots of the distribution of FB-labeled cells in temporal cortex following a large injection of FB into the right IC (injection shown in Fig. 1). Plots are ipsilateral (A) or contralateral (B) to the injection. Each black dot represents one or more labeled cells. Sections are numbered from caudal to rostral and are spaced 600 µm apart. Scale bar=1 mm. Abbreviations: rs, rhinal sulcus; V, VI-cortical layers; wm, white matter; arrowheads, location of pseudosylvian sulcus; arrows, dorsally located layer VI cells.
On the contralateral side (Fig. 2B), labeled cells were restricted almost entirely to layer V. On rare occasion, a labeled cell was visible in layer IV; the cell was morphologically similar to layer V cells. Such cells were also seen on the ipsilateral side, though no examples were present in the sections illustrated in Fig. 2A. In layer V, the number of cells was much lower than on the ipsilateral side, but their depth distribution within the layer was similar to that on the ipsilateral side. The cells were distributed widely across cortex, with rostral and ventral limits similar to those on the ipsilateral side. Dorsally, the distribution extended to the pseudosylvian sulcus, though perhaps not as far as on the ipsilateral side (the small number of cells made it difficult to determine a limit precisely). A difference in the dorsal spread may be greater in more caudal sections (where the pseudosylvian sulcus is absent), but this point would have to be examined with additional studies. In the rostro-caudal dimension, the contralateral cells extended a considerable distance, though perhaps not as far caudally as on the ipsilateral side.
The results described above are based on FB. Similar results were obtained with red beads (RB), green beads (GB) and CTB. Data were available from many other cases in the laboratory, completed as parts of other studies. These cases showed that several other tracers, including FluoroRuby, fluorescein dextran and FluoroGold, all labeled large numbers of layer V cells but rarely labeled layer VI cells. The reasons for the different sensitivities of the tracers are unclear, but are important in comparing results across different studies and species (see Discussion).
Morphology of corticocollicular cells
The cell bodies of layer VI cells were smaller than those in layer V. Many of the cells in both layers had clearly pyramidal morphology, with a readily identifiable apical dendrite (Fig. 1B). In some cells, the tracer was limited to the cell body; these were generally pyramidal in shape, but morphologic type could not be determined with certainty. Further details on layer V morphology will be presented in a later study, which includes intracellular labeling. The remainder of this study is focused on the labeled layer VI cells.
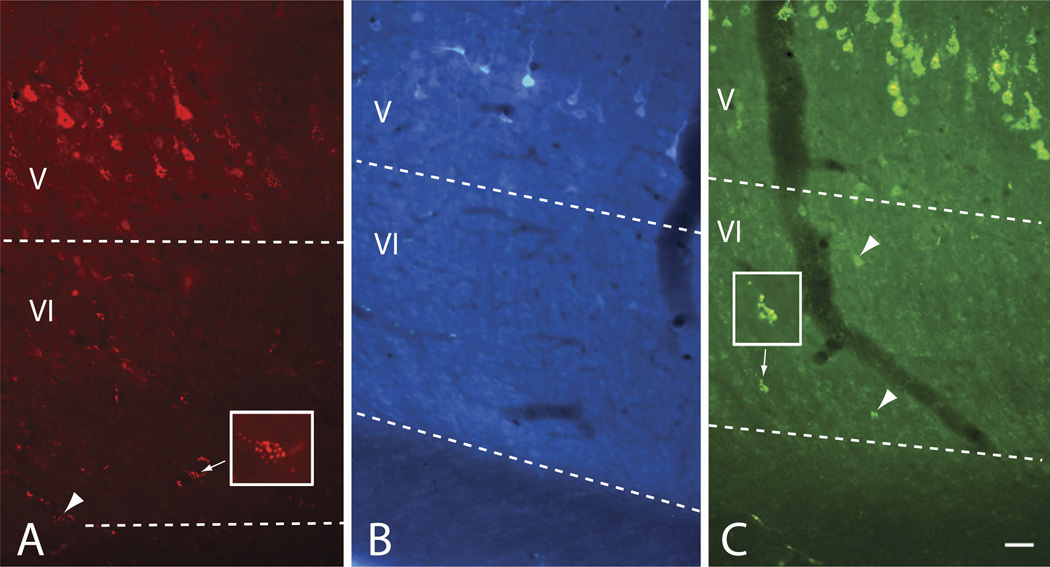
The layer VI cells were variable in morphology. This was best appreciated following injection of CTB into the IC. The CTB labeled fewer cells than did FB, RB or GB, but the dendritic labeling was often more extensive. Fig. 3 shows a CTB injection site and labeled cells in layer VI. The injection site was centered in the ICc but spread into both ICd and ICx. Labeled cells in layer VI varied in somatic morphology (Fig. 3B–E). Some had a pyramidal soma (e.g. Fig. 3, cell “1”) whereas others had a more elongated soma that was sometimes oriented parallel to the layer VI border (Fig. 1C). Fig. 4 shows camera lucida drawings of selected cells to illustrate the variety of somatodendritic architectures. To our knowledge, there is no description of morphologically-defined cell types in guinea-pig auditory cortex layer VI; however, there is a common theme across species (reviewed by Winer, 1992) and it was relatively straightforward to use the detailed description from cats (Prieto and Winer, 1999) to identify likely cell types in our sections. As seen in Fig. 4A, pyramidal cell bodies could exhibit classic pyramidal dendritic architecture (e.g. cells labeled “1” and “p” in Fig. 4). Dendritic spines were not visible; this may be a limitation of the tracer labeling, as spines were not visible on the labeled layer V cells, which are known to possess dendritic spines** [Lu et al., 2007]). Other cells appeared to resemble inverted pyramids (Fig. 4A, “ip”), stellate cells (“s”), vertical cells (“v”) or horizontal cells (“h”).
Fig. 3.
Photomicrographs from an animal with an injection of CTB into the left IC. (A) Image through the center of the injection site (the tissue is cracked due to the brittleness of the reaction product). Transverse section. Scale bar=1 mm. (B–E) Photomicrographs of CTB-labeled cells in layer VI of the left temporal cortex. Transverse sections; cortical surface is to the left. The line in C is the border between layer VI (VI) and white matter (wm). Scale bar in E=40 µm (for B, C); 20 µm (D, E).
Fig. 4.
Camera lucida drawings of CTB-labeled corticocollicular cells from three different transverse sections through temporal cortex. (A) The most caudal of the three sections in this figure, showing a band of layer VI cells. The cell labeled “1” is the same as that shown in the photograph in Fig. 3B (several of the nearby cells are also visible). In each panel, the locations of the cells are shown in low magnification drawings of the sections in which the cortical layers are indicated; individual cells are drawn at higher magnifications in the enlargements. The border between white matter and layer VI is indicated by a dotted line. Several types of morphology are visible: h, horizontal cell; ip, inverted pyramid; p, pyramidal cell; s, stellate cell; v, vertical cell (see text for discussion). (B, C) Intermediate (B) and rostral (C) sections with labeled layer VI cells. Both these sections had clear examples of horizontal cells. CPu, caudate putamen; Den, dorsal endopiriform nucleus; ps, pseudosylvian sulcus; rs, rhinal sulcus; wm, white matter. Scale bars=1 mm (low magnification drawings) or 50 µm (enlarged cells).
Projections to individual subdivisions of the IC
In order to identify cortical cells that project to specific IC subdivisions, we made small injections restricted to a single subdivision. Our cases include injections restricted to ICc, ICd or ICx (Table 1). Results for a given subdivision were similar across all cases. This was true for injections into ICd whether the injection was centered in the dorsomedial part of the IC or in the caudal part (often called caudal cortex). Injections in ICx gave similar results whether they were located in the so-called lateral cortex or in the rostral part of the ICx. Significantly, the results were similar regardless of the angle of approach to the IC: vertical, oblique (40 or 50° posterior to vertical) or horizontal. We illustrate our findings by describing a representative case in which we injected three different fluorescent tracers, one into each subdivision, in a single IC. RB were injected into the ICx; FB was injected into the ICc, and GB were injected into the ICd. The medial superior olivary nucleus, as well as other nuclei in the superior olivary complex, contained many cells labeled with FB, confirming that the ICc was injected with FB. The dorsal column nuclei contained a small number of cells labeled with RB, confirming their injection into ICx (Coleman and Clerici, 1987; Syka et al., 2000; Zhou and Shore, 2006). Results from other cases are described as needed to address specific issues.
For all three tracers, the labeled cells were spread across a wide area of cortex, comparable to that described above after large injections into the IC. The following description focuses on the distribution within the ipsilateral cortex.
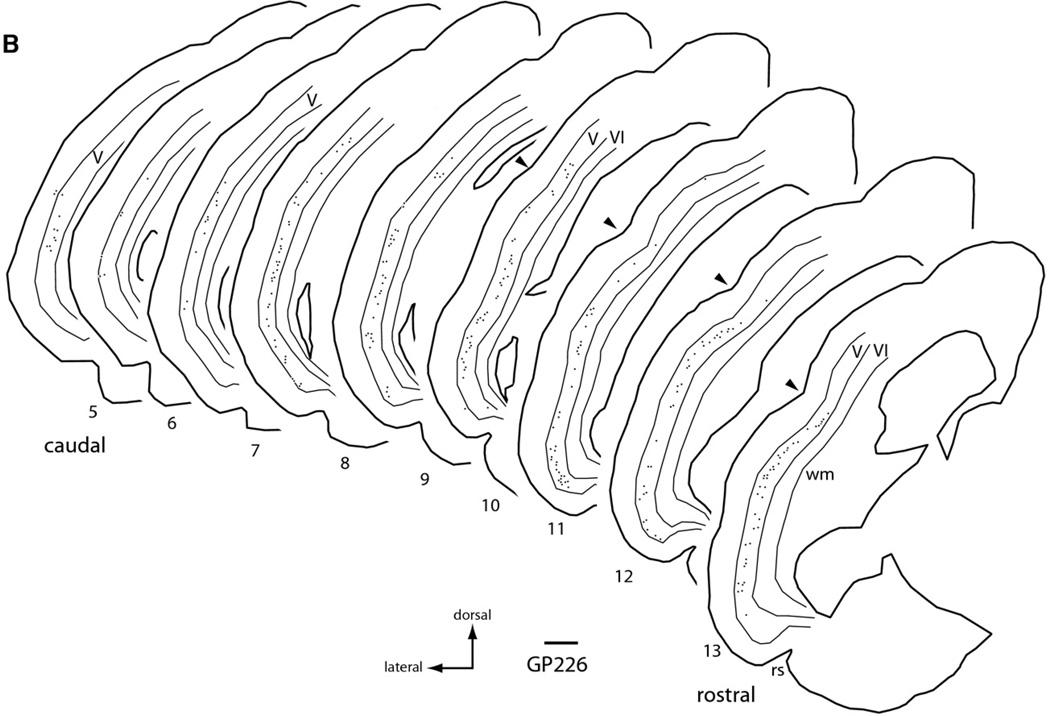
The RB injection in ICx labeled cells in both layers V and VI (Fig. 5A). Fig. 6A shows that the distribution of the RB-labeled cells was roughly similar to that described after large injections. One difference is that, in the case illustrated, the layer VI cells did not extend as far rostrally and rostro-dorsally. Two other cases included very small injections into the rostro-dorsal part of ICx. In case GP364, an injection of FB was located in the right ICx and in case GP374 an injection of RB was confined to a similar part of the ICx in the left IC. In both cases, labeled layer VI cells were present in the most rostral sections of temporal cortex; however, even in these sections there were no labeled layer VI cells near the pseudosylvian sulcus at these rostral levels. The large injections described above did label layer VI cells in the rostro-dorsal temporal cortex; it remains to be determined whether these cells would be labeled by injections into other parts of the ICx.
Fig. 5.
Photomicrographs showing labeled cells after injections in different IC subdivisions. (A–C) Images through three different areas in temporal cortex showing labeled cortical cells after injection of RB in ICx (A), FB in ICc (B) and GB in ICd (C). Layer V cells are labeled in all three images. Layer VI cells were labeled (arrowheads) with RB (A) or with GB (C); two cells are shown at higher magnification in the insets. Transverse sections; cortical surface (lateral) is up. Scale bar=50 µm. Dotted lines indicate laminar borders. All images are from experiment GP370.
Fig. 6.
Plots showing the distribution of labeled cells in right temporal cortex after injections into individual IC subdivisions (GP370). (A) Distribution of RB-labeled cells after an injection confined to the right ICx. Note the significant number of labeled cells in layer VI. (B) Distribution of FB-labeled cells after an injection confined to the right ICc. Labeled cells in layer VI were rare. (C) Distribution of GB-labeled cells after an injection confined to the right ICd. Note the significant number of labeled cells in layer VI. For all panels: Each dot indicates one or more labeled cells. Transverse sections are numbered from caudal to rostral and are spaced 600 µm apart. Thin lines indicate the borders of cortical layers V and VI. Abbreviations: rs, rhinal sulcus; V, VI, cortical layers; arrowheads, location of pseudosylvian sulcus. Scale bar=1 mm.
Injections into the ICc labeled fewer cortical cells overall than injections into other parts of the IC (Fig. 5B). The number of layer VI cells was very small. Fig. 6B shows the distribution of the labeled cells after an injection of FB that was largely confined to the ICc. Similar results were obtained with CTB (case GP534), with a small number of labeled layer VI cells but a possibility that the injection encroached on ICx. A very small injection (36.8 nl) of FB in GP374 appeared to be restricted to ICc. Labeled cells were observed only in layer V, but they were so few in number (15 labeled cells in a series of every sixth section through temporal cortex) that it is unclear if the lack of label in layer VI is meaningful.
Injections into the ICd labeled many cortical cells (Fig. 5C). Fig. 6C shows that the distribution was similar to that obtained with the large injections. Rostrally, the layer VI cells were distributed about as broadly as the layer V cells. Caudally and caudo-dorsally, the layer VI cells did not spread as far as the layer V cells. In case GP379, the spread of the layer VI cells was similar to the layer V cells caudally but not as extensive caudo-dorsally.
Quantitative comparisons
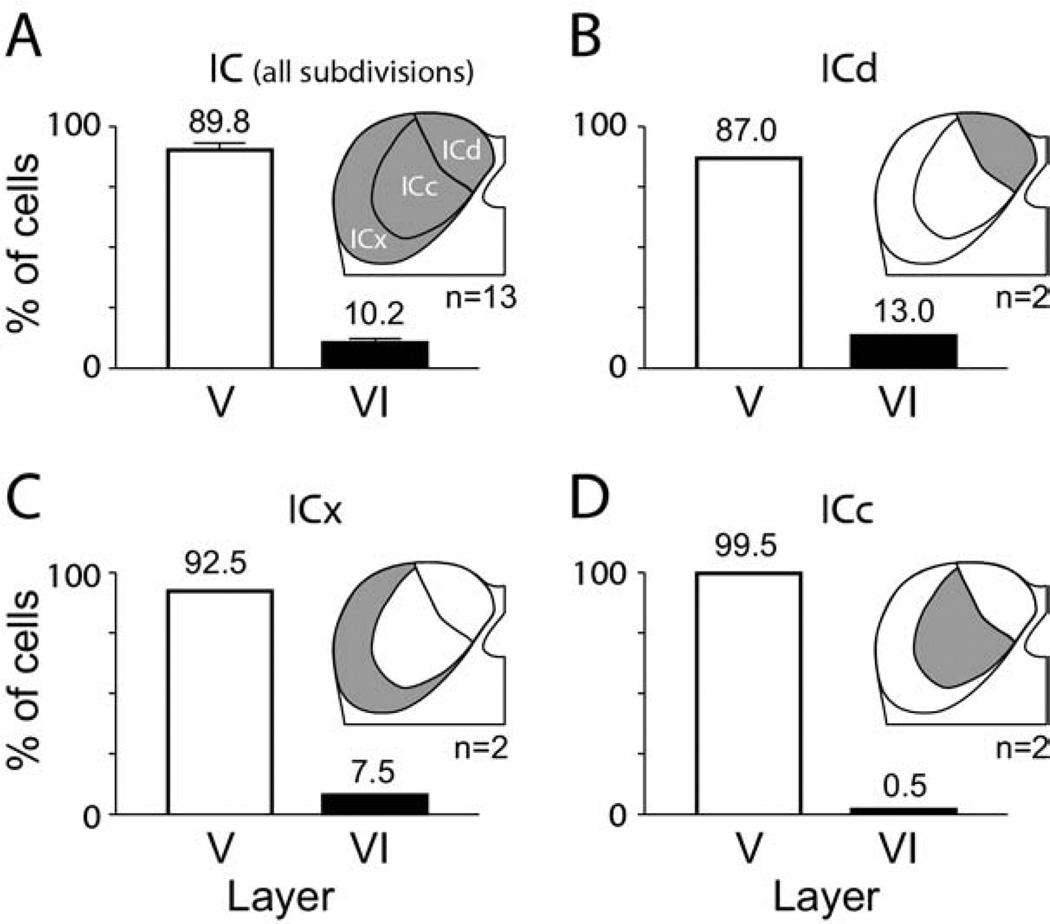
In order to obtain a measure of the relative contribution of layer VI cells to the overall corticocollicular pathway, we compared the number of such cells in layer VI to the number in layer V (Fig. 7). In order to characterize the overall projection, we pooled data from 13 cases in which we collected quantitative data. The data set included cases with large injections that involved two or all three IC subdivisions as well as experiments with smaller injections. The layer VI cells averaged 10.2% of the ipsilateral cells. We then examined the results of injections restricted to individual IC subdivisions. As described above, it can be difficult to be certain about the limits of an injection site, particularly for one restricted to the ICc. Nonetheless, we were able to quantify cells in two cases for each of the three IC subdivisions. The contributions from layer VI were more substantial for ICd (average 13.0%) and ICx (average 7.5%) than for ICc (average 0.5%). The difference between ICx and ICd suggests that layer VI may play a more prominent role in projections to ICd but, given the small number of cases on which this observation is based, this hypothesis should be tested with further experiments.
Fig. 7.
Graphs illustrating the relative number of cells in layer V (white bars) versus layer VI (black bars) labeled by injection of retrograde tracer into the ipsilateral IC. (A) Overview of projections to the entire IC (indicated by shading in the IC drawing) as revealed by pooling results across 13 experiments. Error bars indicate S.E.M. (B–D) Results from small injections restricted to the ICd (B), ICx (C) or ICc (D). For all panels: The numbers above each bar indicate the average percentage. n=Number of experiments.
DISCUSSION
The present results confirm a large corticocollicular projection that terminates in all three IC subdivisions ipsilaterally and in the ICx and ICd contralaterally in guinea pigs. The results extend previous studies by adding the following points: 1) the cells in layer VI project only ipsilaterally; 2) cells in layer VI constitute about 10% of the population projecting to the ipsilateral IC; 3) the projections from layer VI, like those from layer V, arise from a widespread region of temporal cortex; 4) the layer VI cells comprise multiple cell types, including both pyramidal and non-pyramidal cells; 5) the layer VI projections terminate predominantly in the ICd and ICx. The following sections discuss the interpretation of these results and compare them to findings in previous studies and other species.
Technical considerations
There is widespread agreement on a projection from layer V to the IC across species (reviewed by Winer, 1992, 2006). A projection from layer VI is less clear. It is unlikely that axons of layer VI cells travel through the IC to terminate at more caudal levels. The auditory cortex projects to a number of subcollicular targets in guinea pigs (Jacomme et al., 2003; Coomes and Schofield, 2004; Schofield et al., 2006; Peterson and Schofield, 2007). All these studies, which include experiments with the tracers used here, have identified layer V cells as the source of the projections to targets caudal to the IC.
Another concern is that a small amount of tracer may be deposited outside the intended target as the micropipette or microsyringe travels through other brain regions. The commonly-used vertical approach to the IC traverses visual cortex in many species, and it is possible that tracer deposit in visual cortex labels layer VI cells in other cortical areas. This concern has been ruled out in the present study by making injections with oblique and horizontal approaches that traverse the cerebellum rather than cerebral cortex. The fact that our results were similar regardless of approach suggests strongly that auditory cortical layer VI cells project directly to the IC.
Layer VI projections across species
Games and Winer (1988) described labeled layer VI cells after injections of retrograde tracers into rat IC; in fact, they state that up to 10% of the cells were in layer VI, a number matching our findings in the present study. The presence of layer VI corticocollicular cells in rats was confirmed recently by Doucet et al. (2003). Evidence from other species is very limited. Künzle (1995) described a band of layer VI corticocollicular cells (in addition to layer V cells) in the hedgehog tenrec. Bajo and Moore (2005) described a small number of ipsilaterally-projecting layer VI corticocollicular cells in gerbils. Their results were very similar to those in the present study in demonstrating that layer VI cells project ipsilaterally and not contralaterally and terminate terminate in regions outside the ICc. Studies in cats (Kelly and Wong, 1981; Winer and Prieto, 2001) and in ferrets (Bajo et al., 2007) suggest that the corticocollicular projections originate solely from layer V in these species.
The current study highlights the different sensitivities of retrograde tracers, showing that not all the more recently introduced tracers are equally sensitive in labeling the layer VI corticocollicular pathway (see discussion in Schofield et al., 2007; Schofield, 2008). A previous study in guinea pigs (Druga et al., 1988) did not describe layer VI cells following retrograde transport of horseradish peroxidase injected in the IC. Bajo and colleagues (Bajo and Moore, 2005; Bajo et al., 2007) labeled layer VI cells with FluoroGold in gerbils, but did not describe labeled layer VI cells after injections of FluoroGold or RB or GB into the IC of ferrets. We conclude that there are likely to be species differences, but that the sensitivity of tracers must be considered carefully when looking for a layer VI corticocollicular projection. Given that many studies of corticocollicular projections were based on early retrograde tracers, we suggest that a contribution from layer VI cells may be more widespread than would be suggested by the literature.
Origins from wide area of cortex
Most studies conclude that corticocollicular projections originate from multiple auditory areas (reviewed by Winer, 2006). This is well demonstrated for projections from layer V cells in guinea pigs (Druga et al., 1988; Coomes et al., 2005). Studies that describe layer VI corticocollicular cells have mentioned them only briefly, with no details on their distribution across cortical areas. The present data show that layer VI corticocollicular cells are spread almost as widely as the layer V cells. It is clear that labeled layer VI cells were located in both of the large “core” areas—primary auditory cortex and the dorsocaudal field (c.f. Wallace et al., 2000, 2002 for definition of auditory cortical areas in guinea pigs)—as well as several of the surrounding areas. For example, the presence of labeled layer VI cells extending dorsally beyond the pseudosylvian sulcus (the rostro-dorsal limit of the temporal lobe) suggests strongly that the dorsorostral belt (field DRB) and/or the small field (field S) contribute layer VI projections to the IC. Similarly, extension of layer VI label to the rhinal sulcus suggests that the ventrally located belt areas (the ventrocaudal belt VCB, the transitional area “T,” and the ventrorostral belt, VRB) also project to the IC. Projections from other areas, such as the dorsocaudal belt, appear likely from our data, but are more difficult to assess because of the lack of structural correlates related to the borders. In any case, inter-animal variation in the locations of the borders (Wallace et al., 2000) means that confirmation of projections from specific belt areas will require tracer studies combined with physiological identification of the cortical areas. Nonetheless, we can conclude that both core areas and at least some of the belt areas have corticocollicular cells in layers V and VI.
Cell types in layer VI
Layer VI has been called the polymorphic layer because of the relative prominence of non-pyramidal cells (reviewed by Winer, 1992). Both the pyramidal and the non-pyramidal layer VI cells are morphologically distinct from the layer V corticocollicular cells, which are described as medium and large pyramids with apical dendrites that extend into the supragranular layers (reviewed by Winer, 2006; Bajo and Moore, 2005). One of the largest projections from layer VI is to the thalamus. Kelly and Wong (1981) found that the thalamic projection from layer VI arises from both pyramidal and fusiform cells. Using different techniques, Mitani et al. (1985) and Prieto and Winer (1999) confirmed non-pyramidal layer VI cells as projecting to the thalamus, but noted that these cells were greatly outnumbered by pyramidal cells. The present results demonstrate that both pyramidal and non-pyramidal cells in layer VI project to the IC. It would be interesting to know if any of these cells have collateral projections to both the thalamus and the IC. Either way, it is likely that the different cell types serve different functions.
The corticocollicular pathway is considered excitatory (Mitani et al., 1983; Torterolo et al., 1998). There is direct evidence that the layer V cells use glutamate (Feliciano and Potashner, 1995; Saint Marie, 1996). It is likely that many of the layer VI corticocollicular cells are also glutamatergic; however, the identification of non-pyramidal corticocollicular corticocollicular cells raises another possibility because some of these cell types (and variants such as inverted pyramids) include cells that are GABAergic (Prieto et al., 1994).
Terminations in specific IC subdivisions
The present results suggest that layer VI cells terminate preferentially in ICd and ICx. Cortical axons traveling toward the IC comprise several groups differing in trajectory and point of entry to the IC (Saldaña et al., 1996). The groups take various routes within the IC, often traveling in the outer fibrous capsule but also crossing the deeper layers and the central nucleus. It is tempting to speculate that the axons of layer VI cells represent one of the incoming fiber groups, but this idea must be tested. The intermingling of the groups and their complex trajectories through the IC raise an important issue for interpretation of the current data. It is possible that an injection in one IC subdivision labeled axons that terminate there as well as other axons that traverse the area but do not terminate there (i.e. fibers of passage). Several factors argue against this being a major problem. Layer VI cells were labeled by injections that were very small and that were made with small diameter micropipettes. This approach minimizes labeling axons of passage, particularly when used with RB or GB (Katz et al., 1984; Katz and Iarovici, 1990). In addition, we used several different approaches—vertical, horizontal or oblique—to minimize involvement of multiple subdivisions. Consequently, it was easy to inject the ICx or ICd without traversing any other IC subdivision. Of course, it is not possible to reach the ICc without going through other subdivisions. Our injections into the ICc labeled very few layer VI cells, and we cannot distinguish between a small projection from layer VI to ICc and possible labeling of layer VI axons that were traversing ICc en route to another IC subdivision.
Functional implications
Several types of data suggest a multitude of functions for the corticocollicular pathway. The projections originate from different cortical areas. Layer VI cells have different morphology and connections compared with layer V cells (reviewed by Winer, 1992, 2006) and different responses to acoustic stimulation compared with cells in layer V (Sugimoto et al., 1997). Finally, the different morphologic types of corticocollicular cells suggest different functions. A multitude of functions is also apparent in studies of the acoustic responses of IC cells when cortical projections are activated or inactivated. Such manipulation can affect the tuning of IC responses to stimulus frequency, amplitude, inter-stimulus delay and interaural differences (e.g. Yan et al., 2005; Zhou and Jen, 2007; Suga, 2008; Nakamoto et al., 2008). Interestingly, most of the physiological recordings have been from cells in ICc. Direct cortical projections to ICc, described in at least some species, likely underlie some of the effects observed physiologically. Additional effects may be mediated by indirect routes, including cortical projections to ICd and ICx that could affect cells that project in turn to the ICc (Jen et al., 2001). An important challenge for future studies will be to distinguish the effects of the corticocollicular pathway on IC cells in the context of the cells of origin and the pathways by which the effects are elicited. The current data highlight the need to consider cortical layer of origin as well as cortical area in assessing the functions of the corticocollicular projections.
Acknowledgments
I would like to dedicate this paper to the memory of Dr. Jeffery Winer, auditory neuroanatomist, colleague and friend. Special thanks to Arkadiusz Slusarczyk, Ryan Schofield and Colleen Sowick for expert technical assistance. Misty Haas and Diana Peterson participated in some of the experiments. Thanks also to Susan Motts for comments on an earlier draft. This work was supported by the National Institutes of Health DC04391.
Abbreviations
- CTB
cholera toxin B subunit
- FB
Fast Blue
- GB
green beads
- IC
inferior colliculus
- ICc
central nucleus of inferior colliculus
- ICd
dorsal cortex of inferior colliculus
- ICx
external cortex of inferior colliculus
- nNOS
neuronal nitric oxide synthase
- PB
phosphate buffer
- RB
red beads
REFERENCES
- Bajo VM, Moore DR. Descending projections from the auditory cortex to the inferior colliculus in the gerbil, Meriones unguiculatus. J Comp Neurol. 2005;486:101–116. doi: 10.1002/cne.20542. [DOI] [PubMed] [Google Scholar]
- Bajo VM, Nodal FR, Bizley JK, Moore DR, King AJ. The ferret auditory cortex: descending projections to the inferior colliculus. Cereb Cortex. 2007;17:475–491. doi: 10.1093/cercor/bhj164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman JR, Clerici WJ. Sources of projections to subdivisions of the inferior colliculus in the rat. J Comp Neurol. 1987;262:215–226. doi: 10.1002/cne.902620204. [DOI] [PubMed] [Google Scholar]
- Coomes DL, Schofield BR. Projections from the auditory cortex to the superior olivary complex in guinea pigs. Eur J Neurosci. 2004;19:2188–2200. doi: 10.1111/j.0953-816X.2004.03317.x. [DOI] [PubMed] [Google Scholar]
- Coomes DL, Schofield RM, Schofield BR. Unilateral and bilateral projections from cortical cells to the inferior colliculus in guinea pigs. Brain Res. 2005;1042:62–72. doi: 10.1016/j.brainres.2005.02.015. [DOI] [PubMed] [Google Scholar]
- Coote EJ, Rees A. The distribution of nitric oxide synthase in the inferior colliculus of guinea pig. Neuroscience. 2008;154:218–225. doi: 10.1016/j.neuroscience.2008.02.030. [DOI] [PubMed] [Google Scholar]
- Doucet JR, Molavi DL, Ryugo DK. The source of corticocollicular and corticobulbar projections in area Te1 of the rat. Exp Brain Res. 2003;153:461–466. doi: 10.1007/s00221-003-1604-4. [DOI] [PubMed] [Google Scholar]
- Druga R, Syka J. NADPH-diaphorase activity in the central auditory structures of the rat. Neuroreport. 1993;4:999–1002. doi: 10.1097/00001756-199308000-00001. [DOI] [PubMed] [Google Scholar]
- Druga R, Syka J. Effect of auditory cortex lesions on NADPH-diaphorase staining in the inferior colliculus of rat. Neuroreport. 2001;12:1555–1559. doi: 10.1097/00001756-200106130-00009. [DOI] [PubMed] [Google Scholar]
- Druga R, Syka J, Rajkowska-Markow G. Localization of cortical neurons projecting to the inferior colliculus in the rat and guinea pig. In: Syka J, Masterton RB, editors. Auditory pathway: Structure and function. New York: Plenum Press; 1988. pp. 293–298. [Google Scholar]
- Faye-Lund H, Osen KK. Anatomy of the inferior colliculus in rat. Anat Embryol. 1985;171:1–20. doi: 10.1007/BF00319050. [DOI] [PubMed] [Google Scholar]
- Feliciano M, Potashner SJ. Evidence for a glutamatergic pathway from the guinea pig auditory cortex to the inferior colliculus. J Neurochem. 1995;65:1348–1357. doi: 10.1046/j.1471-4159.1995.65031348.x. [DOI] [PubMed] [Google Scholar]
- Feliciano M, Saldaña E, Mugnaini E. Direct projections from the rat primary auditory neocortex to nucleus sagulum, paralemniscal regions, superior olivary complex and cochlear nuclei. Auditory Neurosci. 1995;1:287–308. [Google Scholar]
- Games KD, Winer JA. Layer V in rat auditory cortex: projections to the inferior colliculus and contralateral cortex. Hear Res. 1988;34:1–26. doi: 10.1016/0378-5955(88)90047-0. [DOI] [PubMed] [Google Scholar]
- Haas MD, Coomes DL, Kuwabara N, Schofield BR. Laminar distribution and projection patterns of corticocollicular cells in guinea pig auditory cortex. Assoc Res Otolaryngol Abstr. 2003;26:87. [Google Scholar]
- Jacomme AV, Nodal FR, Bajo VM, Manunta Y, Edeline JM, Babalian A, Rouiller EM. The projection from auditory cortex to cochlear nucleus in guinea pigs: An in vivo anatomical and in vitro electrophysiological study. Exp Brain Res. 2003;153:467–476. doi: 10.1007/s00221-003-1606-2. [DOI] [PubMed] [Google Scholar]
- Jen PH, Sun X, Chen QC. An electrophysiological study of neural pathways for corticofugally inhibited neurons in the central nucleus of the inferior colliculus of the big brown bat, Eptesicus fuscus. Exp Brain Res. 2001;137:292–302. doi: 10.1007/s002210000637. [DOI] [PubMed] [Google Scholar]
- Katz LC, Burkhalter A, Dreyer WJ. Fluorescent latex microspheres as a retrograde neuronal marker for in vivo and in vitro studies of visual cortex. Nature. 1984;310:498–500. doi: 10.1038/310498a0. [DOI] [PubMed] [Google Scholar]
- Katz LC, Iarovici DM. Green fluorescent latex microspheres: a new retrograde tracer. Neuroscience. 1990;34:511–520. doi: 10.1016/0306-4522(90)90159-2. [DOI] [PubMed] [Google Scholar]
- Kelly JP, Wong D. Laminar connections of the cat’s auditory cortex. Brain Res. 1981;212:1–15. doi: 10.1016/0006-8993(81)90027-5. [DOI] [PubMed] [Google Scholar]
- Künzle H. Regional and laminar distribution of cortical neurons projecting to either superior or inferior colliculus in the hedgehog tenrec. Cereb Cortex. 1995;5:338–352. doi: 10.1093/cercor/5.4.338. [DOI] [PubMed] [Google Scholar]
- Lim HH, Anderson DJ. Antidromic activation reveals tonotopically organized projections from primary auditory cortex to the central nucleus of the inferior colliculus in guinea pig. J Neurophysiol. 2007;97:1413–1427. doi: 10.1152/jn.00384.2006. [DOI] [PubMed] [Google Scholar]
- Lu Y, Gao H, Waterman JD, Schofield BR. Society for Neuroscience, Program Number 278.14, 2007 Neuroscience Meeting Planner. San Diego, CA: Society for Neuroscience; 2007. Physiology, morphology, and connections of guinea pig auditory cortical layer V pyramidal neurons. www.sfn.org. [Google Scholar]
- Malmierca MS, Rees A, Le Beau FE, Bjaalie JG. Laminar organization of frequency-defined local axons within and between the inferior colliculi of the guinea pig. J Comp Neurol. 1995;357:124–144. doi: 10.1002/cne.903570112. [DOI] [PubMed] [Google Scholar]
- Mitani A, Shimokouchi M, Itoh K, Nomura S, Kudo M, Mizuno N. Morphology and laminar organization of electrophysiologically identified neurons in the primary auditory cortex in the cat. J Comp Neurol. 1985;235:430–447. doi: 10.1002/cne.902350403. [DOI] [PubMed] [Google Scholar]
- Mitani A, Shimokouchi M, Nomura S. Effects of stimulation of the primary auditory cortex upon colliculogeniculate neurons in the inferior colliculus of the cat. Neurosci Lett. 1983;42:185–189. doi: 10.1016/0304-3940(83)90404-4. [DOI] [PubMed] [Google Scholar]
- Nakamoto KT, Jones SJ, Palmer AR. Descending projections from auditory cortex modulate sensitivity in the midbrain to cues for spatial position. J Neurophysiol. 2008;99:2347–2356. doi: 10.1152/jn.01326.2007. [DOI] [PubMed] [Google Scholar]
- Ojima H. Terminal morphology and distribution of corticothalamic fibers originating from layers 5 and 6 of cat primary auditory cortex. Cereb Cortex. 1994;4:646–663. doi: 10.1093/cercor/4.6.646. [DOI] [PubMed] [Google Scholar]
- Paloff A, Hinova-Palova D. Topographical distribution of NADPH-Diaphorase positive neurons in the cat’s inferior colliculus. J Hirnforschung. 1998;39:231–243. [PubMed] [Google Scholar]
- Peterson DC, Schofield BR. Projections from auditory cortex contact ascending pathways that originate in the superior olive and inferior colliculus. Hear Res. 2007;232:67–77. doi: 10.1016/j.heares.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popelár J, Nwabueze-Ogbo FC, Syka J. Changes in neuronal activity of the inferior colliculus in rat after temporal inactivation of the auditory cortex. Physiol Res. 2003;52:615–628. [PubMed] [Google Scholar]
- Prieto JJ, Peterson BA, Winer JA. Morphology and spatial distribution of GABAergic neurons in cat primary auditory cortex (AI) J Comp Neurol. 1994;344:349–382. doi: 10.1002/cne.903440304. [DOI] [PubMed] [Google Scholar]
- Prieto JJ, Winer JA. Layer VI in cat primary auditory cortex: Golgi study and sublaminar origins of projection neurons. J Comp Neurol. 1999;404:332–358. doi: 10.1002/(sici)1096-9861(19990215)404:3<332::aid-cne5>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Saint Marie RL. Glutamatergic connections of the auditory midbrain: selective uptake and axonal transport of D-[3H]aspartate. J Comp Neurol. 1996;373:255–270. doi: 10.1002/(SICI)1096-9861(19960916)373:2<255::AID-CNE8>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Saldaña E, Feliciano M, Mugnaini E. Distribution of descending projections from primary auditory neocortex to inferior colliculus mimics the topography of intracollicular projections. J Comp Neurol. 1996;371:15–40. doi: 10.1002/(SICI)1096-9861(19960715)371:1<15::AID-CNE2>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Schofield BR. Retrograde axonal tracing with fluorescent markers. Curr Protoc Neurosci. 2008;Chapter 1(Unit 1):17. doi: 10.1002/0471142301.ns0117s43. [DOI] [PubMed] [Google Scholar]
- Schofield BR, Coomes DL. Projections from auditory cortex contact cells in the cochlear nucleus that project to the inferior colliculus. Hear Res. 2005;206:3–11. doi: 10.1016/j.heares.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Schofield BR, Coomes DL, Schofield RM. Cells in auditory cortex that project to the cochlear nucleus in guinea pigs. J Assoc Res Otolaryngol. 2006;7:95–109. doi: 10.1007/s10162-005-0025-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield BR, Schofield RM, Sorensen KA, Motts SD. On the use of retrograde tracers for identification of axon collaterals with multiple fluorescent retrograde tracers. Neuroscience. 2007;146:773–783. doi: 10.1016/j.neuroscience.2007.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SM. The thalamus is more than just a relay. Curr Opin Neurobiol. 2007;17:417–422. doi: 10.1016/j.conb.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SM, Guillery RW. The role of the thalamus in the flow of information to the cortex. Philos Trans R Soc Lond B Biol Sci. 2002;357:1695–1708. doi: 10.1098/rstb.2002.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spatz WB, Vogt DM, Illing RB. Delineation of the striate cortex, and the striate-peristriate projections in the guinea pig. Exp Brain Res. 1991;84:495–504. doi: 10.1007/BF00230961. [DOI] [PubMed] [Google Scholar]
- Suga N. Role of corticofugal feedback in hearing. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2008;194:169–183. doi: 10.1007/s00359-007-0274-2. [DOI] [PubMed] [Google Scholar]
- Suga N, Ma X. Multiparametric corticofugal modulation and plasticity in the auditory system. Nat Rev Neurosci. 2003;4:783–794. doi: 10.1038/nrn1222. [DOI] [PubMed] [Google Scholar]
- Sugimoto S, Sakurada M, Horikawa J, Taniguchi I. The columnar and layer-specific response properties of neurons in the primary auditory cortex of Mongolian gerbils. Hear Res. 1997;112:175–185. doi: 10.1016/s0378-5955(97)00119-6. [DOI] [PubMed] [Google Scholar]
- Sun X, Xia Q, Lai CH, Shum DK, Chan YS, He J. Corticofugal modulation of acoustically induced Fos expression in the rat auditory pathway. J Comp Neurol. 2007;501:509–525. doi: 10.1002/cne.21249. [DOI] [PubMed] [Google Scholar]
- Syka J, Popelar J, Kvasnak E, Astl J. Response properties of neurons in the central nucleus and external and dorsal cortices of the inferior colliculus in guinea pig. Exp Brain Res. 2000;133:254–266. doi: 10.1007/s002210000426. [DOI] [PubMed] [Google Scholar]
- Torterolo P, Zurita P, Pedemonte M, Velluti RA. Auditory cortical efferent actions upon inferior colliculus unitary activity in the guinea pig. Neurosci Lett. 1998;249:172–176. doi: 10.1016/s0304-3940(98)00367-x. [DOI] [PubMed] [Google Scholar]
- Wallace MN, Rutkowski RG, Palmer AR. Identification and localisation of auditory areas in guinea pig cortex. Exp Brain Res. 2000;132:445–456. doi: 10.1007/s002210000362. [DOI] [PubMed] [Google Scholar]
- Wallace MN, Rutkowski RG, Palmer AR. Interconnections of auditory areas in the guinea pig neocortex. Exp Brain Res. 2002;143:106–119. doi: 10.1007/s00221-001-0973-9. [DOI] [PubMed] [Google Scholar]
- Winer JA. The functional architecture of the medial geniculate body and the primary auditory cortex. In: Webster DB, et al., editors. The mammalian auditory pathway: Neuroanatomy. New York: Springer-Verlag; 1992. –409.pp. 222 [Google Scholar]
- Winer JA. Decoding the auditory corticofugal systems. Hear Res. 2006;212:1–8. doi: 10.1016/j.heares.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Winer JA, Prieto JJ. Layer V in cat primary auditory cortex (AI): Cellular architecture and identification of projection neurons. J Comp Neurol. 2001;434:379–412. doi: 10.1002/cne.1183. [DOI] [PubMed] [Google Scholar]
- Wu Y, Yan J. Modulation of the receptive fields of midbrain neurons elicited by thalamic electrical stimulation through corticofugal feedback. J Neurosci. 2007;27:10651–10658. doi: 10.1523/JNEUROSCI.1320-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, Zhang Y, Ehret G. Corticofugal shaping of frequency tuning curves in the central nucleus of the inferior colliculus of mice. J Neurophysiol. 2005;93:71–83. doi: 10.1152/jn.00348.2004. [DOI] [PubMed] [Google Scholar]
- Zhou X, Jen PH. Corticofugal modulation of multi-parametric auditory selectivity in the midbrain of the big brown bat. J Neurophysiol. 2007;98:2509–2516. doi: 10.1152/jn.00613.2007. [DOI] [PubMed] [Google Scholar]
- Zhou J, Shore S. Convergence of spinal trigeminal and cochlear nucleus projections in the inferior colliculus of the guinea pig. J Comp Neurol. 2006;495:100–112. doi: 10.1002/cne.20863. [DOI] [PubMed] [Google Scholar]