Abstract
Recent studies have suggested the presence of cardiac atrophy as a key component of the pathogenesis of the postural orthostatic tachycardia syndrome (POTS), similar to physical deconditioning. It has also been shown that exercise intolerance is associated with a reduced stroke volume (SV) in POTS, and that the high heart rate (HR) observed at rest and during exercise in these patients is due to this low SV. We tested the hypotheses that (a) circulatory control during exercise is normal in POTS; and (b) that physical ‘reconditioning’ with exercise training improves exercise performance in patients with POTS. Nineteen (18 women) POTS patients completed a 3 month training programme. Cardiovascular responses during maximal exercise testing were assessed in the upright position before and after training. Resting left ventricular diastolic function was evaluated by Doppler echocardiography. Results were compared with those of 10 well-matched healthy sedentary controls. A lower SV resulted in a higher HR in POTS at any given oxygen uptake (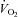 ) during exercise while the cardiac output (
) during exercise while the cardiac output (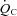 )–
)–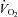 relationship was normal.
relationship was normal. 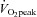 was lower in POTS than controls (26.1 ± 1.0 (SEM) vs. 36.3 ± 0.9 ml kg−1 min−1; P < 0.001) due to a lower peak SV (65 ± 3 vs. 80 ± 5 ml; P = 0.009). After training in POTS, HR became lower at any given
was lower in POTS than controls (26.1 ± 1.0 (SEM) vs. 36.3 ± 0.9 ml kg−1 min−1; P < 0.001) due to a lower peak SV (65 ± 3 vs. 80 ± 5 ml; P = 0.009). After training in POTS, HR became lower at any given 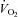 due to increased SV without changes in the
due to increased SV without changes in the 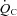 –
–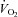 relationship.
relationship. 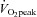 increased by 11% (P < 0.001) due to increased peak SV (P = 0.021) and was proportional to total blood volume. Peak HR was similar, but HR recovery from exercise was faster after training than before training (P = 0.036 for training and 0.009 for interaction). Resting diastolic function was mostly normal in POTS before training, though diastolic suction was impaired (P = 0.023). There were no changes in any Doppler index after training. These results suggest that short-term exercise training improves physical fitness and cardiovascular responses during exercise in patients with POTS.
increased by 11% (P < 0.001) due to increased peak SV (P = 0.021) and was proportional to total blood volume. Peak HR was similar, but HR recovery from exercise was faster after training than before training (P = 0.036 for training and 0.009 for interaction). Resting diastolic function was mostly normal in POTS before training, though diastolic suction was impaired (P = 0.023). There were no changes in any Doppler index after training. These results suggest that short-term exercise training improves physical fitness and cardiovascular responses during exercise in patients with POTS.
Key points
Recent studies have suggested the presence of cardiac atrophy as a key component of the pathogenesis of the postural orthostatic tachycardia syndrome (POTS), similar to physical deconditioning; exercise intolerance is associated with a reduced stroke volume (SV) in POTS which may be the cause of the high heart rate (HR) at rest and during exercise.
We determined whether physical ‘reconditioning’ with exercise training improves exercise performance in POTS.
A lower SV resulted in a higher HR in POTS at any given oxygen uptake (
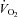 ) during exercise while the cardiac output (
) during exercise while the cardiac output (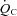 )–
)–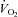 relationship remained normal.
relationship remained normal. 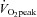 was lower in POTS than healthy sedentary controls.
was lower in POTS than healthy sedentary controls.After 3 months of training in POTS, HR became lower at any given
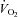 due to increased SV without changes in the
due to increased SV without changes in the 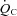 –
–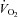 relationship.
relationship. 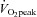 increased due to increased peak SV, and was proportional to total blood volume. HR recovery from exercise was faster after training than before training.
increased due to increased peak SV, and was proportional to total blood volume. HR recovery from exercise was faster after training than before training.Thus, exercise training improves physical fitness and cardiovascular responses during exercise in POTS.
Introduction
The postural orthostatic tachycardia syndrome (POTS) is characterized by an excessive increase in heart rate (HR) during orthostasis, and the inability to stand or remain upright for prolonged periods of time due to intolerable light-headedness, weakness, and near-syncope (Low et al. 1995). This disorder affects over 500,000 Americans (Robertson, 1999), the vast majority of whom are premenopausal women. Although several mechanisms for this excessive orthostatic tachycardia have been postulated (Low et al. 2009), recent findings, including those from our laboratory suggest the presence of cardiac atrophy along with reduced blood volume as key components of the pathogenesis of this syndrome (Raj et al. 2005; Joyner & Masuki, 2008; Fu et al. 2010a), while autonomic regulation, including baroreflex regulation of HR is essentially intact (Fu et al. 2010a; Galbreath et al. 2011). Most of these patients also report high HR during exercise, as well as during quiet standing (Raj et al. 2005; Masuki et al. 2007; Raj & Robertson, 2007; Fu et al. 2010a,b, 2011).
Cardiac atrophy and reduced blood volume, resulting in a low stroke volume (SV) during exercise are clinically relevant physiological changes also observed after long-term bed rest (Saltin et al. 1968; Levine et al. 1997; Dorfman et al. 2008; Shibata et al. 2010). These adaptive responses are directly related to the critical symptoms observed commonly after bed rest as well as in patients with POTS, namely orthostatic intolerance and low physical work performance. A low SV during exercise is responsible for the reduced peak oxygen uptake (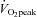 ) after bed rest (Saltin et al. 1968; Shibata et al. 2010). Besides heart size and blood volume, cardiac diastolic relaxation also plays a role in maintaining left ventricular (LV) end diastolic volume and subsequently SV in the upright position and during exercise in humans. Whether cardiac atrophy in POTS would lead to impaired diastolic function, and therefore, low SV during exercise is unclear.
) after bed rest (Saltin et al. 1968; Shibata et al. 2010). Besides heart size and blood volume, cardiac diastolic relaxation also plays a role in maintaining left ventricular (LV) end diastolic volume and subsequently SV in the upright position and during exercise in humans. Whether cardiac atrophy in POTS would lead to impaired diastolic function, and therefore, low SV during exercise is unclear.
Results regarding cardiovascular response to exercise in POTS are limited. There is only one study published showing reduced SV during graded cycling exercise in POTS patients (Masuki et al. 2007). Whether similar observations can be made during upright treadmill exercise is unknown. Physical exercise training has been shown to increase blood volume, cardiac size and mass in different patient populations (Saltin et al. 1968; Dorfman et al. 2007; Fu et al. 2010a). Whether exercise training would be beneficial in improving exercise performance and diastolic function in POTS is unclear. Thus, the purposes of this study were: (a) to evaluate cardiac diastolic function at rest and cardiovascular responses during exercise; and (b) to assess the effects of short-term (i.e. 3 months) exercise training on diastolic function and exercise performance in POTS.
Methods
Participants
Details of the recruitment process were reported previously (Fu et al. 2010a, 2011); patients were consecutive patients referred to our tertiary Autonomic Function Clinic for the diagnosis and management of POTS. At the time of the study, all patients met the inclusion criteria for POTS, and had a HR rise ≥30 beats min−1 or a rate that exceeded 120 beats min−1 that occurred after 10 min of standing without any evidence of orthostatic hypotension (Raj et al. 2005). Patients had POTS-related symptoms for 6 months to 5 years prior to participation in the study. Twenty-five patients (24 women, 1 man) were enrolled in an ‘optimized’ exercise training programme for 3 months with 19 (18 women, 1 man) completing the programme (Fu et al. 2010a). There were no differences in resting cardiac diastolic function parameters between those who finished the protocol versus those who dropped out except that septal mitral annular early diastolic velocity was slightly but significantly greater in the drop-out group. All patients were non-smokers. Although the exercise history in these patients was variable, and many of them reported a previously active life-style, all reported at least a brief period of change in physical activity or bedrest (Gaffney et al. 1985). All were screened with a careful medical history, physical examination, 12-lead electrocardiogram (ECG), and a 10 min stand test. Patients had stopped taking medications that could affect the autonomic nervous system ≥2 weeks before screening and ≥4 weeks before testing. Ten age-matched healthy sedentary individuals (9 women, 1 man) served as controls. All participants were informed of the purpose and procedures used in the study and gave their written informed consent to a protocol approved by the Institutional Review Boards of the University of Texas Southwestern Medical Center and Texas Health Presbyterian Hospital Dallas. This study followed guidelines set forth in the Declaration of Helsinki.
Protocol
All of the subjects were on an isocaloric constant diet consisting of 200 mEq of sodium, 100 mEq of potassium, and 1000 mg of calcium 3 days prior to testing. Foods were prepared by the Clinical and Translational Research Center at the University of Texas Southwestern Medical Center. All underwent an extensive set of autonomic and renal-adrenal measurements (previously published) (Fu et al. 2010a,b, 2011; Galbreath et al. 2011) before diastolic function assessment and maximal exercise testing. Female subjects were studied in the mid-luteal phase of the menstrual cycle (day 19 to 22 after the onset of menstruation, high oestrogen and progesterone). They took a pregnancy test and showed negative results on each study day.
Doppler echocardiography
Echocardiographic images (iE33, Philips Ultrasound) were obtained during supine rest and measurements were made during quiet guided expiration. A colour M-mode image of LV inflow was obtained with the sampling area positioned to extend from mid-atrium to the apex, directly through the mitral valve orifice. The resulting mitral inflow spatiotemporal velocity profile pattern was used to derive the early propagation velocity of mitral inflow (Rivas-Gotz et al. 2003; Prasad et al. 2007). Measurements of septal and lateral mitral annular early diastolic (E′) velocities were obtained via standard tissue Doppler imaging techniques from the apical four-chamber view with a 4.0 mm sample volume (Sohn et al. 1997; Prasad et al. 2007). Mitral inflow velocities were assessed using pulsed wave Doppler with a sample volume of 2.0 mm positioned over the mitral valve leaflet tips. From these measurements were obtained of the peak inflow velocity during the early phase of LV relaxation (E) (Labovitz & Pearson, 1987; Hatle, 1993; Cohen et al. 1996) and during left atrial contraction (A) (Cohen et al. 1996). These values were subsequently used to calculate the E/A ratio (Labovitz & Pearson, 1987; Hatle, 1993; Prasad et al. 2007). Isovolumetric relaxation time (IVRT) was considered as the time interval between the end of aortic outflow during systole and the opening of the mitral valve during diastole (Prasad et al. 2007). IVRT was determined using a five-chamber apical view with the sample volume set at 4.0 mm.
Maximal exercise testing
Cardiac output (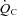 ) was measured with a modified acetylene rebreathing method (Jarvis et al. 2007). SV was calculated with coincident HR measured with 12-lead ECG. Blood pressure (BP) was measured by electrosphygmomanometry (SunTech, Raleigh, NC, USA) with a microphone placed over the brachial artery to detect Korotkoff sounds. Mean arterial pressure (MAP) was calculated as [(systolic BP – diastolic BP)/3 + diastolic BP]. Total peripheral resistance (TPR) was calculated as the quotient of MAP and
) was measured with a modified acetylene rebreathing method (Jarvis et al. 2007). SV was calculated with coincident HR measured with 12-lead ECG. Blood pressure (BP) was measured by electrosphygmomanometry (SunTech, Raleigh, NC, USA) with a microphone placed over the brachial artery to detect Korotkoff sounds. Mean arterial pressure (MAP) was calculated as [(systolic BP – diastolic BP)/3 + diastolic BP]. Total peripheral resistance (TPR) was calculated as the quotient of MAP and 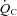 , multiplied by 80 (expressed as dyn s cm−5).
, multiplied by 80 (expressed as dyn s cm−5). 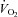 was measured with the Douglas bag technique with gas fractions analysed by mass spectrometry (Marquette MGA1100). Ventilatory volume was measured using a Tissot spirometer.
was measured with the Douglas bag technique with gas fractions analysed by mass spectrometry (Marquette MGA1100). Ventilatory volume was measured using a Tissot spirometer.
Quiet standing 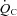 , HR, BP and
, HR, BP and 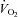 were collected. After that, two submaximal steady-state workloads were determined based on individual fitness level so that ∼30% and 60% of
were collected. After that, two submaximal steady-state workloads were determined based on individual fitness level so that ∼30% and 60% of 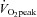 could be achieved at each level. Each work load lasted 5 min, and steady-state
could be achieved at each level. Each work load lasted 5 min, and steady-state 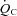 , HR, BP and
, HR, BP and 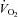 were collected during the last 1 min. Following a brief break, a maximal exercise test was performed by using a modified Astrand-Saltin incremental treadmill protocol (Balke et al. 1965). Subjects walked or jogged at a constant speed, which was determined based on individual fitness level and submaximal steady-state data to achieve a peak work rate at 10–12 min; the grade was subsequently increased by 2% every 2 min until exhaustion. Douglas bags were collected in the second minute of each stage, with consecutive 45 s collections when the subject was nearing maximal effort.
were collected during the last 1 min. Following a brief break, a maximal exercise test was performed by using a modified Astrand-Saltin incremental treadmill protocol (Balke et al. 1965). Subjects walked or jogged at a constant speed, which was determined based on individual fitness level and submaximal steady-state data to achieve a peak work rate at 10–12 min; the grade was subsequently increased by 2% every 2 min until exhaustion. Douglas bags were collected in the second minute of each stage, with consecutive 45 s collections when the subject was nearing maximal effort. 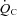 , BP and HR were measured during the final 20 s of maximal exercise. HR recovery from exercise was calculated from peak exercise to minute 2 of recovery (Cole et al. 1999; Cole et al. 2000; Nishime et al. 2000; Rosenwinkel et al. 2001) in the sitting position.
, BP and HR were measured during the final 20 s of maximal exercise. HR recovery from exercise was calculated from peak exercise to minute 2 of recovery (Cole et al. 1999; Cole et al. 2000; Nishime et al. 2000; Rosenwinkel et al. 2001) in the sitting position.
Maximal exercise was defined as the inability to continue exercise despite vigorous encouragement. 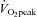 was defined as the highest
was defined as the highest 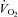 measured from at least a 40 s Douglas bag.
measured from at least a 40 s Douglas bag. 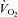 was divided by
was divided by 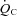 to calculate arteriovenous oxygen content difference (a-v
to calculate arteriovenous oxygen content difference (a-v 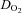 ) according to the Fick equation.
) according to the Fick equation.
Exercise training
Details of the exercise intervention have been previously reported (Fu et al. 2010a). The majority of the training sessions were prescribed as ‘base training’ with target HR equivalent to ∼75% of maximal. Initially, patients trained 2–4 times per week for 30–45 min per session by using a recumbent bike, rowing, or swimming. As the patients became relatively fit, the duration of the base training sessions was prolonged, and subsequently sessions of increased intensity (i.e. maximal steady-state) were added. Upright exercise was added gradually as tolerated, though usually not until the second or third month. By the end of the 3 month training, patients were exercising 5–6 h per week. HR was monitored during every session using a Polar monitor in all the patients and their physical activity level was quantified every 2 weeks. In addition to the en-durance training, resistance training using weight lifting was also undertaken from once a week, 15–20 min per session to twice a week, 30–40 min per session. Additionally, patients were encouraged to increase their dietary salt and water intake, and elevate the head of the bed during sleeping at night.
Doppler echocardiography and the maximal exercise testing were repeated after 3 months of exercise training in all POTS patients. Patients were studied at the same time of the day during the mid-luteal phase of the menstrual cycle. Blood volume was measured by a modified carbon monoxide rebreathing technique (Gore et al. 2006) in POTS patients before and after training and in healthy controls, and these results were previously reported (Fu et al. 2010a).
Statistical analysis
Data are presented as means ± SEM. Statistical probability was assessed with Student's paired t test to compare the differences before and after exercise training in POTS patients, and with an unpaired t-test to compare the differences between patients and controls. HR recovery from exercise in POTS patients before and after training was compared using two-way repeated measures analysis of variance. The Holm–Sidak method was used post hoc for multiple comparisons. The relationship between blood/plasma volume and 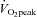 in POTS patients before and after training was determined by least-squares linear regression. All of the statistical analyses were performed with a personal computer based analysis program (SigmaStat; Systat Software Inc., San Jose, CA, USA). A P value of <0.05 was considered statistically significant.
in POTS patients before and after training was determined by least-squares linear regression. All of the statistical analyses were performed with a personal computer based analysis program (SigmaStat; Systat Software Inc., San Jose, CA, USA). A P value of <0.05 was considered statistically significant.
Results
Subject characteristics
There were no significant differences in age, height, weight, body mass index, body surface area, and lean body mass between POTS patients and healthy sedentary controls, while percentage body fat was higher in patients than in controls (Table 1). Anthropometric characteristics did not change in POTS patients after 3 months of exercise training, but physical activity level significantly increased in all the patients by design.
Table 1.
Subject characteristics
| POTS | |||
|---|---|---|---|
| Variables | Pre-training | Post-training | Controls |
| Age (years) | 27 ± 2 | 27 ± 2 | 30 ± 2 |
| Height (cm) | 167 ± 1 | 167 ± 1 | 171 ± 3 |
| Weight (kg) | 69 ± 4 | 68 ± 4 | 65 ± 5 |
| Body mass index (kg m−2) | 25 ± 1 | 24 ± 1 | 22 ± 1 |
| Body fat (%) | 34 ± 2† | 32 ± 2† | 24 ± 3 |
| Body surface area (m2) | 1.78 ± 0.06 | 1.77 ± 0.05 | 1.75 ± 0.08 |
| Lean body mass (kg) | 45 ± 2 | 45 ± 2 | 50 ± 4 |
| Duration of illness (years) | 2.4 ± 0.4 | ||
Values are means ± SEM.
P < 0.05 compared with controls.
Cardiovascular responses during exercise
In the resting standing condition, a lower SV led to a lower 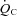 in POTS patients compared with healthy controls, although HR was higher in POTS patients (Table 2). Peak
in POTS patients compared with healthy controls, although HR was higher in POTS patients (Table 2). Peak 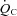 was lower in POTS patients due to a lower peak SV with a similar peak HR compared with healthy controls during peak exercise while peak a-v
was lower in POTS patients due to a lower peak SV with a similar peak HR compared with healthy controls during peak exercise while peak a-v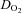 was comparable between groups, resulting in a lower
was comparable between groups, resulting in a lower 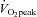 in POTS patients (Table 2).
in POTS patients (Table 2).
Table 2.
Cardiovascular response during exercise
| Variables | POTS | Controls | |
|---|---|---|---|
| Pre-training | Post-training | ||
| Standing resting | |||
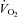 (l min−1) (l min−1) |
0.24 ± 0.01 | 0.24 ± 0.01 | 0.26 ± 0.02 |
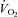 (ml kg−1 min−1) (ml kg−1 min−1) |
3.5 ± 0.1† | 3.5 ± 0.1† | 3.9 ± 0.1 |
a-v 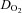 (ml dl−1) (ml dl−1) |
7.2 ± 0.3†† | 7.0 ± 0.3†† | 5.6 ± 0.2 |
| HR (beats min−1) | 116 ± 4†† | 107 ± 4*†† | 91 ± 4 |
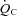 (l min−1) (l min−1) |
3.38 ± 0.14†† | 3.51 ± 0.17†† | 4.70 ± 0.33 |
| SV (ml) | 29 ± 1†† | 33 ± 2*†† | 52 ± 5 |
| Peak exercise | |||
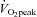 (l min−1) (l min−1) |
1.78 ± 0.08†† | 1.93 ± 0.08*†† | 2.40 ± 0.22 |
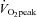 (ml kg−1 min−1) (ml kg−1 min−1) |
26.1 ± 1.0†† | 28.9 ± 1.1**†† | 36.3 ± 0.9 |
Peak a-v 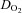 (ml dl−1) (ml dl−1) |
14.2 ± 0.5 | 14.4 ± 0.3 | 15.2 ± 0.7 |
| Peak HR (beats min–1) | 195 ± 3 | 194 ± 3 | 196 ± 4 |
Peak 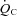 (l min−1) (l min−1) |
12.62 ± 0.58† | 13.57 ± 0.64**† | 15.79 ± 1.02 |
| Peak SV (ml) | 65 ± 3†† | 70 ± 3*†† | 80 ± 5 |
| Peak lactate (mmol) | 7.91 ± 0.45 | 7.87 ± 0.33 | 9.55 ± 1.36 |
Values are means ± SEM. *P < 0.05 and **P < 0.01 compared with pre-training in POTS. †P < 0.05 and ††P < 0.01 compared with controls.
Plots of 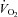 versus SV showed that at any given
versus SV showed that at any given 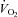 , SV was lower in patients (downward shift) compared with controls (Fig. 1A), while plots of
, SV was lower in patients (downward shift) compared with controls (Fig. 1A), while plots of 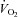 versus HR showed that at any given
versus HR showed that at any given 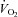 , HR was higher in POTS patients (upward shift) (Fig. 1B). After 3 months of exercise training, an upward shift in the relationship of
, HR was higher in POTS patients (upward shift) (Fig. 1B). After 3 months of exercise training, an upward shift in the relationship of 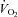 versus SV and a downward shift in plots of
versus SV and a downward shift in plots of 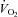 versus HR were observed in POTS patients; however, these relationships were not completely normalized (Fig. 1A and B). Plots of
versus HR were observed in POTS patients; however, these relationships were not completely normalized (Fig. 1A and B). Plots of 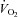 versus
versus
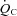 , as well as of
, as well as of 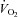 versus a-v
versus a-v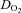 from patients were not different from those of controls; these relationships did not change in POTS patients after 3 months of exercise training (Fig. 1C and D). Training did not alter MAP and TPR responses to exercise in POTS patients (Fig. 1E and F).
from patients were not different from those of controls; these relationships did not change in POTS patients after 3 months of exercise training (Fig. 1C and D). Training did not alter MAP and TPR responses to exercise in POTS patients (Fig. 1E and F). 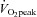 was correlated directly with blood/plasma volume in healthy controls and POTS patients (Fig. 2). This relationship was intact in POTS. Both
was correlated directly with blood/plasma volume in healthy controls and POTS patients (Fig. 2). This relationship was intact in POTS. Both 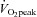 and blood volume increased after training, but the relationship remained unchanged.
and blood volume increased after training, but the relationship remained unchanged.
Figure 1. Changes in stroke volume (SV, A), heart rate (HR, B), cardiac output (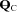 , C), arterio-venous oxygen content difference (a-v
, C), arterio-venous oxygen content difference (a-v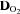 , D), mean arterial pressure (MAP, E), and total peripheral resistance (TPR, F) in relation to changes in oxygen uptake (
, D), mean arterial pressure (MAP, E), and total peripheral resistance (TPR, F) in relation to changes in oxygen uptake (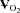 ) during upright treadmill exercise before and after 3 months of exercise training in patients with POTS (n = 19) and healthy sedentary controls (n = 7).
) during upright treadmill exercise before and after 3 months of exercise training in patients with POTS (n = 19) and healthy sedentary controls (n = 7).
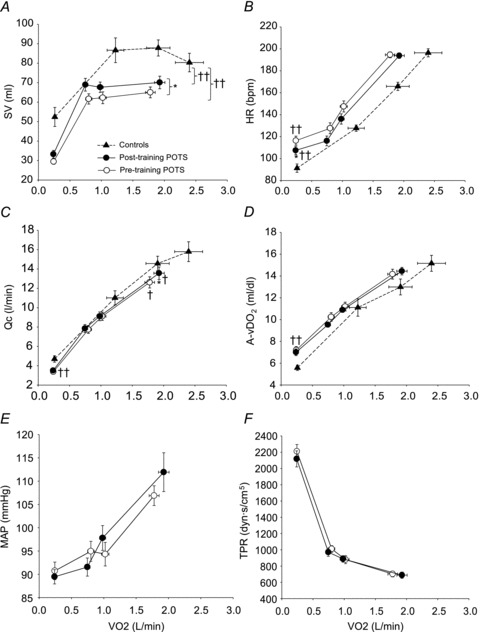
Values are means ± SEM. *P < 0.05, pre-training compared with post-training in POTS. ††P < 0.01, patients compared with controls.
Figure 2.
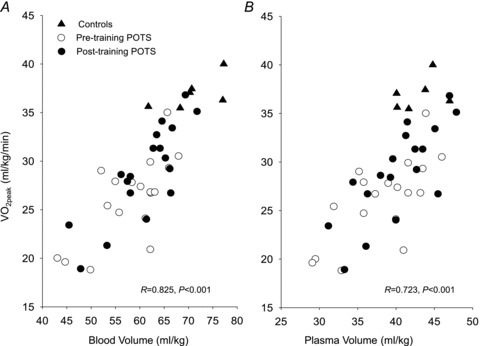
Peak oxygen uptake (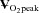 ) as a function of blood volume (A) and plasma volume (B) in healthy controls and patients with POTS before and after 3 months of exercise training
) as a function of blood volume (A) and plasma volume (B) in healthy controls and patients with POTS before and after 3 months of exercise training
HR recovery from maximal exercise testing was significantly faster after training compared with before training in POTS patients (Fig. 3).
Figure 3. Heart rate (HR) recovery from exercise in POTS patients before and after exercise training. Peak EX, maximal exercise.
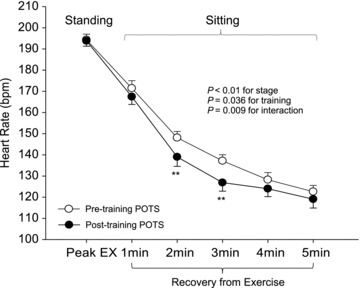
Values are means ± SEM. **P < 0.01 compared with pre-training in POTS.
Left ventricular diastolic function from Doppler echocardiography
Table 3 depicts cardiac diastolic function at rest in healthy sedentary controls and POTS patients before and after exercise training. There was no difference in the E/A ratio, IVRT, and tissue Doppler imaging of early diastole between patients and controls, while propagation velocity of early mitral inflow was lower in patients than in controls (P = 0.023). There were no changes in any Doppler index after training in POTS patients. Consequently, propagation velocity of early mitral inflow remained significantly lower in POTS patients even after training compared with healthy sedentary controls (P = 0.017).
Table 3.
Left ventricular diastolic function from Doppler echocardiography
| Variables | POTS | Controls | |
|---|---|---|---|
| Pre-training | Post-training | ||
| Mitral inflow | |||
| Emax (cm s−1) | 84.4 ± 4.6 | 84.0 ± 3.8 | 90.0 ± 4.4 |
| Amax (cm s−1) | 51.8 ± 2.5 | 53.9 ± 3.4 | 57.1 ± 6.3 |
| E/A ratio | 1.67 ± 0.10 | 1.68 ± 0.14 | 1.73 ± 0.20 |
| IVRT (cm s−1) | 90.4 ± 3.6 | 91.0 ± 5.0 | 90.8 ± 4.9 |
| TDI-E | |||
| Lateral (cm s−1) | 15.3 ± 0.7 | 15.7 ± 0.6 | 15.7 ± 0.7 |
| Septum (cm s−1) | 12.0 ± 0.5 | 11.5 ± 0.5 | 12.0 ± 0.6 |
| Vp (cm s−1) | 60.1 ± 3.1† | 60.3 ± 3.4† | 74.1 ± 4.9 |
Values are means ± SEM. IVRT, isovolumetric relaxation time. TDI-E, tissue Doppler imaging of early (diastole). Vp, propagation velocity of early mitral inflow.
P < 0.05 compared with controls.
Discussion
The major new findings of this study are as follows: (a) Despite a marked functional limitation, cardiovascular control and oxygen delivery were normal and appropriate for the increase in oxygen uptake in patients with POTS; during maximal exercise, the lower peak SV in POTS patients led to a lower peak 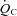 with a similar peak HR compared with healthy sedentary controls. This led to a lower
with a similar peak HR compared with healthy sedentary controls. This led to a lower 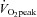 with a similar peak a-v
with a similar peak a-v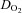 . (b) SV at rest and during exercise increased after 3 months of exercise training, leading to a lower HR at a given
. (b) SV at rest and during exercise increased after 3 months of exercise training, leading to a lower HR at a given 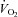 compared with before training, although they did not reach the level of healthy sedentary controls. (c) HR recovery from exercise was faster after training than before training in POTS patients. (d) The relationship between
compared with before training, although they did not reach the level of healthy sedentary controls. (c) HR recovery from exercise was faster after training than before training in POTS patients. (d) The relationship between 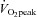 and blood volume was intact in these patients and similar to healthy controls. (e) Resting cardiac diastolic function was mostly normal in POTS patients, though diastolic suction was impaired and not normalized by 3 months of training. These results suggest that short-term exercise training improves physical fitness and exercise performance in POTS.
and blood volume was intact in these patients and similar to healthy controls. (e) Resting cardiac diastolic function was mostly normal in POTS patients, though diastolic suction was impaired and not normalized by 3 months of training. These results suggest that short-term exercise training improves physical fitness and exercise performance in POTS.
Cardiovascular response to exercise in POTS
The lower 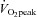 in POTS patients (28%) was primarily attributable to a lower peak
in POTS patients (28%) was primarily attributable to a lower peak 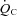 (20%), since peak a-v
(20%), since peak a-v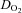 was comparable between patients and controls. Moreover, given the comparable peak HR between groups, the lower peak
was comparable between patients and controls. Moreover, given the comparable peak HR between groups, the lower peak 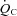 in POTS patients was primarily attributable to a lower SV (19%). Thus, exercise intolerance in POTS patients is most easily explained by a low SV during exercise. A similar observation was made by Masuki and Joyner (Masuki et al. 2007) in their elegant study in which POTS patients showed smaller SV, higher HR, and comparable
in POTS patients was primarily attributable to a lower SV (19%). Thus, exercise intolerance in POTS patients is most easily explained by a low SV during exercise. A similar observation was made by Masuki and Joyner (Masuki et al. 2007) in their elegant study in which POTS patients showed smaller SV, higher HR, and comparable 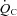 during cycle ergometer with fixed workloads of 25, 50 and 75 watts compared with healthy individuals. This finding coupled with the present study strongly supports the cardiac origin of exercise intolerance in this syndrome. These physiological characteristics are quite similar to those observed after bed rest deconditioning. For instance, previous work including from our laboratory has shown that
during cycle ergometer with fixed workloads of 25, 50 and 75 watts compared with healthy individuals. This finding coupled with the present study strongly supports the cardiac origin of exercise intolerance in this syndrome. These physiological characteristics are quite similar to those observed after bed rest deconditioning. For instance, previous work including from our laboratory has shown that 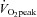 ,
, 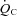 and SV decreased by approximately 26%, 27% and 29%, respectively, after 3 weeks of bed rest (Saltin et al. 1968; Shibata et al. 2010).
and SV decreased by approximately 26%, 27% and 29%, respectively, after 3 weeks of bed rest (Saltin et al. 1968; Shibata et al. 2010).
An important observation in this study is that POTS patients and healthy sedentary controls had a similar linear relationship between 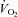 and
and 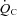 . The slope of this linear relationship has been reported to vary little in healthy adults with ageing, sex, mode of exercise, overall fitness, or degree of effort (Astrand et al. 1964; Julius et al. 1967; Lewis et al. 1983; Proctor et al. 1998; McGuire et al. 2001; Fu & Levine, 2005). Conversely, the slope is augmented, such that more blood flow is required to meet the metabolic demand of exercise, in patients with mitochondrial myopathy (Haller et al. 1991; Taivassalo et al. 2003) presumably due to impaired oxygen utilization by working muscles. In contrast, the slope is depressed in patients with severe heart failure (Chomsky et al. 1996; Mancini et al. 1996; Bhella et al. 2011), reflecting impaired ability of the heart to meet the metabolic demand. Thus, our results indicate that POTS patients have a normal ability to increase
. The slope of this linear relationship has been reported to vary little in healthy adults with ageing, sex, mode of exercise, overall fitness, or degree of effort (Astrand et al. 1964; Julius et al. 1967; Lewis et al. 1983; Proctor et al. 1998; McGuire et al. 2001; Fu & Levine, 2005). Conversely, the slope is augmented, such that more blood flow is required to meet the metabolic demand of exercise, in patients with mitochondrial myopathy (Haller et al. 1991; Taivassalo et al. 2003) presumably due to impaired oxygen utilization by working muscles. In contrast, the slope is depressed in patients with severe heart failure (Chomsky et al. 1996; Mancini et al. 1996; Bhella et al. 2011), reflecting impaired ability of the heart to meet the metabolic demand. Thus, our results indicate that POTS patients have a normal ability to increase 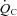 for the oxygen demand during exercise, as well as to utilize oxygen in the periphery, consistent with the finding that a-v
for the oxygen demand during exercise, as well as to utilize oxygen in the periphery, consistent with the finding that a-v 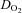 in these patients was comparable with that of healthy controls at any given
in these patients was comparable with that of healthy controls at any given 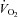 . Therefore, the higher HR in POTS patients at any given
. Therefore, the higher HR in POTS patients at any given 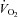 is most likely explained by a normal autonomically mediated compensatory response of the heart to the lower SV in order to maintain the required
is most likely explained by a normal autonomically mediated compensatory response of the heart to the lower SV in order to maintain the required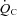 and O2 delivery.
and O2 delivery.
Effects of training on exercise performance in POTS
Numerous studies have shown that 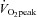 increases after several months of endurance exercise training in both men and women with a wide range of age (Ekblom et al. 1968; Hartley et al. 1969; Saltin et al. 1969; Seals et al. 1984; Ehsani et al. 1991; Stratton et al. 1994; Beere et al. 1999; McGuire et al. 2001; Fujimoto et al. 2010). We also found that
increases after several months of endurance exercise training in both men and women with a wide range of age (Ekblom et al. 1968; Hartley et al. 1969; Saltin et al. 1969; Seals et al. 1984; Ehsani et al. 1991; Stratton et al. 1994; Beere et al. 1999; McGuire et al. 2001; Fujimoto et al. 2010). We also found that 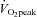 increased by 8% in POTS patients after 3 months of training. The increase in
increased by 8% in POTS patients after 3 months of training. The increase in 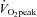 was primarily caused by an increase in peak
was primarily caused by an increase in peak 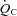 with no changes in peak a-v
with no changes in peak a-v 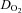 , in contrast with several previous investigations that showed an improvement of peripheral oxygen extraction after training (Ekblom et al. 1968; Seals et al. 1984; Beere et al. 1999; McGuire et al. 2001). The duration of training in those investigations was equal to (Beere et al. 1999) or longer than (Ekblom et al. 1968; Seals et al. 1984; McGuire et al. 2001) that of the present study. One previous report, which similarly demonstrated no changes in a-v
, in contrast with several previous investigations that showed an improvement of peripheral oxygen extraction after training (Ekblom et al. 1968; Seals et al. 1984; Beere et al. 1999; McGuire et al. 2001). The duration of training in those investigations was equal to (Beere et al. 1999) or longer than (Ekblom et al. 1968; Seals et al. 1984; McGuire et al. 2001) that of the present study. One previous report, which similarly demonstrated no changes in a-v 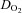 after exercise training in young adults, conducted a relatively short duration of training (∼10 weeks) (Hartley et al. 1969). There are two possible explanations for our findings. One, POTS patients had much lower levels of physical fitness than healthy sedentary controls, and 3 months of training may not have been long enough to normalize their physical fitness levels. Two, patients started training in the semi-recumbent position to avoid the upright posture that elicits their symptoms, while upright exercise was not added until the second or third month of training. Thus, the intensity of training may not have been strong enough to cause changes in peripheral oxygen extraction in these patients within the time frame of this study.
after exercise training in young adults, conducted a relatively short duration of training (∼10 weeks) (Hartley et al. 1969). There are two possible explanations for our findings. One, POTS patients had much lower levels of physical fitness than healthy sedentary controls, and 3 months of training may not have been long enough to normalize their physical fitness levels. Two, patients started training in the semi-recumbent position to avoid the upright posture that elicits their symptoms, while upright exercise was not added until the second or third month of training. Thus, the intensity of training may not have been strong enough to cause changes in peripheral oxygen extraction in these patients within the time frame of this study.
The increase in peak 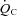 after training was caused by an increase in peak SV of 8% in the present study, consistent with the previous finding that POTS patients showed concentric hypertrophic remodelling in the left ventricle after training (Fu et al. 2010a). The similar increase in peak SV after exercise training was observed in many (Ekblom et al. 1968; Hartley et al. 1969; Seals et al. 1984; Ehsani et al. 1991; Stratton et al. 1994; McGuire et al. 2001; Fujimoto et al. 2010) but not all (Beere et al. 1999) prior studies in healthy individuals. This discrepancy is likely to be explained by different exercise regimens and/or subject backgrounds. In the present study, SV became larger at any given
after training was caused by an increase in peak SV of 8% in the present study, consistent with the previous finding that POTS patients showed concentric hypertrophic remodelling in the left ventricle after training (Fu et al. 2010a). The similar increase in peak SV after exercise training was observed in many (Ekblom et al. 1968; Hartley et al. 1969; Seals et al. 1984; Ehsani et al. 1991; Stratton et al. 1994; McGuire et al. 2001; Fujimoto et al. 2010) but not all (Beere et al. 1999) prior studies in healthy individuals. This discrepancy is likely to be explained by different exercise regimens and/or subject backgrounds. In the present study, SV became larger at any given 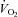 after training including the resting standing condition with the relationship between
after training including the resting standing condition with the relationship between 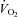 and
and 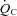 being constant, which consequently led to a lower HR at any given
being constant, which consequently led to a lower HR at any given 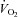 . This observation provides a strong physiological explanation for our previous finding as to why orthostatic tachycardia was substantially improved after training in most POTS patients (Fu et al. 2010a). Increases in cardiac size/mass and blood volume associated with exercise training were responsible for the increase in SV in these patients. We found that
. This observation provides a strong physiological explanation for our previous finding as to why orthostatic tachycardia was substantially improved after training in most POTS patients (Fu et al. 2010a). Increases in cardiac size/mass and blood volume associated with exercise training were responsible for the increase in SV in these patients. We found that 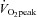 was correlated directly with blood volume in controls and patients before and after training, suggesting that expanded blood volume with training may contribute to the increased physical fitness level and cardiovascular performance in POTS (Hagberg et al. 1998). It is also possible that training might improve leg venous function and increase venous return during exercise in POTS.
was correlated directly with blood volume in controls and patients before and after training, suggesting that expanded blood volume with training may contribute to the increased physical fitness level and cardiovascular performance in POTS (Hagberg et al. 1998). It is also possible that training might improve leg venous function and increase venous return during exercise in POTS.
However, neither 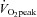 and
and 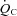 nor SV reached the level of healthy secondary controls after training although all of these physiological parameters were significantly improved. In contrast, it was reported that the decrease in
nor SV reached the level of healthy secondary controls after training although all of these physiological parameters were significantly improved. In contrast, it was reported that the decrease in 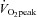 ,
, 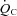 and SV after 3 weeks of bed rest deconditioning was normalized above the level of pre-bed rest by 8 weeks of exercise training (Saltin et al. 1968). It is possible that chronic physical deconditioning may require a longer period of training to restore the deconditioned heart compared to the acute physical deconditioning of short-term bed rest.
and SV after 3 weeks of bed rest deconditioning was normalized above the level of pre-bed rest by 8 weeks of exercise training (Saltin et al. 1968). It is possible that chronic physical deconditioning may require a longer period of training to restore the deconditioned heart compared to the acute physical deconditioning of short-term bed rest.
We also found that HR recovery from exercise was significantly faster after training than before training in POTS patients. These results suggest that there are neural changes that occur after training that are relevant. It has been proposed that early recovery of the HR after acute exercise is dominated by vagal reactivation with sympathetic withdrawal becoming more important later in recovery (Imai et al. 1994; Pierpont et al. 2000). The rate of HR recovery from exercise has also been proposed to be associated with fitness level and global measures of health (Shetler et al. 2001). Thus, the faster HR recovery from exercise after training in our patients indicates an increase in vagal reactivation, a decrease in sympathetic tone, and an improvement in physical fitness level.
Cardiac diastolic function in POTS
Propagation velocity was lower in POTS patients compared with controls, while other Doppler indices were comparable between groups. Propagation velocity is primarily determined by passive filling and intra-ventricular pressure gradients within the ventricular cavity that actively draw blood from the base to the apex (Garcia et al. 2000). These intra-ventricular pressure gradients were previously shown to reflect the magnitude of diastolic suction during early diastole (Popovic et al. 2006). Filling pressure is usually high during supine exercise; however, Masuki et al. (2007) found that even while supine POTS patients tended to have low SVs. Because all other parameters of diastolic function were normal in our patients, the abnormality of diastolic function in POTS appeared limited to diastolic suction, rather than an intrinsic abnormality of relaxation.
Previous longitudinal studies have consistently shown that exercise training improves Doppler variables of LV diastolic function in young healthy individuals (Naylor et al. 2005; Kivisto et al. 2006; Baggish et al. 2008; Weiner et al. 2010). It was also reported that short-term physical deconditioning due to bed rest led to impaired LV diastolic suction, which could be prevented by daily exercise training in young healthy subjects (Dorfman et al. 2008). However, there were no changes in any Doppler indices after training in POTS patients. Given the fact that this training paradigm increased, but did not entirely normalize, blood volume in these patients (Fu et al. 2010a), we suspect that the persistently abnormal resting propagation velocity was a function of a persistently low equilibrium volume. We speculate that more training, especially in the upright position would be required to completely normalize diastolic suction.
Perspectives
Results from this study confirm that in this referral population of patients with POTS, cardiovascular deconditioning plays an important role in the functional disability, and both orthostatic and exercise tachycardia observed in this condition. It is important to emphasize that even 20 h of bedrest induces substantial cardiovascular deconditioning in healthy individuals (Gaffney et al. 1985). This process may induce a downward spiral of hypovolaemia, cardiac atrophy (∼1%/week in bed), worsening orthostatic intolerance and physical disability which can be quite profound. By demonstrating a normal cardiac output response to exercise, and a normal relationship between blood volume and 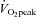 , this study also demonstrates that the exertional tachycardia in POTS patients is appropriate for the metabolic demand. This tachycardia therefore appears ‘appropriate’ rather than ‘inappropriate’ and reflects the normal autonomic adjustments to the stimulus of exercise. Targeted therapy directed at the underlying pathophysiology improved, but did not completely normalize, the exercise responses in these patients and we speculate that perhaps longer durations of training may be necessary for a full functional recovery. What levels of physical activity must be maintained in patients with POTS to achieve and maintain normal functional capacity is uncertain; however we speculate that a lifetime adherence to an active life-style will be necessary.
, this study also demonstrates that the exertional tachycardia in POTS patients is appropriate for the metabolic demand. This tachycardia therefore appears ‘appropriate’ rather than ‘inappropriate’ and reflects the normal autonomic adjustments to the stimulus of exercise. Targeted therapy directed at the underlying pathophysiology improved, but did not completely normalize, the exercise responses in these patients and we speculate that perhaps longer durations of training may be necessary for a full functional recovery. What levels of physical activity must be maintained in patients with POTS to achieve and maintain normal functional capacity is uncertain; however we speculate that a lifetime adherence to an active life-style will be necessary.
Limitations
First, although the two groups were matched for age, sex and body mass index, percentage body fat was greater in POTS patients than in healthy controls presumably due to physical deconditioning. Second, the control group did not undergo a similar exercise training programme. However, previous studies from our laboratory showed that 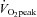 increased similarly in initially healthy sedentary individuals and POTS patients (13%versus 11%) after 3 months of training (Iwasaki et al. 2003).
increased similarly in initially healthy sedentary individuals and POTS patients (13%versus 11%) after 3 months of training (Iwasaki et al. 2003).
Conclusion
In conclusion, the present findings emphasize that cardiovascular control during exercise is normal in patients with POTS. Moreover, they highlight the similarity between POTS and deconditioning by showing lower physical performance due to a low stroke volume during exercise in patients with POTS. Although physical fitness level and cardiovascular responses during exercise were improved after short-term (i.e. 3 months) exercise training, diastolic suction remained unchanged in these patients. Whether prolonged upright exercise training is needed to completely normalize exercise capacity and cardiac function in POTS patients remains to be determined.
Acknowledgments
The authors thank Robin P. Shook, M. Dean Palmer, and Kazunobu Okazaki for their valuable laboratory assistance and contributions to data collection. Funding was from the following sources: NASA-NSBRI postdoctoral fellowship grant (PR01101), NASA-NSBRI career development award (EO00007), the National Institutes of Health K23 grant (HL075283), and the Clinical and Translational Research Center (formerly, the General Clinical Research Center) grant (RR00633).
Glossary
- HR
heart rate
- IVRT
isovolumetric relaxation time
- POTS
postural orthostatic tachycardia syndrome
- SV
stroke volume
- TPR
total peripheral resistance
Author contributions
Shigeki Shibata contributed to 1) conception and design of the experiments; 2) collection, analysis and interpretation of data; and 3) drafting the article and revising it critically for important intellectual content.
Qi Fu contributed to 1) conception and design of the experiments; 2) collection, analysis and interpretation of data; and 3) drafting the article and revising it critically for important intellectual content.
Tiffany B. Bivens contributed to collection and analysis of data.
Jeffrey L. Hastings contributed to 1) collection, analysis and interpretation of data; and 2) revising it critically for important intellectual content.
Wade Wang contributed to collection and analysis of data.
Benjamin D. Levine contributed to 1) conception and design of the experiments; 2) collection, analysis and interpretation of data; and 3) revising the article for important intellectual content.
All authors approved the final version of the manuscript.
References
- Astrand PO, Cuddy TE, Saltin B, Stenberg J. Cardiac output during submaximal and maximal work. J Appl Physiol. 1964;19:268–274. doi: 10.1152/jappl.1964.19.2.268. [DOI] [PubMed] [Google Scholar]
- Baggish AL, Wang F, Weiner RB, Elinoff JM, Tournoux F, Boland A, Picard MH, Hutter AM, Jr, Wood MJ. Training-specific changes in cardiac structure and function: a prospective and longitudinal assessment of competitive athletes. J Appl Physiol. 2008;104:1121–1128. doi: 10.1152/japplphysiol.01170.2007. [DOI] [PubMed] [Google Scholar]
- Balke B, Nagle FJ, Daniels J. Altitude and maximum performance in work and sports activity. JAMA. 1965;194:646–649. [PubMed] [Google Scholar]
- Beere PA, Russell SD, Morey MC, Kitzman DW, Higginbotham MB. Aerobic exercise training can reverse age-related peripheral circulatory changes in healthy older men. Circulation. 1999;100:1085–1094. doi: 10.1161/01.cir.100.10.1085. [DOI] [PubMed] [Google Scholar]
- Bhella PS, Prasad A, Heinicke K, Hastings JL, Arbab-Zadeh A, Adams-Huet B, Pacini EL, Shibata S, Palmer MD, Newcomer BR, Levine BD. Abnormal haemodynamic response to exercise in heart failure with preserved ejection fraction. Eur J Heart Fail. 2011;13:1296–1304. doi: 10.1093/eurjhf/hfr133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomsky DB, Lang CC, Rayos GH, Shyr Y, Yeoh TK, Pierson RN, 3rd, Davis SF, Wilson JR. Hemodynamic exercise testing. A valuable tool in the selection of cardiac transplantation candidates. Circulation. 1996;94:3176–3183. doi: 10.1161/01.cir.94.12.3176. [DOI] [PubMed] [Google Scholar]
- Cohen GI, Pietrolungo JF, Thomas JD, Klein AL. A practical guide to assessment of ventricular diastolic function using Doppler echocardiography. J Am Coll Cardiol. 1996;27:1753–1760. doi: 10.1016/0735-1097(96)00088-5. [DOI] [PubMed] [Google Scholar]
- Cole CR, Blackstone EH, Pashkow FJ, Snader CE, Lauer MS. Heart-rate recovery immediately after exercise as a predictor of mortality. N Engl J Med. 1999;341:1351–1357. doi: 10.1056/NEJM199910283411804. [DOI] [PubMed] [Google Scholar]
- Cole CR, Foody JM, Blackstone EH, Lauer MS. Heart rate recovery after submaximal exercise testing as a predictor of mortality in a cardiovascularly healthy cohort. Ann Intern Med. 2000;132:552–555. doi: 10.7326/0003-4819-132-7-200004040-00007. [DOI] [PubMed] [Google Scholar]
- Dorfman TA, Levine BD, Tillery T, Peshock RM, Hastings JL, Schneider SM, Macias BR, Biolo G, Hargens AR. Cardiac atrophy in women following bed rest. J Appl Physiol. 2007;103:8–16. doi: 10.1152/japplphysiol.01162.2006. [DOI] [PubMed] [Google Scholar]
- Dorfman TA, Rosen BD, Perhonen MA, Tillery T, McColl R, Peshock RM, Levine BD. Diastolic suction is impaired by bed rest: MRI tagging studies of diastolic untwisting. J Appl Physiol. 2008;104:1037–1044. doi: 10.1152/japplphysiol.00858.2006. [DOI] [PubMed] [Google Scholar]
- Ehsani AA, Ogawa T, Miller TR, Spina RJ, Jilka SM. Exercise training improves left ventricular systolic function in older men. Circulation. 1991;83:96–103. doi: 10.1161/01.cir.83.1.96. [DOI] [PubMed] [Google Scholar]
- Ekblom B, Astrand PO, Saltin B, Stenberg J, Wallstrom B. Effect of training on circulatory response to exercise. J Appl Physiol. 1968;24:518–528. doi: 10.1152/jappl.1968.24.4.518. [DOI] [PubMed] [Google Scholar]
- Fu Q, Levine BD. Cardiovascular response to exercise in women. Med Sci Sports Exerc. 2005;37:1433–1435. doi: 10.1249/01.mss.0000174886.08219.85. [DOI] [PubMed] [Google Scholar]
- Fu Q, Vangundy TB, Galbreath MM, Shibata S, Jain M, Hastings JL, Bhella PS, Levine BD. Cardiac origins of the postural orthostatic tachycardia syndrome. J Am Coll Cardiol. 2010a;55:2858–2868. doi: 10.1016/j.jacc.2010.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Q, VanGundy TB, Shibata S, Auchus RJ, Williams GH, Levine BD. Menstrual cycle affects renal-adrenal and hemodynamic responses during prolonged standing in the postural orthostatic tachycardia syndrome. Hypertension. 2010b;56:82–90. doi: 10.1161/HYPERTENSIONAHA.110.151787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Q, Vangundy TB, Shibata S, Auchus RJ, Williams GH, Levine BD. Exercise training versus propranolol in the treatment of the postural orthostatic tachycardia syndrome. Hypertension. 2011;58:167–175. doi: 10.1161/HYPERTENSIONAHA.111.172262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto N, Prasad A, Hastings JL, Arbab-Zadeh A, Bhella PS, Shibata S, Palmer D, Levine BD. Cardiovascular effects of 1 year of progressive and vigorous exercise training in previously sedentary individuals older than 65 years of age. Circulation. 2010;122:1797–1805. doi: 10.1161/CIRCULATIONAHA.110.973784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffney FA, Nixon JV, Karlsson ES, Campbell W, Dowdey AB, Blomqvist CG. Cardiovascular deconditioning produced by 20 hours of bedrest with head-down tilt (-5 degrees) in middle-aged healthy men. Am J Cardiol. 1985;56:634–638. doi: 10.1016/0002-9149(85)91025-2. [DOI] [PubMed] [Google Scholar]
- Galbreath MM, Shibata S, VanGundy TB, Okazaki K, Fu Q, Levine BD. Effects of exercise training on arterial-cardiac baroreflex function in POTS. Clin Auton Res. 2011;21:73–80. doi: 10.1007/s10286-010-0091-5. [DOI] [PubMed] [Google Scholar]
- Garcia MJ, Smedira NG, Greenberg NL, Main M, Firstenberg MS, Odabashian J, Thomas JD. Color M-mode Doppler flow propagation velocity is a preload insensitive index of left ventricular relaxation: animal and human validation. J Am Coll Cardiol. 2000;35:201–208. doi: 10.1016/s0735-1097(99)00503-3. [DOI] [PubMed] [Google Scholar]
- Gore CJ, Rodriguez FA, Truijens MJ, Townsend NE, Stray-Gundersen J, Levine BD. Increased serum erythropoietin but not red cell production after 4 wk of intermittent hypobaric hypoxia (4,000–5,500 m) J Appl Physiol. 2006;101:1386–1393. doi: 10.1152/japplphysiol.00342.2006. [DOI] [PubMed] [Google Scholar]
- Hagberg JM, Goldberg AP, Lakatta L, O’Connor FC, Becker LC, Lakatta EG, Fleg JL. Expanded blood volumes contribute to the increased cardiovascular performance of endurance-trained older men. J Appl Physiol. 1998;85:484–489. doi: 10.1152/jappl.1998.85.2.484. [DOI] [PubMed] [Google Scholar]
- Haller RG, Henriksson KG, Jorfeldt L, Hultman E, Wibom R, Sahlin K, Areskog NH, Gunder M, Ayyad K, Blomqvist CG, et al. Deficiency of skeletal muscle succinate dehydrogenase and aconitase. Pathophysiology of exercise in a novel human muscle oxidative defect. J Clin Invest. 1991;88:1197–1206. doi: 10.1172/JCI115422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley LH, Grimby G, Kilbom A, Nilsson NJ, Astrand I, Bjure J, Ekblom B, Saltin B. Physical training in sedentary middle-aged and older men. 3. Cardiac output and gas exchange asubmaximal and maximal exercise. Scand J Clin Lab Invest. 1969;24:335–344. doi: 10.3109/00365516909080170. [DOI] [PubMed] [Google Scholar]
- Hatle L. Doppler echocardiographic evaluation of diastolic function in hypertensive cardiomyopathies. Eur Heart J. 1993;14(Suppl J):88–94. [PubMed] [Google Scholar]
- Imai K, Sato H, Hori M, Kusuoka H, Ozaki H, Yokoyama H, Takeda H, Inoue M, Kamada T. Vagally mediated heart rate recovery after exercise is accelerated in athletes but blunted in patients with chronic heart failure. J Am Coll Cardiol. 1994;24:1529–1535. doi: 10.1016/0735-1097(94)90150-3. [DOI] [PubMed] [Google Scholar]
- Iwasaki K, Zhang R, Zuckerman JH, Levine BD. Dose-response relationship of the cardiovascular adaptation to endurance training in healthy adults: how much training for what benefit? J Appl Physiol. 2003;95:1575–1583. doi: 10.1152/japplphysiol.00482.2003. [DOI] [PubMed] [Google Scholar]
- Jarvis SS, Levine BD, Prisk GK, Shykoff BE, Elliott AR, Rosow E, Blomqvist CG, Pawelczyk JA. Simultaneous determination of the accuracy and precision of closed-circuit cardiac output rebreathing techniques. J Appl Physiol. 2007;103:867–874. doi: 10.1152/japplphysiol.01106.2006. [DOI] [PubMed] [Google Scholar]
- Joyner MJ, Masuki S. POTS versus deconditioning: the same or different? Clin Auton Res. 2008;18:300–307. doi: 10.1007/s10286-008-0487-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius S, Amery A, Whitlock LS, Conway J. Influence of age on the hemodynamic response to exercise. Circulation. 1967;36:222–230. doi: 10.1161/01.cir.36.2.222. [DOI] [PubMed] [Google Scholar]
- Kivisto S, Perhonen M, Holmstrom M, Lauerma K. Assessment of the effect of endurance training on left ventricular relaxation with magnetic resonance imaging. Scand J Med Sci Sports. 2006;16:321–328. doi: 10.1111/j.1600-0838.2005.00493.x. [DOI] [PubMed] [Google Scholar]
- Labovitz AJ, Pearson AC. Evaluation of left ventricular diastolic function: clinical relevance and recent Doppler echocardiographic insights. Am Heart J. 1987;114:836–851. doi: 10.1016/0002-8703(87)90795-2. [DOI] [PubMed] [Google Scholar]
- Levine BD, Zuckerman JH, Pawelczyk JA. Cardiac atrophy after bed-rest deconditioning: a nonneural mechanism for orthostatic intolerance. Circulation. 1997;96:517–525. doi: 10.1161/01.cir.96.2.517. [DOI] [PubMed] [Google Scholar]
- Lewis SF, Taylor WF, Graham RM, Pettinger WA, Schutte JE, Blomqvist CG. Cardiovascular responses to exercise as functions of absolute and relative work load. J Appl Physiol. 1983;54:1314–1323. doi: 10.1152/jappl.1983.54.5.1314. [DOI] [PubMed] [Google Scholar]
- Low PA, Opfer-Gehrking TL, Textor SC, Benarroch EE, Shen WK, Schondorf R, Suarez GA, Rummans TA. Postural tachycardia syndrome (POTS) Neurology. 1995;45:S19–25. [PubMed] [Google Scholar]
- Low PA, Sandroni P, Joyner M, Shen WK. Postural tachycardia syndrome (POTS) J Cardiovasc Electrophysiol. 2009;20:352–358. doi: 10.1111/j.1540-8167.2008.01407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini D, Katz S, Donchez L, Aaronson K. Coupling of hemodynamic measurements with oxygen consumption during exercise does not improve risk stratification in patients with heart failure. Circulation. 1996;94:2492–2496. doi: 10.1161/01.cir.94.10.2492. [DOI] [PubMed] [Google Scholar]
- Masuki S, Eisenach JH, Schrage WG, Johnson CP, Dietz NM, Wilkins BW, Sandroni P, Low PA, Joyner MJ. Reduced stroke volume during exercise in postural tachycardia syndrome. J Appl Physiol. 2007;103:1128–1135. doi: 10.1152/japplphysiol.00175.2007. [DOI] [PubMed] [Google Scholar]
- McGuire DK, Levine BD, Williamson JW, Snell PG, Blomqvist CG, Saltin B, Mitchell JH. A 30-year follow-up of the Dallas Bedrest and Training Study: II. Effect of age on cardiovascular adaptation to exercise training. Circulation. 2001;104:1358–1366. [PubMed] [Google Scholar]
- Naylor LH, Arnolda LF, Deague JA, Playford D, Maurogiovanni A, O’Driscoll G, Green DJ. Reduced ventricular flow propagation velocity in elite athletes is augmented with the resumption of exercise training. J Physiol. 2005;563:957–963. doi: 10.1113/jphysiol.2004.078360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishime EO, Cole CR, Blackstone EH, Pashkow FJ, Lauer MS. Heart rate recovery and treadmill exercise score as predictors of mortality in patients referred for exercise ECG. JAMA. 2000;284:1392–1398. doi: 10.1001/jama.284.11.1392. [DOI] [PubMed] [Google Scholar]
- Pierpont GL, Stolpman DR, Gornick CC. Heart rate recovery post-exercise as an index of parasympathetic activity. J Auton Nerv Syst. 2000;80:169–174. doi: 10.1016/s0165-1838(00)00090-4. [DOI] [PubMed] [Google Scholar]
- Popovic ZB, Prasad A, Garcia MJ, Arbab-Zadeh A, Borowski A, Dijk E, Greenberg NL, Levine BD, Thomas JD. Relationship among diastolic intraventricular pressure gradients, relaxation, and preload: impact of age and fitness. Am J Physiol Heart Circ Physiol. 2006;290:H1454–1459. doi: 10.1152/ajpheart.00902.2005. [DOI] [PubMed] [Google Scholar]
- Prasad A, Popovic ZB, Arbab-Zadeh A, Fu Q, Palmer D, Dijk E, Greenberg NL, Garcia MJ, Thomas JD, Levine BD. The effects of aging and physical activity on Doppler measures of diastolic function. Am J Cardiol. 2007;99:1629–1636. doi: 10.1016/j.amjcard.2007.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor DN, Beck KC, Shen PH, Eickhoff TJ, Halliwill JR, Joyner MJ. Influence of age and gender on cardiac output-VO2 relationships during submaximal cycle ergometry. J Appl Physiol. 1998;84:599–605. doi: 10.1152/jappl.1998.84.2.599. [DOI] [PubMed] [Google Scholar]
- Raj SR, Biaggioni I, Yamhure PC, Black BK, Paranjape SY, Byrne DW, Robertson D. Renin-aldosterone paradox and perturbed blood volume regulation underlying postural tachycardia syndrome. Circulation. 2005;111:1574–1582. doi: 10.1161/01.CIR.0000160356.97313.5D. [DOI] [PubMed] [Google Scholar]
- Raj SR, Robertson D. Blood volume perturbations in the postural tachycardia syndrome. Am J Med Sci. 2007;334:57–60. doi: 10.1097/MAJ.0b013e318063c6c0. [DOI] [PubMed] [Google Scholar]
- Rivas-Gotz C, Manolios M, Thohan V, Nagueh SF. Impact of left ventricular ejection fraction on estimation of left ventricular filling pressures using tissue Doppler and flow propagation velocity. Am J Cardiol. 2003;91:780–784. doi: 10.1016/s0002-9149(02)03433-1. [DOI] [PubMed] [Google Scholar]
- Robertson D. The epidemic of orthostatic tachycardia and orthostatic intolerance. Am J Med Sci. 1999;317:75–77. doi: 10.1097/00000441-199902000-00001. [DOI] [PubMed] [Google Scholar]
- Rosenwinkel ET, Bloomfield DM, Arwady MA, Goldsmith RL. Exercise and autonomic function in health and cardiovascular disease. Cardiol Clin. 2001;19:369–387. doi: 10.1016/s0733-8651(05)70223-x. [DOI] [PubMed] [Google Scholar]
- Saltin B, Blomqvist G, Mitchell JH, Johnson RL, Jr, Wildenthal K, Chapman CB. Response to exercise after bed rest and after training. Circulation. 1968;38(Suppl VII):1–78. [PubMed] [Google Scholar]
- Saltin B, Hartley LH, Kilbom A, Astrand I. Physical training in sedentary middle-aged and older men. II. Oxygen uptake, heart rate, and blood lactate concentration at submaximal and maximal exercise. Scand J Clin Lab Invest. 1969;24:323–334. doi: 10.3109/00365516909080169. [DOI] [PubMed] [Google Scholar]
- Seals DR, Hagberg JM, Hurley BF, Ehsani AA, Holloszy JO. Endurance training in older men and women. I. Cardiovascular responses to exercise. J Appl Physiol. 1984;57:1024–1029. doi: 10.1152/jappl.1984.57.4.1024. [DOI] [PubMed] [Google Scholar]
- Shetler K, Marcus R, Froelicher VF, Vora S, Kalisetti D, Prakash M, Do D, Myers J. Heart rate recovery: validation and methodologic issues. J Am Coll Cardiol. 2001;38:1980–1987. doi: 10.1016/s0735-1097(01)01652-7. [DOI] [PubMed] [Google Scholar]
- Shibata S, Perhonen M, Levine BD. Supine cycling plus volume loading prevent cardiovascular deconditioning during bed rest. J Appl Physiol. 2010;108:1177–1186. doi: 10.1152/japplphysiol.01408.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn DW, Chai IH, Lee DJ, Kim HC, Kim HS, Oh BH, Lee MM, Park YB, Choi YS, Seo JD, Lee YW. Assessment of mitral annulus velocity by Doppler tissue imaging in the evaluation of left ventricular diastolic function. J Am Coll Cardiol. 1997;30:474–480. doi: 10.1016/s0735-1097(97)88335-0. [DOI] [PubMed] [Google Scholar]
- Stratton JR, Levy WC, Cerqueira MD, Schwartz RS, Abrass IB. Cardiovascular responses to exercise. Effects of aging and exercise training in healthy men. Circulation. 1994;89:1648–1655. doi: 10.1161/01.cir.89.4.1648. [DOI] [PubMed] [Google Scholar]
- Taivassalo T, Jensen TD, Kennaway N, DiMauro S, Vissing J, Haller RG. The spectrum of exercise tolerance in mitochondrial myopathies: a study of 40 patients. Brain. 2003;126:413–423. doi: 10.1093/brain/awg028. [DOI] [PubMed] [Google Scholar]
- Weiner RB, Hutter AM, Jr, Wang F, Kim J, Weyman AE, Wood MJ, Picard MH, Baggish AL. The impact of endurance exercise training on left ventricular torsion. JACC Cardiovasc Imaging. 2010;3:1001–1009. doi: 10.1016/j.jcmg.2010.08.003. [DOI] [PubMed] [Google Scholar]


