
Mehul Patel (left) is a registrar on the North West Thames respiratory training programme. He is currently investigating skeletal muscle dysfunction in chronic obstructive pulmonary disease (COPD) at the Royal Brompton Hospital and Imperial College under the supervision of Professor Michael Polkey. Nicholas Hart is a Clinical Research Consultant in Respiratory & Critical Care Medicine within Guy's and St Thomas’ NHS Foundation Trust and King's College London National Institute of Health Research Comprehensive Biomedical Research Centre. His current research programme is focused on muscle wasting, advanced physiological monitoring and chronic respiratory failure. Michael Polkey (right) was appointed Consultant Respiratory Physician at the Royal Brompton Hospital in 2000 and received a personal chair from Imperial College in 2007. He is associate editor of the European Respiratory Journal and Clinical Science. His research spans a range of questions associated with advanced lung disease, especially COPD.
If effective, respiratory muscle training (RMT) is surely the most unfortunate therapy in medicine. Over 35 years have elapsed since the seminal work of Leith and Bradley (Leith & Bradley, 1976), yet despite being relatively cheap and free of side effects, RMT finds limited favour beyond specialist sporting activities and remains the tool of the enthusiast.
Several caveats require consideration when assessing whether RMT improves exercise tolerance. The first is whether RMT can improve performance in healthy humans and, as claimed, a range of diseases. In this context, although a 0.7% reduction in swimming time was not deemed worthwhile, a 1.5% reduction was (Kilding et al. 2010). In contrast, an improvement of over 10% may be required to be considered worthwhile clinically (Redelmeier et al. 1997). Secondly, RMT tends to improve aspects of respiratory muscle performance which are inherent to the training; i.e. inspiratory pressure generation training enhances the ability to generate inspiratory pressure, while flow-generating tasks enhance the ability to generate flow (Romer & McConnell, 2003). Similarly, in two admittedly small studies, RMT improved static inspiratory mouth pressure, but not an effort-independent measure of diaphragm strength (Hart et al. 2001; Verges et al. 2009). These observations do not preclude the possibility that the brain may be trained; indeed cortical excitability is known to be increased in respiratory disease (Hopkinson et al. 2004) and RMT may improve pulmonary and exercise performance after brain injury (Sutbeyaz et al. 2010). Nevertheless, several arguments support the case against the general application of RMT.
First, despite many trials, there is no clear consensus that RMT improves exercise performance. The quality of the studies may partly explain this. We reviewed over 130 papers for this article; only three trials included more than 50 participants, surprising given the simplicity and safety of RMT. Of these, two (Larson et al. 1999; Correa et al. 2011) showed no effect of RMT on exercise performance, while interpretation of the exercise findings in the third were not clear cut (Gething et al. 2004). Some studies reported findings of outstanding clinical significance with remarkably low participant numbers; for example, Dall’Ago and colleagues found six minute walk distance (6MWD) increased by 19% with RMT despite randomising only 32 patients with heart failure (Dall'Ago et al. 2006); in contrast only 12 of 46 studies with ACE inhibition and 3 of 15 with β blockers (proven treatments) reported improved 6MWD with active therapy mainly because of improvements following placebo (Fig. 1; Olsson et al. 2005).
Figure 1. Mean 6MWD difference after placebo in interventional trials in chronic heart failure.
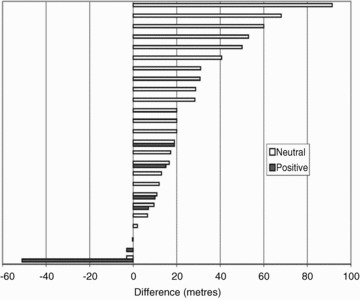
There is a smaller difference in trials reporting positive findings after active therapy. Reproduced from Olsson et al. (2005), with permission from Oxford University Press.
This leads nicely to methodological difficulties when evaluating RMT: devising an adequate placebo. Several options are available to investigators (including assessor blinding). Generally, it seems likely that a genuine intervention will have a higher placebo benefit than either ‘doing nothing’ or a sham requiring no resistance or minimal repetitions. Thus, with an appropriate placebo, perceived as active, improved exercise performance resulted and no additional benefit was conferred by RMT (Sonetti et al. 2001).
Third, for many indications recommended by enthusiasts, there are insufficient data showing that the respiratory muscles are weak, pertinent examples being heart failure and chronic obstructive pulmonary disease (COPD). In heart failure, the diaphragm when assessed non-volitionally shows only a modest degree of weakness (Hughes et al. 1999), while biopsies reveal evidence of a training effect with more type I fibres and oxidative enzymes (Tikunov et al. 1997). In COPD, the diaphragm exhibits similar changes (Levine et al. 1997; Stubbings et al. 2008); more importantly chronic and exercise-induced dynamic hyperinflation entirely explain diaphragm ‘weakness’ (Polkey et al. 1996).
Another objection to RMT in disease is that changes in respiratory muscle function and performance correlate poorly. Importantly, Orozco-Levi et al. found that despite architectural adaptation of the intercostal inspiratory muscles, there was no improvement in 6MWD following RMT (Orozco-Levi et al. 1999). In another study, RMT increased inspiratory muscle endurance in COPD patients, without improving any functional performance measure (McKeon et al. 1986). Similarly, when investigating expiratory muscle training in COPD (Orozco-Levi et al. 1999), 6MWD improved substantially even though classical airway physiology dictates that, in COPD, increasing expiratory force cannot increase expiratory flow.
Finally, RMT, at least clinically, must compete with other proven therapies such as pulmonary rehabilitation (PR), which has universally recognised benefit in COPD. Several data suggest PR confers benefit by improving peripheral muscle function rather than lung function (which would be a correlate of successful RMT). Several studies (the largest perhaps being that of Larson and co-workers (Larson et al. 1999) and meta-analyses, most recently Gosselink et al. (2011), show that RMT adds no benefit to PR, thus merely distracting from the clinical effectiveness of PR.
As already noted, gains not relevant clinically may be worthwhile to athletes. Discrepancies in the sports literature are probably explained by the subjective nature of the performance tests, diverse training regimes, baseline performance levels and the presence and attributes of control groups; in this context we have already highlighted the study by Sonetti and colleagues (Sonetti et al. 2001). This literature displays similar problems to the clinical literature. When assessing 5000 m performance in untrained males receiving cardiovascular training with either RMT or sham-RMT, there was no correlation between performance and change in maximal inspiratory pressure generated (PImax) (Edwards et al. 2008), suggesting a training effect with regard to performing the PImax test or a behavioural mechanism. It is difficult to delineate psychological from physiological factors in many other studies. Professional swimmers receiving RMT for 6 weeks improved 100 m and 200 m performance by 1.7% and 1.5%, respectively, but not 400 m performance despite an 8.9% improvement in PImax (Kilding et al. 2010). The relevance of this is unclear given that after intervention those receiving sham-RMT performed better than the training group at each distance and percentage change in PImax did not relate to change in performance. Furthermore, elite swimmers receiving 12 weeks of rigorous swim training alone or in addition to resistive RMT or sham-RMT revealed no differences between groups in either respiratory muscle strength or endurance (Mickleborough et al. 2008). The additional burdens of controlled breathing and hydrostatic pressure on the respiratory pump may mean that elite swimmers represent a unique scenario; however, this gives credence to the benefits of specific and relevant exercise training over RMT. Lastly, an interesting contextual variation concerns participants. As expected, the sporting literature involves mostly sports enthusiasts, who may not have many calls on their time beyond training. We identified two studies of RMT in healthy young adults who were not sportsmen (Sperlich et al. 2009; Lomax, 2010). In neither case was a worthwhile benefit identified; we speculate that military professionals may have more diverse calls on their time or more scepticism with regard to treatment efficacy.
Interpreting a limited number of trials without adequate sample size, sham control, double-blinding, or balanced baseline characteristics, as evidence of improved exercise capacity following RMT is hazardous. The current literature is littered with such data. A large multicentre, double-blinded randomised controlled trial of RMT against a matched placebo group undergoing appropriate sham intervention considered active by the participants is required to validate any relevant effect of RMT before advocating its use in each relevant group. This minimum requirement, expected of any pharmacological intervention, would apply to those with disease with and without inspiratory weakness and athletes. We suggest that the lack of such a trial after 35 years reflects the view of the majority of physiologists and clinicians that we should not advocate RMT either therapeutically, or for performance enhancement.
Call for comments
Readers are invited to give their views on this and the accompanying CrossTalk articles in this issue by submitting a brief comment. Comments must not exceed 250 words, with a maximum of six references from peer reviewed publications only. To submit a comment, use the online form available in the centre panel on the HighWire site. If other responses have already been submitted, a ‘view comments’ link will be visible.
All comments will be moderated and those deemed to add significantly to the discussion will be published online-only as footnotes to the articles. Comments may be posted up to 6 weeks after publication of the article, at which point the discussion will close and authors will be invited to submit a ‘final word’.
Questions about this call should be directed to Jerry Dempsey at jdempsey@wisc.edu.
To submit a comment, go to: http://jp.physoc.org/letters/submit/jphysiol;590/15/3393
Acknowledgments
M.I.P. and M.S.P.'s contribution to this article was supported by the NIHR Respiratory Disease Biomedical Research Unit at the Royal Brompton and Harefield NHS Foundation Trust and Imperial College London. M.I.P.'s salary is part funded by the NIHR Respiratory Disease Biomedical Research Unit at the Royal Brompton and Harefield NHS Foundation Trust and Imperial College London. N.H. acknowledges financial support from the Department of Health via the National Institute for Health Research (NIHR) comprehensive Biomedical Research Centre award to Guy's & St Thomas’ NHS Foundation Trust in partnership with King's College London and King's College Hospital NHS Foundation Trust.
Glossary
- COPD
chronic obstructive pulmonary disease
- 6MWD
six minute walk distance
- PImax
maximal inspiratory pressure
- PR
pulmonary rehabilitation
- RMT
respiratory muscle training
References
- Correa AP, Ribeiro JP, Balzan FM, Mundstock L, Ferlin EL, Moraes RS. Inspiratory muscle training in type 2 diabetes with inspiratory muscle weakness. Med Sci Sports Exerc. 2011;43:1135–1141. doi: 10.1249/MSS.0b013e31820a7c12. [DOI] [PubMed] [Google Scholar]
- Dall'Ago P, Chiappa GRS, Guths H, Stein R, Ribeiro JP. Inspiratory muscle training in patients with heart failure and inspiratory muscle weakness: A randomized trial. J Am Coll Cardiol. 2006;47:757–763. doi: 10.1016/j.jacc.2005.09.052. [DOI] [PubMed] [Google Scholar]
- Edwards AM, Wells C, Butterly R. Concurrent inspiratory muscle and cardiovascular training differentially improves both perceptions of effort and 5000 m running performance compared with cardiovascular training alone. Br J Sports Med. 2008;42:823–827. doi: 10.1136/bjsm.2007.045377. [DOI] [PubMed] [Google Scholar]
- Gething AD, Passfield L, Davies B. The effects of different inspiratory muscle training intensities on exercising heart rate and perceived exertion. Eur J Appl Physiol. 2004;92:50–55. doi: 10.1007/s00421-004-1044-2. [DOI] [PubMed] [Google Scholar]
- Gosselink R, De Vos J, van den Heuvel SP, Segers J, Decramer M, Kwakkel G. Impact of inspiratory muscle training in patients with COPD: what is the evidence? Eur Respir J. 2011;37:416–425. doi: 10.1183/09031936.00031810. [DOI] [PubMed] [Google Scholar]
- Hart N, Sylvester K, Ward S, Cramer D, Moxham J, Polkey MI. Evaluation of an inspiratory muscle trainer in healthy humans. Respir Med. 2001;95:526–531. doi: 10.1053/rmed.2001.1069. [DOI] [PubMed] [Google Scholar]
- Hopkinson NS, Sharshar T, Ross ET, Nickol AH, Dayer MJ, Porcher R, Jonville S, Moxham J, Polkey MI. Corticospinal control of respiratory muscles in chronic obstructive pulmonary disease. Respir Physiol Neurobiol. 2004;141:1–12. doi: 10.1016/j.resp.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Hughes PD, Polkey MI, Harrus ML, Coats AJ, Moxham J, Green M. Diaphragm strength in chronic heart failure. Am J Respir Crit Care Med. 1999;160:529–534. doi: 10.1164/ajrccm.160.2.9810081. [DOI] [PubMed] [Google Scholar]
- Kilding AE, Brown S, McConnell AK. Inspiratory muscle training improves 100 and 200 m swimming performance. Eur J Appl Physiol. 2010;108:505–511. doi: 10.1007/s00421-009-1228-x. [DOI] [PubMed] [Google Scholar]
- Larson JL, Covey MK, Wirtz SE, Berry JK, Alex CG, Langbein WE, Edwards L. Cycle ergometer and inspiratory muscle training in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160:500–507. doi: 10.1164/ajrccm.160.2.9804067. [DOI] [PubMed] [Google Scholar]
- Leith DE, Bradley M. Ventilatory muscle strength and endurance training. J Appl Physiol. 1976;41:508–516. doi: 10.1152/jappl.1976.41.4.508. [DOI] [PubMed] [Google Scholar]
- Levine S, Kaiser L, Leferovich J, Tikunov B. Cellular adaptations in the diaphragm in chronic obstructive pulmonary disease. N Engl J Med. 1997;337:1799–1806. doi: 10.1056/NEJM199712183372503. [DOI] [PubMed] [Google Scholar]
- Lomax M. Inspiratory muscle training, altitude, and arterial oxygen desaturation: a preliminary investigation. Aviat Space Environ Med. 2010;81:498–501. doi: 10.3357/asem.2718.2010. [DOI] [PubMed] [Google Scholar]
- McKeon JL, Turner J, Kelly C, Dent A, Zimmerman PV. The effect of inspiratory resistive training on exercise capacity in optimally treated patients with severe chronic airflow limitation. Aust N Z J Med. 1986;16:648–652. doi: 10.1111/j.1445-5994.1986.tb00005.x. [DOI] [PubMed] [Google Scholar]
- Mickleborough T, Stager J, Chatham K, Lindley M, Ionescu A. Pulmonary adaptations to swim and inspiratory muscle training. Eur J Appl Physiol. 2008;103:635–646. doi: 10.1007/s00421-008-0759-x. [DOI] [PubMed] [Google Scholar]
- Olsson LG, Swedberg K, Clark AL, Witte KK, Cleland JG. Six minute corridor walk test as an outcome measure for the assessment of treatment in randomized, blinded intervention trials of chronic heart failure: a systematic review. Eur Heart J. 2005;26:778–793. doi: 10.1093/eurheartj/ehi162. [DOI] [PubMed] [Google Scholar]
- Orozco-Levi M, Gea J, Lloreta JL, Felez M, Minguella J, Serrano S, Broquetas JM. Subcellular adaptation of the human diaphragm in chronic obstructive pulmonary disease. Eur Resp J. 1999;13:371–378. doi: 10.1183/09031936.99.13237199. [DOI] [PubMed] [Google Scholar]
- Polkey MI, Kyroussis D, Hamnegard CH, Mills GH, Green M, Moxham J. Diaphragm strength in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1996;154:1310–1317. doi: 10.1164/ajrccm.154.5.8912741. [DOI] [PubMed] [Google Scholar]
- Redelmeier DA, Bayoumi AM, Goldstein RS, Guyatt GH. Interpreting small differences in functional status: the Six Minute Walk test in chronic lung disease patients. Am J Respir Crit Care Med. 1997;155:1278–1282. doi: 10.1164/ajrccm.155.4.9105067. [DOI] [PubMed] [Google Scholar]
- Romer LM, McConnell AK. Specificity and reversibility of inspiratory muscle training. Med Sci Sports Exerc. 2003;35:237–244. doi: 10.1249/01.MSS.0000048642.58419.1E. [DOI] [PubMed] [Google Scholar]
- Sonetti DA, Wetter TJ, Pegelow DF, Dempsey JA. Effects of respiratory muscle training versus placebo on endurance exercise performance. Respir Physiol. 2001;127:185–199. doi: 10.1016/s0034-5687(01)00250-x. [DOI] [PubMed] [Google Scholar]
- Sperlich B, Fricke H, de Marees M, Linville JW, Mester J. Does respiratory muscle training increase physical performance? Mil Med. 2009;174:977–982. doi: 10.7205/milmed-d-04-6408. [DOI] [PubMed] [Google Scholar]
- Stubbings AK, Moore AJ, Dusmet M, Goldstraw P, West TG, Polkey MI, Ferenczi MA. Physiological properties of human diaphragm muscle fibres and the effect of chronic obstructive pulmonary disease. J Physiol. 2008;586:2637–2650. doi: 10.1113/jphysiol.2007.149799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutbeyaz ST, Koseoglu F, Inan L, Coskun O. Respiratory muscle training improves cardiopulmonary function and exercise tolerance in subjects with subacute stroke: a randomized controlled trial. Clin Rehabil. 2010;24:240–250. doi: 10.1177/0269215509358932. [DOI] [PubMed] [Google Scholar]
- Tikunov B, Levine S, Mancini D. Chronic congestive heart failure elicits adaptations of endurance exercise in diaphragmatic muscle. Circulation. 1997;95:910–916. doi: 10.1161/01.cir.95.4.910. [DOI] [PubMed] [Google Scholar]
- Verges S, Renggli AS, Notter DA, Spengler CM. Effects of different respiratory muscle training regimes on fatigue-related variables during volitional hyperpnoea. Respir Physiol Neurobiol. 2009;169:282–290. doi: 10.1016/j.resp.2009.09.005. [DOI] [PubMed] [Google Scholar]


