Summary
Background
Women born around 1940 in countries such as the UK and USA were the first generation in which many smoked substantial numbers of cigarettes throughout adult life. Hence, only in the 21st century can we observe directly the full effects of prolonged smoking, and of prolonged cessation, on mortality among women in the UK.
Methods
For this prospective study, 1·3 million UK women were recruited in 1996–2001 and resurveyed postally about 3 and 8 years later. All were followed to Jan 1, 2011, through national mortality records (mean 12 woman-years, SD 2). Participants were asked at entry whether they were current or ex-smokers, and how many cigarettes they currently smoked. Those who were ex-smokers at both entry and the 3-year resurvey and had stopped before the age of 55 years were categorised by the age they had stopped smoking. We used Cox regression models to obtain adjusted relative risks that compared categories of smokers or ex-smokers with otherwise similar never-smokers.
Findings
After excluding 0·1 million women with previous disease, 1·2 million women remained, with median birth year 1943 (IQR 1938–46) and age 55 years (IQR 52–60). Overall, 6% (66 489/1 180 652) died, at mean age 65 years (SD 6). At baseline, 20% (232 461) were current smokers, 28% (328 417) were ex-smokers, and 52% (619 774) were never-smokers. For 12-year mortality, those smoking at baseline had a mortality rate ratio of 2·76 (95% CI 2·71–2·81) compared with never-smokers, even though 44% (37 240/85 256) of the baseline smokers who responded to the 8-year resurvey had by then stopped smoking. Mortality was tripled, largely irrespective of age, in those still smoking at the 3-year resurvey (rate ratio 2·97, 2·88–3·07). Even for women smoking fewer than ten cigarettes per day at baseline, 12-year mortality was doubled (rate ratio 1·98, 1·91–2·04). Of the 30 most common causes of death, 23 were increased significantly in smokers; for lung cancer, the rate ratio was 21·4 (19·7–23·2). The excess mortality among smokers (in comparison with never-smokers) was mainly from diseases that, like lung cancer, can be caused by smoking. Among ex-smokers who had stopped permanently at ages 25–34 years or at ages 35–44 years, the respective relative risks were 1·05 (95% CI 1·00–1·11) and 1·20 (1·14–1·26) for all-cause mortality and 1·84 (1·45–2·34) and 3·34 (2·76–4·03) for lung cancer mortality. Thus, although some excess mortality remains among these long-term ex-smokers, it is only 3% and 10% of the excess mortality among continuing smokers. If combined with 2010 UK national death rates, tripled mortality rates among smokers indicate 53% of smokers and 22% of never-smokers dying before age 80 years, and an 11-year lifespan difference.
Interpretation
Among UK women, two-thirds of all deaths of smokers in their 50s, 60s, and 70s are caused by smoking; smokers lose at least 10 years of lifespan. Although the hazards of smoking until age 40 years and then stopping are substantial, the hazards of continuing are ten times greater. Stopping before age 40 years (and preferably well before age 40 years) avoids more than 90% of the excess mortality caused by continuing smoking; stopping before age 30 years avoids more than 97% of it.
Funding
Cancer Research UK, Medical Research Council.
Introduction
Smoking, mainly of cigarettes, remains the leading preventable cause of death in countries such as the UK and USA, despite declines in smoking prevalence and in the machine-measured tar yields of manufactured cigarettes.1–3 Among smokers who are now in their 60s, the excess hazards depend strongly not only on their recent smoking habits, but also on their smoking habits in early adult life, more than 40 years ago.4 Many men born during the first quarter of the 20th century started smoking substantial numbers of cigarettes from a young age, so the full hazards in middle and old age have already been seen among men,5–7 but few women began smoking until the second quarter of the century. Smoking prevalence in young women did not peak until the 1960s,8 so previous studies of women might have considerably underestimated the full eventual risks of smoking. Direct measurements of the excess hazards for women who smoke throughout adult life therefore require studies of mortality during the 21st century among women born after the first quarter of the 20th century in countries such as the UK or USA, rather than studies of mortality decades earlier, or of populations in which cigarette smoking became widespread even more recently.
The hazards of smoking for women in the USA during the late 20th century have been reported by several reliable studies, including two large prospective ones: the ACS Cancer Prevention Study II (CPS-II)7 of adults recruited in the 1980s, and the Nurses’ Health Study9,10 of women recruited in the 1970s. We report the hazards of smoking and the benefits of having stopped at various ages in a prospective study of a million women in the UK, based on 21st century mortality rates.
Methods
Study design and participants
In 1996–2001, participants were recruited into the Million Women Study through the National Health Service Breast Screening Programme (NHSBSP), signing consent and completing a questionnaire about lifestyle, medical history, and sociodemographic factors, and were resurveyed postally about 3 and 8 years later. Study participants had unique NHS numbers that link to the NHS Central Register. Dates of any deaths were routinely notified to us, with underlying causes already coded to the International Classification of Diseases, ICD-10.11 All women, including those who did not respond to the resurveys, were followed up for mortality to Jan 1, 2011 (mean 12 woman-years, SD 2) through UK national records.
Million Women Study methods are described elsewhere,12 and questionnaires are available online. Study participants signed consent to follow-up. Ethics approval was obtained from Oxford and Anglia Multicentre Research Ethics Committee.
Procedures
At entry, women were asked if they were a current or ex-smoker, and how many cigarettes they now smoked (in categories of amount smoked: none, <5, 5–9, 10–14, 15–19, 20–24, or ≥25 cigarettes per day; table). These questions were used to define baseline smoking status for analyses of 12-year mortality, although in the UK almost half the women who were smokers in the 1990s gave up by 2010.13 At the 3-year resurvey, women were asked at what ages they had first smoked regularly and had stopped. Those who were ex-smokers at both entry and the 3-year resurvey and had stopped before the age of 55 years were categorised by the age they stopped smoking (<25, 25–34, 35–44, or 45–54 years). We did not analyse results by pack-years, since having smoked ten cigarettes a day for 40 years could produce at age 60 years a risk of lung cancer vastly greater than that from having smoked 20 cigarettes a day for the past 20 years.4
Table.
Smoking patterns at the postal resurvey about 3 years after recruitment, by smoking status reported at recruitment
| Never smoker at recruitment | Ex-smoker at recruitment |
Current smoker at recruitment, by cigarettes per day |
||||
|---|---|---|---|---|---|---|
| <10 | 10–19 | ≥20 | All amounts | |||
| Number of women | 619 774 | 328 417 | 58 957 | 116 122 | 57 382 | 232 461 |
| Percentage of current smokers when resurveyed | <0·1% (223/424 277) | 2·2% (4756/217 248) | 65·2% (19 417/29 773) | 79·4% (44 401/55 953) | 83·6% (23 229/27 795) | 76·7% (87 047/113 521) |
| Age at which participant first started to smoke regularly, years | NA | 18·7 (4·4) | 20·2 (6·5) | 18·9 (5·1) | 18·1 (4·5) | 19·1 (5·4) |
| Cigarettes smoked per day at resurvey | NA | NA* | 8·0 (4·4) | 14·8 (4·6) | 22·2 (6·6) | 15·2 (7·2) |
Data are % of women (n/N), or mean (SD). NA=not applicable.
Mean consumption had been 13·5 (SD 9·2) cigarettes per day when these women last smoked.
To assess the causal effects of continued smoking and of cessation at various ages (while avoiding the biasing effects of reverse causality, whereby disease causes cessation), women were excluded if at baseline they had already had any cancer registered (other than non-melanoma skin cancer), or reported any history of heart disease, stroke, or current respiratory disease treatment.
The remainder contributed woman-years until Jan 1, 2011, or death, or emigration, irrespective of whether they responded to subsequent surveys. National mortality statistics provide virtually complete follow-up of UK deaths (including deaths of UK citizens temporarily abroad) and departure dates for the few who leave permanently (1·4% [16 275/1 180 652], who were included in our analyses until their last date of follow-up). The main analyses of current smokers versus never-smokers related smoking at baseline to 12-year risk, even though many smokers stopped during follow-up, thereby reducing their risk. Analyses of the residual hazards in those who had stopped smoking at various ages were restricted to those not smoking at recruitment and still not smoking at the 3-year resurvey. We excluded women who stopped smoking after the age of 55 years, so as to avoid including substantial numbers of women who had stopped recently because of ill health. Analyses of the effects among smokers by the age they first smoked regularly were restricted to those smoking both at recruitment and at the 3-year resurvey.
Statistical analysis
We used Cox regression models (with attained age as the underlying time variable) to obtain adjusted relative risks that compared categories of smokers or ex-smokers with never-smokers (treated as a fixed reference group). Adjustment was by geographical region (ten UK cancer registry regions), age (in single years) and other variables (in categories) such as body-mass index (<20, 20–24·9, 25–29·9, or ≥30 kg/m2), socioeconomic status (quintiles of 1991 Townsend deprivation index for area of residence at recruitment),14 current alcohol intake (none, <2, 2–7, 8–14, or ≥15 units per week), weekly strenuous physical activity (rarely or more often), height (<160, 160–164·9, or ≥165 cm), oral contraceptive use (never or ever), menopausal status (pre-menopausal, peri-menopausal, or post-menopausal), and menopausal hormone therapy use (never or ever). For every variable, missing values formed a separate category. We excluded women with bilateral oophorectomy at baseline from ovarian cancer analyses, and those with hysterectomy from endometrial cancer analyses.
When plotting relative risks by amount smoked, we defined the categories and calculated the risks by consumption reported at recruitment. We then plotted these risks against the mean number of cigarettes reported at the 3-year resurvey by those still smoking, taken as an estimate of long-term mean consumption before the study started among all in that category.
To assess the relevance of the age at which participants first started smoking regularly among those who smoke similar amounts, we adjusted relative risks for cigarettes smoked per day (by taking a weighted average of the log relative risks within each category of amount smoked, weighted by the proportion of women in each category).
As smoking correlates with drinking, we did sensitivity analyses for causes of death where alcohol is a known risk factor such as liver disease, external causes (mainly accidents and suicide), and cancers of the upper aero-digestive tract, liver, or breast. These sensitivity analyses were restricted to women who reported drinking less than three units per week (UK unit: 10 mL of alcohol).
Relative risks are plotted as squares. In figure 1, the area of each square is inversely proportional to the variance of the log risk in each separate category of amount smoked or age at which participant began smoking (indicating the informativeness of that one category alone).15 In figure 2, it is proportional to the variance of the log relative risk (indicating the informativeness of the comparison between two categories). Corresponding 95% CIs are plotted as lines. The text cites only conventional 95% CIs that compare two categories. We used Stata version 12.1 for calculations.
Figure 1.
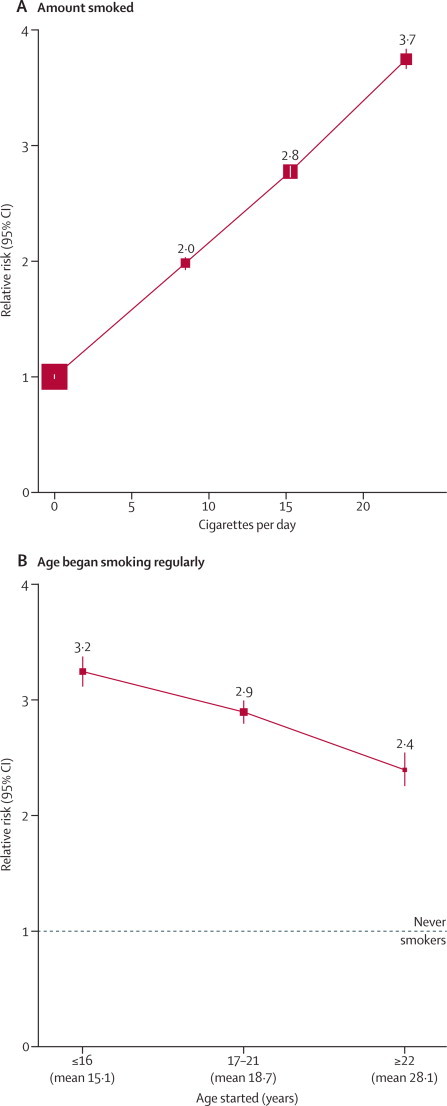
All-cause mortality, current versus never-smoker
(A) 12-year relative risk by amount smoked (at recruitment). (B) 9-year relative risk by the age at which women first began smoking regularly (as reported at the 3-year postal resurvey). For each category, the area of the square is inversely proportional to the variance of the category-specific log risk (which also determines the CI).
Figure 2.
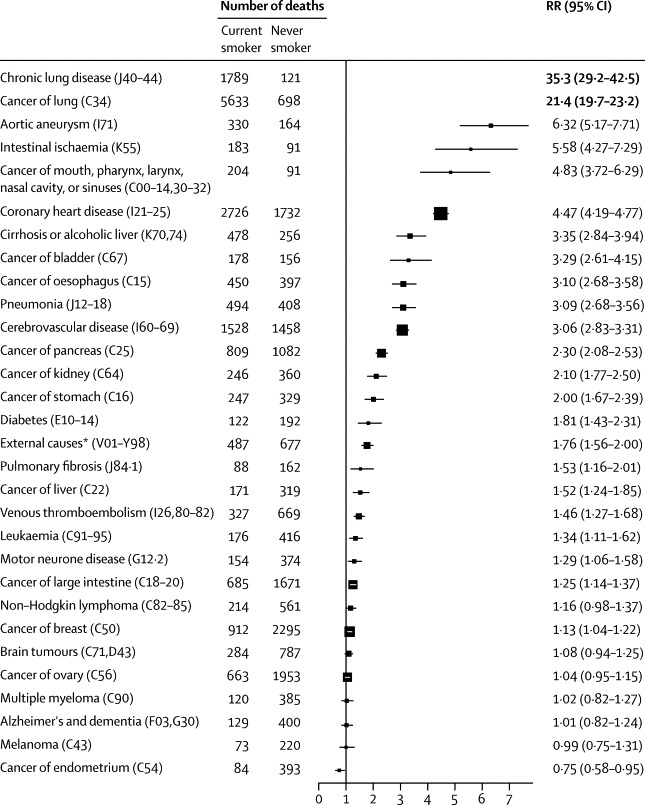
30 most common specific causes of death (ICD-10): 12-year relative risk, current versus never-smoker
RR=relative risk. ICD=International Classification of Diseases. *Suicide (ICD-10 X60–64,Y10–34): RR 1·40 (1·12–1·75); transport accident (V01–99): 0·85 (0·60–1·21); and other external: 2·51 (2·11–2·99). The area of each square is inversely proportional to the variance of the log relative risk (vs never-smokers), which also determines the CI.
To illustrate the absolute effects of our relative risk estimate, we apply it to a hypothetical population where non-smokers and smokers each have an appropriate fraction of the UK 2010 female mortality rates.16
Role of funding sources
The sponsors of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
1 311 943 women aged 50–69 years were recruited and answered questions on smoking status. Women with previous disease at baseline were excluded, of whom 58 730 reported cancer (other than non-melanoma skin cancer), 57 751 reported history of heart disease, 10 984 reported stroke, and 3826 women were currently under treatment for respiratory disease.
After exclusion of these previous disease categories, 1 180 652 women remained. They were on average born in 1943 (IQR 1938–1946), recruited in 1998 (range 1996–2001) at age 55 years (IQR 52–60), and followed for 12 years (SD 2) to Jan 1, 2011, during which time 6% (66 489) died, at mean age 65 (SD 6) years.
At baseline, 20% (232 461) of women were current smokers, 28% (328 417) were ex-smokers, and 52% (619 774) were never-smokers. The appendix (p 3) shows characteristics of the participants; the main differences were that smokers were more likely than non-smokers to live in deprived areas, drink more than 14 units of alcohol weekly, and avoid strenuous exercise. Analyses of the effects of smoking were, therefore, adjusted for these and other differences. Exposure to second-hand smoke from a partner was uncommon in never-smokers, with only 13% (44 031/341 232) of never-smokers reporting it at the 3-year resurvey. Random samples of participants were invited 9 years after baseline to give blood or for anthropometry. Comparing those who had been current smokers, ex-smokers, and never-smokers at baseline, we noted no substantial differences in lipid profile, blood pressure, or measured body-mass index (appendix pp 3–4).
The table describes smoking patterns at the first postal resurvey, at which 49% (113 521/232 461) of current smokers, 66% (217 248/328 417) of ex-smokers, and 68% (424 277/619 774) of never-smokers at baseline, replied and answered questions on smoking status. The consistency of the replies shows that there was little misclassification of never-smokers and ex-smokers, and that few ex-smokers restarted (table). But, of the current smokers at baseline who replied at the 3-year resurvey, 23% (26 474/113 521) had stopped smoking, as had 44% (37 240/85 256) at the 8-year resurvey, with cessation more common among lighter smokers (appendix p 3). Those still smoking at the 3-year resurvey reported on average having started at age 19 years (SD 5) and currently smoking 15 cigarettes per day (SD 7).
All study participants, including responders and non-responders to resurveys, were followed for mortality. During the 12-year mortality follow-up, those who had been current smokers at baseline had almost three times the overall mortality rate of never-smokers (adjusted mortality rate ratio of 2·76, 95% CI 2·71–2·81), even though within just a few years of recruitment many had stopped smoking, thereby reducing their risk. Had we inappropriately included women with previous disease at baseline, the relative risk would have been 2·62 (2·58–2·67). Those who were still smoking at the 3-year resurvey had a mortality rate ratio of 2·97 (2·88–3·07) during the remainder of the study, even though some later stopped smoking. These analyses were fully adjusted for the measures available to us of socioeconomic status, alcohol intake, and inactivity, thereby somewhat attenuating them (sensitivity analyses in appendix p 5).
The risks in smokers increased steeply with the amount smoked and happened to fall nearly on a straight line (figure 1A). Even those smoking fewer than ten cigarettes per day at baseline had double the overall mortality rate of never-smokers. Smoking was categorised as shown in the table, which suggests that when those in the lowest category were smoking, they consumed about eight cigarettes per day. At the individual level it is unclear how much past and current consumption differed, but, on average, the amount consumed per smoker seemed to have changed little during adult life (14 cigarettes per day [SD 7] at age 20 years and 14 cigarettes per day [SD 7] currently, in a subsample of 7437 women who responded to a resurvey about 12 years after study entry, and were smokers then and at age 20 years).
The age at which women had first started smoking regularly affected overall mortality decades later. Those who had started at about age 15 years were at greater risk than those who had started only 4 years later (figure 1B); this effect of age first started was more extreme for lung cancer than for overall mortality (appendix p 6).
Of the 30 most common underlying causes, or groups of causes, of death, many were neoplastic, but many were vascular or respiratory (figure 2). For 23 of these 30 causes, 12-year mortality rates were significantly higher in smokers than in never-smokers; the only cause significantly lower in smokers was, as expected,17 endometrial cancer (figure 2). The most extreme risk ratios were for chronic lung disease (risk ratio 35·3) and lung cancer (21·4), with, respectively, 1789 and 5633 deaths among current smokers. Excluding the first 3 years of follow-up did not materially alter any of these 30 risk ratios (appendix p 7).
For most of the 23 smoking-associated causes, there was a significant trend in risk among current smokers with amount smoked (appendix p 8). The four main diseases in smokers were chronic lung disease, lung cancer, heart disease, and stroke, which can all be caused by smoking.4–7 For these, figure 3 shows mortality rate ratios by amount smoked. Even women in the lowest category of amount smoked had, compared with never-smokers, substantial excess mortality from all four conditions.
Figure 3.
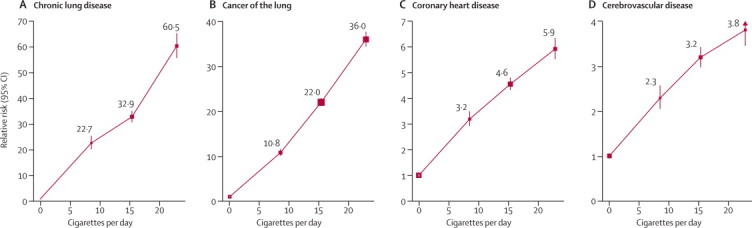
12-year relative risk, current smoker versus never-smoker, by amount smoked
(A) Chronic lung disease. (B) Cancer of the lung. (C) Coronary heart disease. (D) Cerebrovascular disease. For each category, the area of the square is inversely proportional to the variance of the category-specific log risk (which also determines the CI).
Particularly for chronic lung disease, which has a long natural history, the lowest smoking category (and the recent ex-smokers) could include some who used to smoke more but cut down their consumption (or stopped) because of early effects of the disease that would eventually cause death. To limit such biases, our analyses exclude women who reported at baseline any current treatment for respiratory disease, or any history of cancer, heart disease or stroke.
The appendix (p 9) shows the mortality ratios (current smokers versus never-smokers) for the same four main diseases at different ages. Vascular mortality rate ratios decreased steeply with age. For coronary heart disease, they were 6·66 (95% CI 5·60–7·93) at age 50–59 years, 4·79 (4·40–5·22) at age 60–69 years, and 3·30 (2·92–3·73) at somewhat older ages (ages 70+ years, where mean age of those who died was 73 years). For stroke, they were 3·90 (3·24–4·70) at age 50–59 years, 3·28 (2·94–3·65) at age 60–69 years, and 2·43 (2·11–2·79) at somewhat older ages. For overall mortality, however, the current smoker versus never-smoker mortality rate ratio varied little with age.
All results were adjusted by alcohol intake, but for causes for which alcohol is a known risk factor, we did additional sensitivity analyses among women who did not drink alcohol or consumed less than three units of alcohol per week. Although this restriction weakened some of these associations, all except that for breast cancer remained significant. The changes in the rate ratios were: from 4·83 to 3·76 (95% CI 2·65–5·31) for cancer of the mouth, pharynx, larynx, nasal cavity, or sinuses; from 3·35 to 3·37 (2·58–4·41) for cirrhosis and alcoholic liver disease; from 3·10 to 2·74 (2·27–3·30) for oesophagus cancer; from 1·76 to 1·60 (1·36–1·90) for external causes; from 1·52 to 1·43 (1·11–1·85) for liver cancer; and from 1·13 to 1·06 (0·95–1·18) for breast cancer.
Age at stopping smoking was first sought at the 3-year resurvey. At that resurvey, those who were still current smokers reported consuming on average 15·2 cigarettes per day, and during the remainder of the study had relative risks of 3·0 (95% CI 2·9–3·1) for overall mortality and 24·0 (21·3–27·1) for lung cancer mortality. Women who had stopped at ages under 25, 25–34, 35–44, and 45–54 years (ie, at around ages 20, 30, 40, or 50 years, respectively) had on average started at ages 17, 18, 19, and 19 years and smoked 9·4, 12·5, 14·6, and 15·5 cigarettes per day, so those who had stopped at ages 35–54 previously smoked from the same age and as many cigarettes per day as the continuing smokers.
We assessed the residual hazard in later middle age in women who had stopped smoking in these four age ranges; figure 4 gives ex-smoker versus never-smoker mortality ratios for overall mortality and lung cancer mortality; the appendix (p 10) gives the same for chronic lung disease, heart disease, and stroke.
Figure 4.
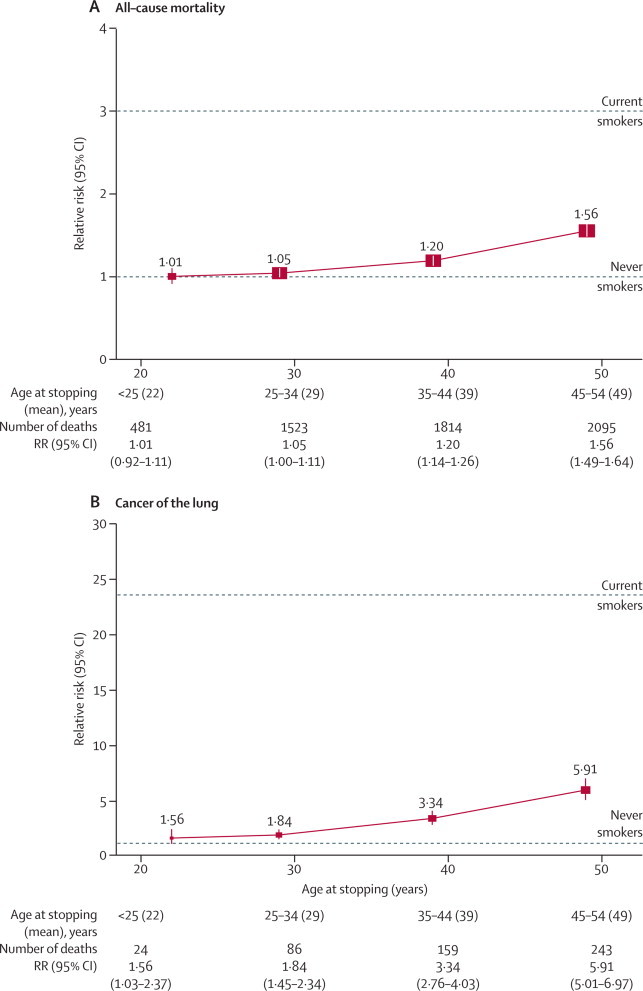
9-year relative risk of (A) all-cause mortality and (B) cancer of the lung for ex-smokers by age at stopping (as reported at the 3-year postal resurvey) versus never-smokers
The area of each square is inversely proportional to the variance of the log relative risk (vs never-smokers), which also determines the CI.
Women who had stopped at ages 45–54 (mean 49) years were, like other women, mainly in their 60s during follow-up, at which time they still had substantially higher overall and lung cancer mortality rates than never-smokers: relative risk 1·56 (95% CI 1·49–1·64) for overall mortality and 5·91 (5·01–6·97) for lung cancer mortality. Nevertheless, even the upper limit for this excess overall mortality (compared with never-smokers) was only about a third that of continuing smokers, so they avoided at least two-thirds of the excess mortality among smokers late in middle age.
Women who had stopped at ages 35–44 (mean 39) years also still had, 20 or 30 years later, higher overall mortality and lung cancer mortality than never-smokers. Their relative risk was 1·20 (95% CI 1·14–1·26) for overall mortality and 3·34 (2·76–4·03) for lung cancer mortality, both significantly increased (p<0·0001). Although these are not small residual risks, these ex-smokers did avoid about 90% of the excess lung cancer mortality and excess overall mortality among continuing smokers. The appendix shows, likewise, that for chronic lung disease, coronary heart disease, and stroke, about 90% of the excess risk was avoided by stopping at around age 40 years (and more by stopping earlier).
Women who had stopped at ages 25–34 (mean 29) years still had, decades later, measurably higher lung cancer mortality than did never-smokers: 86 deaths due to lung cancer were recorded among them as against about 46 predicted from never-smoker rates, relative risk 1·84 (1·45–2·34), p<0·0001. They also had slightly higher overall mortality, relative risk 1·05 (1·00–1·11), p=0·05. They avoided, however, about 97% of the excess lung cancer mortality in continuing smokers, and seemed also to avoid about 97% of the excess overall mortality.
Finally, few women in this generation stopped smoking before age 25 years, so direct estimates of whatever small excess risks remained in later life are not statistically reliable. Those who did so had on average been light smokers who had stopped at mean age 22 years. They had a slight excess risk of lung cancer in later life, but this was based on only 24 deaths due to lung cancer observed as against about 15 predicted, so it is not statistically trustworthy: relative risk 1·56 (1·03–2·37), p=0·04. Their relative risk for overall mortality was 1·01 (0·92–1·11). These confidence limits are uninformatively wide, given the narrower confidence limits already seen for the residual hazard in those who smoked substantial numbers and stopped smoking at ages 25–34 years.
Discussion
The excess mortality among smokers was chiefly from diseases that are known to be affected by smoking, such as lung cancer, chronic lung disease, heart disease, stroke, and various other neoplastic, respiratory, or vascular conditions.18 Moreover, results from randomly invited subsamples showed little difference between smokers and others in potential confounding factors such as adiposity, blood pressure, or lipid profile. There were differences between smokers and non-smokers in factors such as alcohol intake, physical activity, and socioeconomic status, but these were adjusted for (attenuating only slightly the hazards among smokers). Thus, although some of the associations of smoking (eg, with suicide or the fatal effects of alcohol) might be partly or wholly non-causal, the large majority of the excess overall mortality among smokers is actually caused by smoking (ie, made more probable among otherwise similar people of the same age).
The purpose of the present analyses has been to help predict the eventual causal effects of prolonged cigarette smoking on female mortality in many different countries, not just the UK. For assessment of smoker versus non-smoker relative risks of death from particular diseases, the fact that smokers were somewhat under-represented in comparison with the general UK population (as the large majority of these women had been screened for breast cancer at entry, and all had chosen to participate in the present study) was not an important limitation, nor was the exclusion of women with various previous diseases. Although the relative risks for the effects of prolonged smoking on particular diseases cannot be generalised exactly to populations with very different background rates of those diseases, they should be approximately generalisable to many (though not all) countries where women smoke.
For several of the diseases caused by smoking, and for all-cause mortality, the proportional excess risk in smokers was more marked than in many previous studies,17–19 but recently updated analyses of 21st century mortality in six smaller cohorts of US smokers now suggest, in aggregate, similar hazards from smoking and benefits of stopping,20,21 as does a recent study in Japanese men and women.22 Women who were smoking at baseline and still smoking at the 3-year resurvey had triple the overall mortality of never-smokers, despite many having subsequently reduced their risks by stopping after that resurvey. Hence, if none had stopped then the all-cause smoker versus never-smoker mortality ratio would have been somewhat greater than 3.
Although a little of this excess would have been non-causal, an overall mortality ratio somewhat greater than 3 implies that continued smoking would actually cause smokers to have roughly triple the mortality rate of otherwise similar never-smokers of the same age. Hence, among continuing smokers in this population who died, two-thirds died at the age they did because smoking caused their death. This is despite large declines in machine-measured tar yields in recent decades, with women in the UK smoking cigarettes containing less than 10 mg of tar per cigarette, on average, throughout this study.2 Low-tar cigarettes are not low-risk cigarettes, and the Million Women Study shows that more than half of those who smoke them will eventually be killed by them, unless they stop smoking in time to avoid this.
Most women were recruited at ages 50–65 years and were then followed for 9–15 years, so this study was of mortality at ages 50–80 years (but mainly 55–74 years). Within this range, the smoker versus never-smoker mortality ratio was roughly independent of age, so the conclusion that smoking triples mortality rates can be applied as a reasonable approximation not only throughout the age range 50–80 years but also at somewhat younger ages (and, perhaps, somewhat older ages).
The absolute death rates in women included in these analyses are lower than the UK average, partly because those with various previous illnesses were excluded, and also because participants were recruited through a national screening programme, and so were relatively healthy volunteers. The absolute effects at current UK death rates of a three-fold difference in age-specific mortality rates can, however, be illustrated by comparing the probabilities of survival from ages 35–80 years that would be seen in a hypothetical population in which non-smokers have exactly two-thirds of the UK 2010 age-specific female mortality rates and smokers have three times the rates of non-smokers (figure 5). (We note that if, at each age, 75% are at low risk and have two-thirds of the UK rates and the remainder have rates three times as great, the overall mortality rates would match the overall UK rates.)
Figure 5.
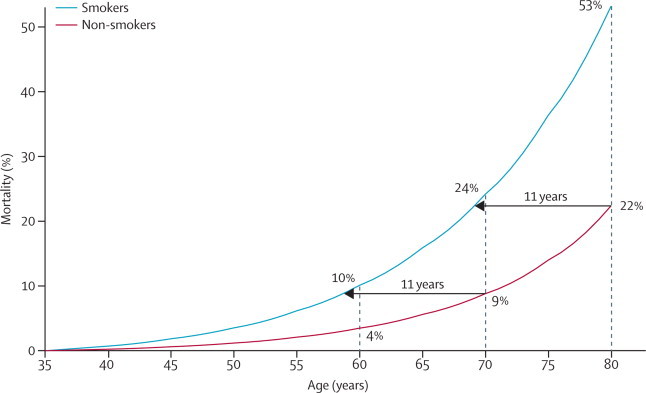
All-cause mortality: Illustration of the effects of a 3-fold difference in annual death rates on mortality from age 35 years to age 80 years
This hypothetical example takes age-specific death rates in non-smokers to be two-thirds of the UK 2010 female rates and those in smokers to be three times as great. NB In a population where at each age 75% have two-thirds of the UK rates and 25% have rates three times as great, the overall death rates in that population would match the UK rates. The horizontal arrows show that the non-smoker rates of death at ages 70 or 80 years are experienced 11 years earlier by smokers, suggesting an 11-year loss of lifespan.
In figure 5, the smokers lose about 11 years of lifespan. The probabilities of death before age 70 years are 24% for smokers and 9% for never-smokers (absolute difference 15%), and the probabilities of death before age 80 years are 53% and 22% (absolute difference 31%). These probabilities of female death before age 80 years in smokers and non-smokers are similar to the corresponding probabilities of male death before age 70 years for British doctors born in 1900–30.5 Although the relative risks seem to be more extreme for women than for men, the eventual absolute excess risks seem similar for male and female smokers.
Because the absolute hazards of prolonged smoking are substantial, so too are the absolute benefits of cessation. Even cessation at about 50 years of age avoids at least two-thirds of the continuing smoker's excess mortality in later middle age. The benefits are, however, considerably greater for those who stop much earlier (panel).
Panel. Research in context.
Systematic review
There have already been many reviews17–19 of previous studies of mortality associated with smoking in women. Although we routinely undertake online searches of such studies, we did not undertake another review of their results. For, the hazards among smokers in later middle age depend strongly not only on their recent smoking patterns, but also on their patterns of smoking in early adult life,4 so previous studies could not yet observe directly the full eventual hazards of smoking or the full benefits of cessation among women. These can first be seen only as the generation born around 1940 in countries such as the UK and USA is followed into the 21st century, as this is the first generation in which many women began to smoke substantial numbers of cigarettes in early adult life.
Interpretation
A major aim of the Million Women Study was to assess directly the full eventual hazards of prolonged smoking and the full benefits of stopping at various ages. Women who have smoked cigarettes throughout adult life have as a result three times the overall mortality rate of otherwise similar women who have never smoked, or who stopped well before middle age. Stopping before 40 years of age, and preferably well before, avoids more than 90% of this excess mortality; stopping before 30 years of age avoids more than 97% of it. Nevertheless, the residual hazards among those who smoke until age 40 years and then stop are substantial as decades later, they still have 1·2 times the mortality rate of never-smokers. Reports of 21st century mortality among women in the USA are currently in press elsewhere,20–21 corroborating our findings about the hazards of smoking and benefits of stopping among women in the UK. In both countries, if women smoke like men, they die like men—but, stopping early enough gains 10 extra years of life expectancy for women or men.
Smokers who stop at about 40 years of age avoid about 90% of the excess hazard among continuing smokers, whereas those who stop at about 30 years of age avoid about 97% of it. Hence, stopping well before age 40 years would avoid well over 90% of the excess hazard in continuing smokers, and stopping well before age 30 years would avoid well over 97% of it.
This does not, however, mean that it is safe to smoke until age 40 years and then stop, for women who do so have throughout the next few decades a mortality rate 1·2 times that of never-smokers. This is a substantial excess risk, causing one in six of the deaths among these ex-smokers. The excess risk is, however, ten times as great in women who smoke until age 40 years and do not stop, for at any given age their mortality rate is three times that of never-smokers.
In every country, the full hazards of smoking can be observed directly only when the first generation to smoke cigarettes throughout adult life has reached old age. In such populations, it is also possible to observe directly the substantial benefits of early cessation. However, in any population in the world in which young adults smoke cigarettes, the benefits of early cessation will be substantial, not necessarily in comparison with the current hazards noted among smokers in that population, but in comparison with their own eventual risks if those young smokers were to continue.23
Acknowledgments
Acknowledgments
This report is dedicated to Richard Doll, who would have turned 100 on Oct 28, 2012. He died on July 24, 2005, but discussed preliminary results from this study with some authors a few days beforehand. Our short illustrated account of his life and science is available online.24,25
The study was supported by Cancer Research UK, the British Heart Foundation, and the Medical Research Council. We thank Adrian Goodill (Cancer Epidemiology Unit, Oxford University) for help in preparing the figures. The chief acknowledgment is to the women who took part, the project staff, the screening centres, and the remarkable services routinely provided to UK medical research by the availability of automatic notification of all deaths and incident cancers (and, more recently, episodes of NHS treatment).
Million Women Study Steering Committee
Emily Banks, Valerie Beral, Judith Church, Ruth English, Jane Green, Julietta Patnick, Richard Peto, Gillian Reeves, Martin Vessey, and Matthew Wallis.
NHS Breast Screening Centres collaborating in the Million Women Study
Avon, Aylesbury, Barnsley, Basingstoke, Bedfordshire & Hertfordshire, Cambridge & Huntingdon, Chelmsford & Colchester, Chester, Cornwall, Crewe, Cumbria, Doncaster, Dorset, East Berkshire, East Cheshire, East Devon, East of Scotland, East Suffolk, East Sussex, Gateshead, Gloucestershire, Great Yarmouth, Hereford & Worcester, Kent (Canterbury, Rochester, Maidstone), Kings Lynn, Leicestershire, Liverpool, Manchester, Milton Keynes, Newcastle, North Birmingham, North East Scotland, North Lancashire, North Middlesex, North Nottingham, North of Scotland, North Tees, North Yorkshire, Nottingham, Oxford, Portsmouth, Rotherham, Sheffield, Shropshire, Somerset, South Birmingham, South East Scotland, South East Staffordshire, South Derbyshire, South Essex, South Lancashire, South West Scotland, Surrey, Warrington Halton St Helens & Knowsley, Warwickshire Solihull & Coventry, West Berkshire, West Devon, West London, West Suffolk, West Sussex, Wiltshire, Winchester, and Wirral and Wycombe.
Million Women Study Co-ordinating Centre staff
Simon Abbott, Miranda Armstrong, Angela Balkwill, Vicky Benson, Valerie Beral, Judith Black, Anna Brown, Diana Bull, Benjamin Cairns, Kathy Callaghan, Dexter Canoy, Andrew Chadwick, James Chivenga, Barbara Crossley, Francesca Crowe, Dave Ewart, Sarah Ewart, Lee Fletcher, Sarah Floud, Toral Gathani, Laura Gerrard, Adrian Goodill, Jane Green, Lynden Guiver, Sau Wan Kan, Oksana Kirichek, Carol Keene, Mary Kroll, Nicky Langston, Isobel Lingard, Pauline Lowe, Maria Jose Luque, Kath Moser, Lynn Pank, Kirstin Pirie, Gillian Reeves, Emma Sherman, Evie Sherry-Starmer, Julie Schmidt, Moya Simmonds, Helena Strange, Sian Sweetland, Alison Timadjer, Sarah Tipper, Ruth Travis, Lyndsey Trickett, Lucy Wright, and Owen Yang.
Contributors
All authors had full access to the data. All were involved in analysis of data, interpretation, and writing of the report, and had final responsibility for the decision to submit for publication.
Conflicts of interest
We declare that we have no conflicts of interest.
Supplementary Material
References
- 1.The NHS information centre for health and social care. Statistics on smoking: England. 2011. http://www.ic.nhs.uk/pubs/smoking11 (accessed March 12, 2012).
- 2.Jarvis M. Trends in sales weighted tar, nicotine, and carbon monoxide yields of UK cigarettes. Thorax. 2001;56:960–963. doi: 10.1136/thorax.56.12.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peto R, Whitlock G, Jha P. Effects of obesity and smoking on U.S. life expectancy. N Engl J Med. 2010;362:855–856. doi: 10.1056/NEJMc1000079. [DOI] [PubMed] [Google Scholar]
- 4.Doll R, Peto R. The causes of cancer: quantitative estimates of avoidable risks of cancer in the United States today. JNCI. 1981;66:1191–1308. [PubMed] [Google Scholar]
- 5.Doll R, Peto R, Boreham J, Wheatley K, Gray R, Sutherland I. Mortality in relation to smoking: 40 years’ observations on male British doctors. BMJ. 1994;309:901–911. doi: 10.1136/bmj.309.6959.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ. 2004;328:1519–1527. doi: 10.1136/bmj.38142.554479.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thun MJ, Day-Lally CA, Calle EE, Flanders WD, Heath CW. Excess mortality in cigarette smokers: changes in a 20-year interval. Am J Public Health. 1995;85:1223–1230. doi: 10.2105/ajph.85.9.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wald N, Nicolaides-Bouman A. UK Smoking Statistics. 2nd edn. Oxford University Press; Oxford: 1991. [Google Scholar]
- 9.Kenfield SA, Stampfer MJ, Rosner BA, Colditz GA. Smoking and smoking cessation in relation to mortality in women. JAMA. 2008;299:2037–2047. doi: 10.1001/jama.299.17.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kenfield SA, Wei EK, Rosner BA, Glynn RJ, Stampfer MJ, Colditz GA. Burden of smoking on cause-specific mortality: application to the Nurses’ Health Study. Tob Control. 2010;19:248–254. doi: 10.1136/tc.2009.032839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.WHO . International statistical classification of diseases and related health problems, 10th revision. World Health Organization; Geneva: 1992. [Google Scholar]
- 12.The Million Women Study Collaborative Group The Million Women Study: design and characteristics of the study population. Breast Cancer Res. 1999;1:73–80. doi: 10.1186/bcr16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Office for National Statistics General lifestyle survey. Technical appendices 2010. http://www.ons.gov.uk/ons/rel/ghs/general-lifestyle-survey/2010/index.html (accessed Sept 18, 2012).
- 14.Townsend P, Phillimore P, Beattie A. Health and deprivation: inequality and the north. Croon Helm; London: 1988. [Google Scholar]
- 15.Prospective Studies Collaboration Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373:1083–1096. doi: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.WHO Mortality statistics and UN population estimates. http://www.who.int/whosis/mort/download/en/index.html (accessed Sept 27, 2012).
- 17.International Agency for Research on Cancer . International Agency for Research on Cancer (IARC) monographs on the evaluation of carcinogenic risks to humans. Vol. 100E: a review of human carcinogens: personal habits and indoor combustions. IARC; Lyon: 2012. [PMC free article] [PubMed] [Google Scholar]
- 18.US Department of Health and Human Services . The health consequences of smoking: a report of the surgeon general. US Dept of Health and Human Services, US Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; Washington, DC: 2004. [Google Scholar]
- 19.International Agency for Research on Cancer . Monographs on the evaluation of carcinogenic risks to humans. Vol. 83: tobacco smoke and involuntary smoking. IARC; Lyon: 2004. [PMC free article] [PubMed] [Google Scholar]
- 20.Thun MJ, Carter BD, Feskanich D, et al. Recent trends in mortality risks associated with active cigarette smoking in the United States. N Engl J Med (in press).
- 21.Jha P, Ramasundarahettige C, Landsman V, Rostron B, Thun M, Peto R. Direct observation of the hazard of smoking and benefits of stopping in the US during the 21st century: nationally representative prospective cohort study. N Engl J Med (in press). [DOI] [PubMed]
- 22.Sakata R, McGale P, Grant EJ, Ozasa K, Peto R, Darby SC. The effect of smoking on mortality and life expectancy in Japan. BMJ. 2012;345:e7093. doi: 10.1136/bmj.e7093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.International Agency for Research on Cancer. The hazards of smoking and the benefits of stopping: cancer mortality and overall mortality. IARC handbooks of cancer prevention, tobacco control. Vol 11: reversal of risk after quitting smoking. Lyon, France, 2007.
- 24.Peto R, Beral V. Sir Richard Doll, 1912–2005. Biogr Mems Fell R Soc. 2010;56:63–83. [Google Scholar]
- 25.Peto R. Nature, nurture and luck: Richard Peto celebrates Richard Doll, who made sense of the causes of cancer. Oxford Today (Oxford), Michaelmas issue: 13, 2005.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


