Abstract
Dietary inorganic nitrate has profound effects on health and physiological responses to exercise. Here, we examined if nitrate, in doses readily achievable via a normal diet, could improve Ca2+ handling and contractile function using fast- and slow-twitch skeletal muscles from C57bl/6 male mice given 1 mm sodium nitrate in water for 7 days. Age matched controls were provided water without added nitrate. In fast-twitch muscle fibres dissected from nitrate treated mice, myoplasmic free [Ca2+] was significantly greater than in Control fibres at stimulation frequencies from 20 to 150 Hz, which resulted in a major increase in contractile force at ≤50 Hz. At 100 Hz stimulation, the rate of force development was ∼35% faster in the nitrate group. These changes in nitrate treated mice were accompanied by increased expression of the Ca2+ handling proteins calsequestrin 1 and the dihydropyridine receptor. No changes in force or calsequestrin 1 and dihydropyridine receptor expression were measured in slow-twitch muscles. In conclusion, these results show a striking effect of nitrate supplementation on intracellular Ca2+ handling in fast-twitch muscle resulting in increased force production. A new mechanism is revealed by which nitrate can exert effects on muscle function with applications to performance and a potential therapeutic role in conditions with muscle weakness.
Key points
Dietary supplementation with inorganic nitrate has beneficial effects on skeletal muscle responses to exercise.
Both mitochondrial and extra-mitochondrial explanations have been proposed.
Contractile force of fast-twitch muscles was enhanced in mice supplemented with 1 mm NaNO3 in drinking water for 7 days.
Myoplasmic free [Ca2+] during tetanic stimulation was increased in fast-twitch muscles of nitrate-supplemented mice and this was accompanied by increased expression of calsequestrin 1 and the dihydropyridine receptor.
These results provide a new mechanism by which nitrate exerts beneficial effects on muscle function with applications to sports performance and a potential therapeutic role in conditions with muscle weakness.
Introduction
Inorganic nitrate (NO3−) and nitrite (NO2−) are circulating oxidation products from endogenous nitric oxide (NO) production. Emerging data show that these anions can be recycled back to NO, thereby constituting a large pool of potential NO bioactivity, existing in parallel with the more classical l-arginine–NO synthase pathway (Lundberg et al. 2008). Interestingly, this pool can be supplemented by the diet where green leafy vegetables such as spinach and beetroot are especially rich in inorganic nitrate. Numerous studies now show that administration of nitrate or nitrite has NO-like bioactivity in animals and humans including a reduction of blood pressure, protection against ischaemia–reperfusion injury, and modulation of mitochondrial function (Weitzberg et al. 2010).
Nitrate supplementation, either as a sodium salt (NaNO3) or as a natural resource (e.g. beetroot juice), reduces the oxygen cost of exercise (Larsen et al. 2007, 2010, 2011; Bailey et al. 2009, 2010; Vanhatalo et al. 2010; Lansley et al. 2011b; Cemak et al. 2012), and enhances exercise performance (Bailey et al. 2010; Lansley et al. 2011a; Cemak et al. 2012). Both mitochondrial (Larsen et al. 2011) and extra-mitochondrial effects (Bailey et al. 2010; Lansley et al. 2011b) have been reported after nitrate supplementation. Specifically, Larsen et al. (2011) showed that dietary nitrate supplementation for 3 days in healthy human subjects significantly improved skeletal muscle mitochondrial efficiency and this correlated to a measured reduction in whole body O2 consumption. In these experiments, the expression of the mitochondrial uncoupling protein adenine nucleotide translocase was reduced after nitrate treatment. On the other hand, attenuated [PCr] changes during exercise (Bailey et al. 2010) and the absence of alterations in [PCr] recovery rate (Lansley et al. 2011b) indicate an extra-mitochondrial effect of nitrate supplementation.
Modified intracellular Ca2+ handling has been suggested as a mechanism by which nitrate supplementation may enhance muscle performance (Bailey et al. 2010; Ferreira & Behnke, 2011). To date, however, this suggestion has not been experimentally explored. Here, we examine the role of nitrate ingestion for 7 days on contractile force and Ca2+ handling in skeletal muscles from the mouse. It was hypothesized that nitrate supplementation would result in improved contractile performance due to increased Ca2+ activation of contractile proteins.
Methods
Ethical approval
All experiments complied with the Swedish Animal Welfare Act, the Swedish Welfare ordinance, and applicable regulations and recommendations from Swedish authorities. The study was approved by the Stockholm North Ethical Committee on Animal Experiments. The experiments comply with the policies of The Journal of Physiology (Drummond, 2009). Mice were killed by rapid neck disarticulation. A total of 45 mice were used.
Nitrate ingestion
C57bl/6 male mice were given 1 mm NaNO3 (∼3.75 μmol nitrate day−1) dissolved in distilled water for 7 days (Nitrate) and age-matched controls were provided distilled water without nitrate (Control). The dose of nitrate is similar to what has been studied in human exercise studies and corresponds to a daily ingestion of three to four beetroots or 200–300 g of spinach in a human. Mice were given chow containing 0.2 μmol nitrate g−1 and ingested ∼0.75 μmol nitrate day−1 from food.
Contractile function in intact muscles
Intact fast-twitch EDL and slow-twitch soleus muscles were isolated from Control and Nitrate mice. After dissection, stainless steel hooks were tied to each end of the soleus and EDL muscles with suture thread. The muscle was then mounted at optimal length in a stimulation chamber containing Tyrode solution (mm): NaCl, 121; KCl, 5.0; CaCl2, 1.8; MgCl2, 0.5; NaH2PO4, 0.4, NaHCO3, 24.0; EDTA, 0.1; glucose, 5.5 and fetal calf serum (0.2%). The solution was bubbled with 95% O2, 5% CO2 which gives an extracellular pH of 7.4. Experiments were performed at room temperature (24–26°C). The muscle was equilibrated for 45 min prior to data collection. Tetanic stimulation was achieved by supramaximal current pulses (duration 0.5 ms) delivered via platinum electrodes lying parallel to the muscle. The force–frequency relationship was tested by producing one contraction every minute. The duration of contractions was 300 ms for EDL and 1 s for soleus. Frequencies tested ranged from 1 to 150 Hz for EDL and 1 to 100 Hz for soleus. Tetanic force was measured when force was at its peak.
Force and [Ca2+]i measurements in dissected fibres
Single flexor digitorum brevis (FDB) fibres were mechanically dissected as previously described (Lännergren & Westerblad, 1987). The isolated fibre was mounted in a stimulation chamber at optimum length and superfused with Tyrode solution (see above) at room temperature (24–26°C). Tetanic stimulation was achieved by supramaximal current pulses (duration 0.5 ms) delivered via platinum electrodes lying parallel to the muscle fibre. The fluorescent Ca2+ indicator indo-1 (Invitrogen, Carlsbad, CA, USA) was microinjected into the isolated fibre. The fluorescence of indo-1 was converted to free myoplasmic [Ca2+] ([Ca2+]i) using an intracellularly established calibration curve (Andrade et al. 1998a). Tetanic [Ca2+]i was measured as the mean indo-1 fluorescence during tetanic stimulation trains, and basal [Ca2+]i as the mean over ∼200 ms immediately prior to tetanic stimulation. Possible changes in sarcoplasmic reticulum (SR) Ca2+ pumping and/or passive SR Ca2+ leak were assessed by measuring the tails of elevated [Ca2+]i after 30 and 100 Hz tetani (Klein et al. 1991; Westerblad & Allen, 1994). Tetanic force was measured as the mean over 100 ms where force was maximal.
The fibre was allowed to rest for at least 30 min after being injected with indo-1. It was then stimulated by individual 350 ms trains at 15–150 Hz at 1 min intervals while force and [Ca2+]i were recorded. Force–[Ca2+]i curves were constructed from these contractions by fitting data points to the following equation:
where P is the force, Pmax is the force at saturating [Ca2+]i, Ca50 is the [Ca2+]i giving 50% of Pmax, and N is the Hill coefficient that describes the steepness of the function.
In another set of fibres, the amount of releasable Ca2+ stored within the SR was measured. Caffeine at 5 mm was dissolved directly in Tyrode solution and applied for 1 min before stimulation at 100 Hz. Tetanic stimulation in the presence of caffeine can be used to assess the SR Ca2+ load and force at saturating [Ca2+]i (Allen & Westerblad, 1995).
Western blot
EDL and soleus muscles were homogenized with a ground glass homogenizer in ice-cold homogenisation buffer at pH 7.4 (20 μl per mg wet weight) consisting of (mm): Hepes, 20; NaCl, 150; EDTA, 5; KF, 25; Na3VO4, 1; 20% glycerol, 0.5% Triton X-100, and protease inhibitor cocktail (Roche, Basel, Switzerland) 1 tablet/50 ml. The homogenate was centrifuged at 700 g for 10 min at 4°C. Protein content of the supernatant was determined using the Bradford assay (Bio-Rad, Hercules, CA, USA). Samples were diluted 1:1 in Laemmli buffer (Bio-Rad) with 5% 2-mercaptoethanol and heated to 95 °C for 5 min.
Thirty micrograms of protein of were loaded into each well and separated by electrophoresis using NuPAGE Novex 4–12% Bis-Tris Gels (Invitrogen) and transferred onto polyvinylidine fluoride membranes (Immobilon FL, Millipore, Billerica, MA, USA). Membranes were blocked for 1 h at room temperature in Li-Cor Blocking buffer (LI-COR Biosciences, Lincoln, NE, USA), followed by incubation overnight at 4°C with the following antibodies diluted in blocking buffer: mouse anti-SR Ca2+-ATPase 1 (SERCA1; 1:2500, no. ab2818, Abcam, Cambridge, UK), rabbit anti-calsequestrin (CASQ; 1:2500, no. ab3516, Abcam), mouse anti-dihydropyridine receptor (DHPR; 1:500, no. ab2864, Abcam), and mouse anti-ryanodine receptor (RyR, 1:1000, MA3-925, Thermo Scientific, Rockford, IL, USA). Membranes were then washed in TBS-T and incubated for 1 h at room temperature with IRDye 680-conjugated goat anti-mouse IgG and IRDye 800-conjugated goat anti-rabbit IgG (1:15,000, LI-COR) in blocking buffer and 0.01% SDS. Following incubation, membranes were washed in TBS-T. Immunoreactive bands were visualized using infrared fluorescence (IR-Odyssey scanner, LI-COR).
Band densities were analysed with ImageJ (NIH, USA; http://rsb.info.nih.gov/ij/). Equal protein loading was verified with protein staining of membranes (SimplyBlue Safe Stain, Invitrogen) and subsequent scanning of densities for each lane. Band densities were normalized to the total protein measured by Coomassie staining. Precautions were taken to ensure that bands did not reach saturation, but experiments were not performed to establish exact linearity between band density and protein concentration and hence the analyses were only semi-quantitative. The CASQ antibody used (no. ab3516, Abcam) detects CASQ1 and CASQ2 which were identified based on their molecular weights (http://www.uniprot.org; CASQ1: O09165, CASQ2: O09161). The CASQ2 band was markedly stronger than the CASQ1 band even in fast-twitch EDL muscles where CASQ1 should be the dominating isoform (Murphy et al. 2009) and no difference in CASQ2 band densities between muscles was detected. Control Western blot experiments were performed on heart muscle, where CASQ2 is the dominating isoform, and showed no clear CASQ1 band, whereas the CASQ2 band density was similar to that in EDL muscles blotted on the same gel (data not shown). Thus, it is doubtful whether the antibody used accurately detects CASQ2 expression and therefore we have not measured CASQ2 band densities.
Statistics
Data are presented as means ± SEM. Two-way repeated measures ANOVA (SigmaStat 3.1, Systat Software, Chicago, IL, USA) was used when comparing repeated measures (frequency and Nitrate vs. Control). Student's unpaired t test was used to detect differences in single measurements between Nitrate and Control conditions. P < 0.05 was considered significant.
Results
Nitrate ingestion increases contractile force in fast-twitch muscle
Muscle mass did not differ between Control (n = 7) and Nitrate mice (n = 6 soleus; 7 EDL) for fast-twitch EDL (11.6 ± 0.7 vs. 11.6 ± 0.3 mg) or slow-twitch soleus (9.6 ± 0.2 vs. 8.9 ± 0.2 mg) muscles. Cross sectional area also did not differ between Control and Nitrate mice for EDL (1.2 ± 0.1 vs. 1.2 ± 0.1 mm2) or soleus (1.1 ± 0.05 vs. 1.0 ± 0.02 mm2). Time to peak force during a twitch did not differ between Control and Nitrate mice for EDL (21.1 ± 0.9 vs. 23.1 ± 0.7 ms) or soleus (35.6 ± 1.8 vs. 33.7 ± 0.8 ms). Figure 1 depicts mean contractile force data for EDL (Fig. 1A) and soleus (Fig. 1B). Force in Nitrate EDL muscles was significantly greater over the range 1–50 Hz compared to Control mice (P < 0.05). In contrast, there were no significant differences in force at any stimulation frequency between Nitrate and Control soleus muscles.
Figure 1. Nitrate feeding increases contractile force in fast-twitch EDL muscles.
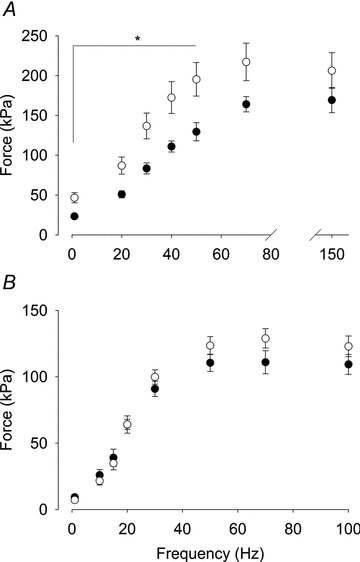
Mean force data (±SEM) for EDL (A) and soleus (B) vs. stimulation frequency. Note the break in the x-axis in panel A. Muscles from Control (filled symbols, n = 7) and Nitrate mice (open symbols; n = 7 for EDL, n = 6 for soleus). *P < 0.05.
The greater force at low stimulation frequencies (≤50 Hz) in EDL muscles from Nitrate mice suggests alterations in cellular Ca2+ handling (Allen et al. 2008). We therefore investigated the expression of proteins involved in SR Ca2+ release (CASQ, DHPR and RyR). Representative Western blots and plotted means relative to total protein are presented in Fig. 2. The expression of CASQ1, the dominating isoform in fast-twitch muscle (Beard et al. 2009), was significantly greater (P < 0.05) in EDL muscles from Nitrate compared to Control mice. DHPR protein expression was also significantly greater (P < 0.05) in EDL muscles from Nitrate compared to those from Control mice. No significant difference (P > 0.05) was found between groups for total RyR. We then examined CASQ1 and DHPR protein expression in the soleus. In contrast to the results from EDL, but in agreement with the lack of effect of nitrate ingestion on contractile force in the soleus, no difference in CASQ1 or DHPR protein expression was found (P > 0.05) (Fig. 2).
Figure 2. Nitrate feeding increases expression of SR Ca2+ handling proteins.
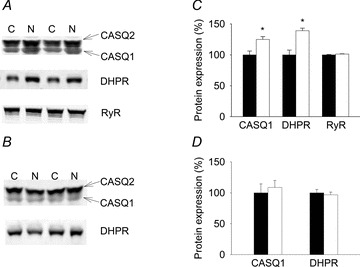
Representative Western blots (EDL: panel A; soleus: panel B). C, Control; N, Nitrate. Mean data (±SEM; EDL: panel C; soleus: panel D) for CASQ1, DHPR, and RyR. EDL for Control (n = 3–7) and Nitrate mice (n = 3–7). Soleus for Control (n = 6) and Nitrate mice (n = 4). Control is filled bar mean set to 100%; Nitrate is open bar. *P < 0.05.
Nitrate ingestion increases contractile force by increasing tetanic [Ca2+]i in fast-twitch muscle
Figure 3 depicts tetanic [Ca2+]i (Fig. 3A) and force (Fig. 3B) responses to 30 Hz stimulation in a representative Control and Nitrate fibre. Mean [Ca2+]i and force for Control and Nitrate fibres vs. stimulation frequency are shown in Fig. 3C and D, respectively. ANOVA revealed significantly higher tetanic [Ca2+]i from 20–150 Hz for Nitrate compared to Control (P < 0.05). Force up to 50 Hz stimulation was significantly greater for Nitrate compared to Control fibres (P < 0.05). However, at 70 Hz and higher frequencies, force was not significantly larger in the Nitrate than in the Control group, despite markedly higher [Ca2+]i in the former. This apparent discrepancy can be explained by the shape of the force–[Ca2+]i relationship and the fact that at higher frequencies, force approaches its maximum level. Therefore, force and [Ca2+]i obtained in contractions at 15–150 Hz were used to construct force–[Ca2+]i curves for each fibre. This analysis showed no difference in maximum force (Pmax: 394 ± 50 vs. 378 ± 11 kPa) or the [Ca2+]i giving 50% of Pmax (Ca50: 0.62 ± 0.03 vs. 0.57 ± 0.03 μm) between Control (n = 7) and Nitrate (n = 5) fibres, whereas the relationship was less steep in Nitrate fibres (N: 5.5 ± 0.8 vs. 2.9 ± 0.1). The mean force–[Ca2+]i curves for Control and Nitrate fibres are shown in Fig. 4A, where we have also included mean data for 30 and 100 Hz tetani. It can then be seen that the markedly higher force at 30 Hz in Nitrate fibres is due to increased [Ca2+]i combined with data points at this frequency lying on the steep part of the force–[Ca2+]i relationship. On the other hand, the lack of higher forces at 100 Hz in the Nitrate group despite higher [Ca2+]i is due to these data points lying on the part of the curve where force is close to the maximum.
Figure 3. Tetanic [Ca2+]i and force are increased in FDB fibres of nitrate fed mice.
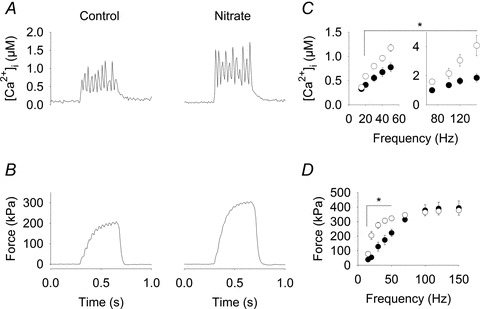
[Ca2+]i (A) and force traces (B) from representative Control and Nitrate fibres during 30 Hz stimulation. Mean data (±SEM) of tetanic [Ca2+]i (C) and force (D) vs. stimulation frequency. Note that in C, two axes are used for tetanic [Ca2+]i in order to show the marked differences between the groups both at low and high frequencies. Fibres from Control (filled symbols, n = 7) and Nitrate mice (open symbols, n = 5). *P < 0.05.
Figure 4. The effect of nitrate feeding on force is mediated via increased [Ca2+]i.
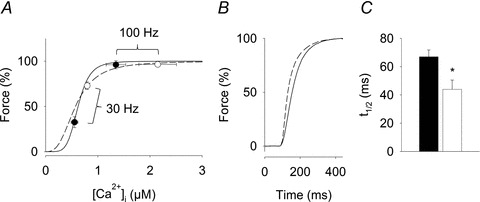
A, mean force–Ca2+ curves constructed from 15–150 Hz contractions given at 1 min intervals. Mean (±SEM) data for 30 and 100 Hz are included to illustrate how the force response to an increase in [Ca2+]i depends on the location on the force–Ca2+ curve. B, mean force records illustrating the faster rate of development of force in 100 Hz tetani. C, mean data (±SEM) for time to 50% max force (t1/2) in these tetani. Control (n = 7, filled symbols and bar, continuous line) and Nitrate (n = 5, open symbols and bar, dashed line). *P < 0.05.
Figure 4A illustrates the effect of the less steep force–[Ca2+]i relationship in Nitrate fibres. At [Ca2+]i higher than Ca50, the corresponding force is slightly lower than it would be with a steeper relationship (e.g. the 30 Hz data point). The opposite is true when [Ca2+]i is lower than Ca50, which in the present experiments only occurred with 15 Hz stimulation (see Fig. 3C and D).
The increase of force at the onset of contraction was used to assess whether the difference in tetanic [Ca2+]i between the groups at 100 Hz still had some effect on force production. This analysis revealed a markedly faster rate of force increase in Nitrate fibres with the time to achieve 50% of maximum force (t1/2) being ∼35% less in Nitrate than in Control fibres (Fig. 4B and C).
The greater CASQ1 expression and tetanic [Ca2+]i in fast-twitch muscles from Nitrate mice indicates a greater store of releasable Ca2+ within the SR. This assumption was examined in another group of fibres by generating 100 Hz tetani during exposure to 5 mm caffeine. During the caffeine experiments, indo-1 approached saturation and hence the conversion of ratios to [Ca2+]i became uncertain (i.e. small changes in ratio translate to large differences in [Ca2+]i). Data are therefore presented as ratios and 100 Hz stimulation in the presence of caffeine gave significantly greater ratios in the Nitrate (4.3 ± 0.2, n = 7) compared to the Control group (3.7 ± 0.1, n = 5), indicating greater SR Ca2+ stores in the Nitrate group.
There was a tendency for resting [Ca2+]i to be higher in Nitrate fibres (79 ± 9 nm) than in Control fibres (60 ± 9 nm) but this difference did not reach statistical significance (P = 0.16). A higher resting [Ca2+]i can, in principle, contribute to a higher tetanic [Ca2+]i by increasing the basal occupancy of Ca2+ binding sites in the cytoplasm and hence the same release of Ca2+ from the SR during stimulation would result in a higher [Ca2+]i. To test this possibility, we plotted [Ca2+]i in 120 Hz tetani vs. resting [Ca2+]i for the individual fibres (Fig. 5A). A positive correlation of tetanic to resting [Ca2+]i would be expected if tetanic [Ca2+]i critically depended on resting [Ca2+]i. Although there was a tendency for a positive correlation for Control fibres (slope 17 ± 10), Nitrate fibres showed the opposite tendency (−38 ± 10), and neither of these correlations was statistically significant. Similar results were obtained with other tetanic stimulation frequencies. Thus, these results do not support an important causative relation between elevated resting and increased tetanic [Ca2+]i.
Figure 5. Sarcoplasmic reticulum Ca2+ uptake is not altered in muscles of Nitrate mice.
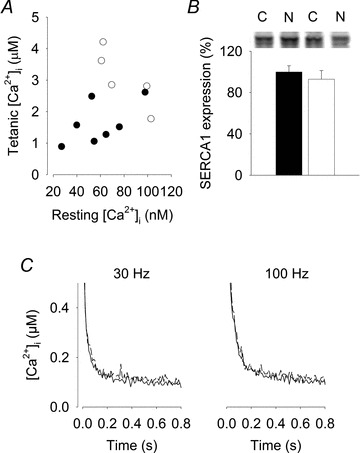
A, individual 120 Hz tetanic vs. resting [Ca2+]i data points for Control (filled circles) and Nitrate (open circles) fibres. B, representative Western blots and mean data (±SEM) of SERCA1 protein expression in EDL muscles from Control (C, n = 7, filled bar mean set to 100%) and Nitrate (N, n = 6, open bar). C, average records of [Ca2+]i tails after 30 and 100 Hz stimulation. Control (n = 7, continuous line) and Nitrate mice (n = 5, dashed line).
SERCA1 protein expression was not significantly different between Control and Nitrate groups (Fig. 5A). To investigate the possibility of changes in SR [Ca2+]i pumping and/or passive SR leak as a contributor for the measured increase in tetanic [Ca2+]i, [Ca2+]i tails after stimulation for Control and Nitrate fibres were examined at 30 and 100 Hz (Fig. 5B). [Ca2+]i transient decay (as assessed by [Ca2+]i 200 ms after the end of stimulation) was not different post-30 Hz (119 ± 12 vs. 107 ± 14 nm) or 100 Hz (155 ± 22 vs. 157 ± 17 nm) between Control and Nitrate fibres, respectively, indicating no difference in SR Ca2+ pumping or leak.
Discussion
The effect of nitrate ingestion over 7 days on skeletal muscle function was examined in this study. The amount of inorganic nitrate per kg body mass used in these experiments is similar to that given to humans in studies on blood pressure and performance and represents amounts that are easily achieved with a normal diet (Larsen et al. 2007; Larsen et al. 2011). Contractile force was measured in fast-twitch EDL and FDB, and slow-twitch soleus of Nitrate and Control mice. Contractile force was increased at low stimulation frequencies in fast-twitch, but not in slow-twitch muscles. This result was accompanied by increased expression of the Ca2+ handling proteins CASQ1 and DHPR, and increased SR Ca2+ content and tetanic [Ca2+]i.
The results from these experiments demonstrate a targeted effect of nitrate supplementation on contractile function in fast-twitch skeletal muscles. Ingestion of nitrate over a 7 day period enhanced SR Ca2+ release and tetanic force production via modifications to cellular Ca2+ handling components. The effect of elevated [Ca2+]i on lower frequency forces is larger due to the sigmoidal nature of the force–Ca2+ relationship (Westerblad et al. 1993): up to 50 Hz stimulation the relationship is steep and an increase in [Ca2+]i results in a marked force increase, whereas above 50 Hz forces approach the maximum and thus increased [Ca2+]i will have little effect (see Fig. 4A). Interestingly, the low frequencies of stimulation (15–50 Hz), where force is most augmented by nitrate ingestion, are similar to those recorded from rat EDL motoneurones during normal movements in vivo (see Table 1 in Hennig & Lømo, 1985). Furthermore, it should be noted that force production was not completely unaffected by the difference in [Ca2+]i at high stimulation frequencies because the rate of force development was markedly faster in the Nitrate than in the Control group, which is consistent with the faster force development when [Ca2+]i is increased by application of caffeine (Allen & Westerblad, 1995). A more rapid force development also has an important positive effect on muscle performance, especially during locomotion where contractions are of short duration (Slawinska & Kasicki, 2002).
The results of the current experiments with caffeine showed an increased Ca2+ content within the SR of FDB fibres in Nitrate mice. In accordance with this result, CASQ1 expression was increased in muscles from Nitrate mice. Increased expression of CASQ has been reported previously in skeletal muscle under a variety of conditions (Ferretti et al. 2009; Goodman et al. 2009; Novak & Soukup, 2011). CASQ is a high-capacity, low affinity Ca2+ buffer which maintains free [Ca2+] in the SR lumen at 1 mm during contraction when Ca2+ is first released from the SR and subsequently pumped back (Beard et al. 2004). Other workers have demonstrated that CASQ and SR Ca2+ regulate the SR Ca2+ release channels (RyR) and thus affect [Ca2+]i and force during contractions (Beard et al. 2004; Paolini et al. 2007; Aydin et al. 2009; Canato et al. 2010). Thus, increased CASQ1 in muscles from nitrate fed mice results in increased SR Ca2+ storage and in turn this increases Ca2+ release. The lack of an increase in CASQ1 expression in soleus coupled with no alterations in force production indicates SR Ca2+ storage and release are unaffected in slow-twitch muscle. The reason for the lack of effect in soleus is unclear, but may be related to the fact that the slow-twitch soleus has: (1) lower nitric oxide synthase content than fast-twitch muscle (Punkt et al. 2006) and (2) a high content of glutathione and antioxidant enzymes (Ji et al. 1992), which could counteract effects of circulating NO2− and NO.
Another interesting finding in the present set of experiments is that DHPR expression was significantly increased in fast-twitch muscles from Nitrate mice whereas RyR was unchanged. DHPRs are voltage sensors located in the t-tubules which relay action potential activation to the RyRs, opening them and producing SR Ca2+ release (Lamb, 2000). DHPRs in skeletal muscle form close connections with RyRs (Tanabe et al. 1990) with four DHPRs forming a complex that is associated with every other RyR (Block et al. 1988), and thus some ‘free’ RyRs exist. Whether the RyRs not directly associated with DHPRs contribute to Ca2+ release is not clear (Dulhunty et al. 2002) and increased DHPR content in the absence of increased RyR content could potentially lead to greater SR Ca2+ release. While there are no previous reports showing that increased DHPR content results in increased force production, DiFranco et al. (2011) showed that over-expression of the DHPR α subunit in mouse muscle resulted in increased charge movement but this extra charge movement did not cause any extra Ca2+ release. On the other hand, reductions in DHPR content have been shown to result in reduced force in skeletal muscle (Pietri-Rouxel et al. 2010).
Ingested inorganic nitrate is converted in the body to NO2−, NO and other nitrogen oxides. The physiological effects of nitrate intake are supposed to be mediated via these two metabolites (Weitzberg et al. 2010). It is worth noting that the present effects of nitrate feeding on muscle function are not similar to those obtained with acute exposure to traditional NO donors. For example, application of the NO donors S-nitroso-N-acetylcysteine and nitroprusside resulted in increased tetanic [Ca2+]i, reduced Ca2+ sensitivity and hence no change in force production (Andrade et al. 1998b). These effects were rapidly reversed after washout (Andrade et al. 1998b). Conversely, the major effect in the present study was an increase in tetanic [Ca2+]i and the effect remained throughout the experiments, which were performed in standard Tyrode solution without added NO2− or NO. Moreover, the increase in tetanic [Ca2+]i in the present study was accompanied by increased expression of the Ca2+ handling proteins CASQ1 and DHPR, which would not occur with acute exposure of NO donors. Thus, the longer-term nitrate supplementation used in the present study resulted in ‘structural’ (i.e. protein-linked) changes to skeletal muscle.
Conclusions
We show that fast-twitch skeletal muscles from mice provided nitrate in drinking water for 7 days display increased tetanic [Ca2+]i, which resulted in increased contractile force at low stimulation frequencies, which are those used most often for normal movement (Hennig & Lømo, 1985). These effects on intracellular Ca2+ handling are consistent with recent proposals (Bailey et al. 2010; Ferreira & Behnke, 2011). It must be emphasized that the changes measured here were due to altered protein expression and not simply acute effects of circulating nitrate and NO intermediates. Translating to the in vivo situation, our results show that fast-twitch muscle of Nitrate mice can be activated at a lower frequency to achieve the same force output, which would reduce the effort required for a given task. An additional benefit is that for a given torque or force output, the number of motor units needed to be recruited will be reduced without any increase in the time taken to achieve the target force.
Practical application of the present results is not limited to exercise performance. The changes in Ca2+ handling and involved proteins (e.g. CASQ1 and DHPR) indicate a potential therapeutic role for nitrate. For example, impaired Ca2+ handling in skeletal muscle has recently been reported in mitochondrial myopathy (Aydin et al. 2009). Specifically, Aydin et al. found that reduced CASQ1 mRNA and protein expression caused reduced tetanic [Ca2+]i and muscle weakness in a mouse model of mitochondrial myopathy. Thus, nitrate supplementation may be used to improve contractile function in diseases with muscle weakness involving impaired Ca2+ handling. This is also interesting from a nutritional perspective since several leafy green vegetables are high in inorganic nitrate and the amount of nitrate used in the present study can be easily achieved by adopting a ‘green’ diet.
Acknowledgments
This work was supported by funds from the Swedish Research Council, the Swedish National Center for Sports Research, Association Française Contre les Myopathies (AFM), and by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) Grant 1F32AR057619 (to Andrés Hernández). The authors thank Michael Hezel for consultation and assistance with the nitrate ingestion protocol.
Glossary
- [Ca2+]i
myoplasmic free [Ca2+]
- CASQ
calsequestrin
- DHPR
dihydropyridine receptor
- EDL
extensor digitorum longus
- FDB
flexor digitorum brevis
- SR
sarcoplasmic reticulum
- t1/2
half-time
Author contributions
All authors except A.J.C. contributed to the conception and design of the study. A.H., N.I., A.J.C. and J.D.B. were responsible for collection of data. A.H., N.I., J.D.B. and H.W. had the main responsibility for analysis and interpretation of data but all authors contributed. All authors were involved in drafting and/or revising the manuscript, and all approved the final version. Experiments were performed at the Cellular Muscle Function Laboratory in the Department of Physiology and Pharmacology at Karolinska Institutet, Stockholm, Sweden.
References
- Allen DG, Lamb GD, Westerblad H. Skeletal muscle fatigue: cellular mechanisms. Physiol Rev. 2008;88:287–332. doi: 10.1152/physrev.00015.2007. [DOI] [PubMed] [Google Scholar]
- Allen DG, Westerblad H. The effects of caffeine on intracellular calcium, force and the rate of relaxation of mouse skeletal muscle. J Physiol. 1995;487:331–342. doi: 10.1113/jphysiol.1995.sp020883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade FH, Reid MB, Allen DG, Westerblad H. Effect of hydrogen peroxide and dithiothreitol on contractile function of single skeletal muscle fibres from the mouse. J Physiol. 1998a;509:565–575. doi: 10.1111/j.1469-7793.1998.565bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade FH, Reid MB, Allen DG, Westerblad H. Effect of nitric oxide on single skeletal muscle fibres from the mouse. J Physiol. 1998b;509:577–586. doi: 10.1111/j.1469-7793.1998.577bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aydin J, Andersson DC, Hänninen SL, Wredenberg A, Tavi P, Park CB, Larsson NG, Bruton JD, Westerblad H. Increased mitochondrial Ca2+ and decreased sarcoplasmic reticulum Ca2+ in mitochondrial myopathy. Human Mol Genet. 2009;18:278–288. doi: 10.1093/hmg/ddn355. [DOI] [PubMed] [Google Scholar]
- Bailey SJ, Fulford J, Vanhatalo A, Winyard PG, Blackwell JR, DiMenna FJ, Wilkerson DP, Benjamin N, Jones AM. Dietary nitrate supplementation enhances muscle contractile efficiency during knee-extensor exercise in humans. J Appl Physiol. 2010;109:135–148. doi: 10.1152/japplphysiol.00046.2010. [DOI] [PubMed] [Google Scholar]
- Bailey SJ, Winyard P, Vanhatalo A, Blackwell JR, DiMenna FJ, Wilkerson DP, Tarr J, Benjamin N, Jones AM. Dietary nitrate supplementation reduces the O2 cost of low-intensity exercise and enhances tolerance to high-intensity exercise in humans. J Appl Physiol. 2009;107:1144–1155. doi: 10.1152/japplphysiol.00722.2009. [DOI] [PubMed] [Google Scholar]
- Beard NA, Laver DR, Dulhunty AF. Calsequestrin and the calcium release channel of skeletal and cardiac muscle. Prog Biophys Mol Biol. 2004;85:33–69. doi: 10.1016/j.pbiomolbio.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Beard NA, Wei L, Dulhunty AF. Ca2+ signaling in striated muscle: the elusive roles of triadin, junctin, and calsequestrin. Eur Biophys J. 2009;39:27–36. doi: 10.1007/s00249-009-0449-6. [DOI] [PubMed] [Google Scholar]
- Block BA, Imagawa T, Campbell KP, Franzini-Armstrong C. Structural evidence for direct interaction between the molecular components of the transverse tubule/sarcoplasmic reticulum junction in skeletal muscle. J Cell Biol. 1988;107:2587–2600. doi: 10.1083/jcb.107.6.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canato M, Scorzeto M, Giacomello M, Protasi F, Reggiani C, Stienen GJ. Massive alterations of sarcoplasmic reticulum free calcium in skeletal muscle fibers lacking calsequestrin revealed by a genetically encoded probe. Proc Natl Acad Sci U S A. 2010;107:22326–22331. doi: 10.1073/pnas.1009168108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cemak NM, Gibala MJ, van Loon LJ. Nitrate supplementation's improvement of a 10-km time-trial performance in trained cyclists. Int J Sports Exerc Metab. 2012;22:64–71. doi: 10.1123/ijsnem.22.1.64. [DOI] [PubMed] [Google Scholar]
- DiFranco M, Tran P, Quinonez M, Vergara JL. Functional expression of transgenic 1sDHPR channels in adult mammalian skeletal muscle fibres. J Physiol. 2011;589:1421–1442. doi: 10.1113/jphysiol.2010.202804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulhunty AF, Haarmann CS, Green D, Laver DR, Board PG, Casarotto MG. Interactions between dihydropyridine receptors and ryanodine receptors in striated muscle. Prog Biophys Mol Biol. 2002;79:45–75. doi: 10.1016/s0079-6107(02)00013-5. [DOI] [PubMed] [Google Scholar]
- Ferreira LF, Behnke BJ. A toast to health and performance! Beetroot juice lowers blood pressure and the O2 cost of exercise. J Appl Physiol. 2011;110:585–586. doi: 10.1152/japplphysiol.01457.2010. [DOI] [PubMed] [Google Scholar]
- Ferretti R, Marques MJ, Pertille A, Santo Neto H. Sarcoplasmic-endoplasmic-reticulum Ca2+-ATPase and calsequestrin are overexpressed in spared intrinsic laryngeal muscles of dystrophin-deficient mdx mice. Muscle Nerve. 2009;39:609–615. doi: 10.1002/mus.21154. [DOI] [PubMed] [Google Scholar]
- Goodman CA, Horvath D, Stathis C, Mori T, Croft K, Murphy RM, Hayes A. Taurine supplementation increases skeletal muscle force production and protects muscle function during and after high-frequency in vitro stimulation. J Appl Physiol. 2009;107:144–154. doi: 10.1152/japplphysiol.00040.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennig R, Lømo T. Firing patterns of motor units in normal rats. Nature. 1985;314:164–166. doi: 10.1038/314164a0. [DOI] [PubMed] [Google Scholar]
- Ji LL, Fu R, Mitchell EW. Glutathione and antioxidant enzymes in skeletal muscle: effects of fiber type and exercise intensity. J Appl Physiol. 1992;73:1854–1859. doi: 10.1152/jappl.1992.73.5.1854. [DOI] [PubMed] [Google Scholar]
- Klein MG, Kovacs L, Simon BJ, Schneider MF. Decline of myoplasmic Ca2+, recovery of calcium release and sarcoplasmic Ca2+ pump properties in frog skeletal muscle. J Physiol. 1991;441:639–671. doi: 10.1113/jphysiol.1991.sp018771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb GD. Excitation-contraction coupling in skeletal muscle: comparisons with cardiac muscle. Clin Exp Pharmacol Physiol. 2000;27:216–224. doi: 10.1046/j.1440-1681.2000.03224.x. [DOI] [PubMed] [Google Scholar]
- Lansley KE, Winyard PG, Bailey SJ, Vanhatalo A, Wilkerson DP, Blackwell JR, Gilchrist M, Benjamin N, Jones AM. Acute dietary nitrate supplementation improves cycling time trial performance. Med Sci Sports Exerc. 2011a;43:1125–1131. doi: 10.1249/MSS.0b013e31821597b4. [DOI] [PubMed] [Google Scholar]
- Lansley KE, Winyard PG, Fulford J, Vanhatalo A, Bailey SJ, Blackwell JR, DiMenna FJ, Gilchrist M, Benjamin N, Jones AM. Dietary nitrate supplementation reduces the O2 cost of walking and running: a placebo-controlled study. J Appl Physiol. 2011b;110:591–600. doi: 10.1152/japplphysiol.01070.2010. [DOI] [PubMed] [Google Scholar]
- Larsen FJ, Schiffer TA, Borniquel S, Sahlin K, Ekblom B, Lundberg JO, Weitzberg E. Dietary inorganic nitrate improves mitochondrial efficiency in humans. Cell Metab. 2011;13:149–159. doi: 10.1016/j.cmet.2011.01.004. [DOI] [PubMed] [Google Scholar]
- Larsen FJ, Weitzberg E, Lundberg JO, Ekblom B. Effects of dietary nitrate on oxygen cost during exercise. Acta Physiol. 2007;191:59–66. doi: 10.1111/j.1748-1716.2007.01713.x. [DOI] [PubMed] [Google Scholar]
- Larsen FJ, Weitzberg E, Lundberg JO, Ekblom B. Dietary nitrate reduces maximal oxygen consumption while maintaining work performance in maximal exercise. Free Radic Biol Med. 2010;48:342–347. doi: 10.1016/j.freeradbiomed.2009.11.006. [DOI] [PubMed] [Google Scholar]
- Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov. 2008;7:156–167. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- Lännergren J, Westerblad H. The temperature dependence of isometric contractions of single, intact fibres dissected from a mouse foot muscle. J Physiol. 1987;390:285–293. doi: 10.1113/jphysiol.1987.sp016700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy RM, Larkins NT, Mollica JP, Beard NA, Lamb GD. Calsequestrin content and SERCA determine normal and maximal Ca2+ storage levels in sarcoplasmic reticulum of fast- and slow-twitch fibres of rat. J Physiol. 2009;587:443–460. doi: 10.1113/jphysiol.2008.163162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak P, Soukup T. Calsequestrin distribution, structure and function, its role in normal and pathological situations and the effect of thyroid hormones. Physiol Res. 2011;60:439–452. doi: 10.33549/physiolres.931989. [DOI] [PubMed] [Google Scholar]
- Paolini C, Quarta M, Nori A, Boncompagni S, Canato M, Volpe P, Allen PD, Reggiani C, Protasi F. Reorganized stores and impaired calcium handling in skeletal muscle of mice lacking calsequestrin-1. J Physiol. 2007;583:767–784. doi: 10.1113/jphysiol.2007.138024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietri-Rouxel F, Gentil C, Vassilopoulos S, Baas D, Mouisel E, Ferry A, Vignaud A, Hourde C, Marty I, Schaeffer L, Voit T, Garcia L. DHPR α1S subunit controls skeletal muscle mass and morphogenesis. EMBO J. 2010;29:643–654. doi: 10.1038/emboj.2009.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punkt K, Fritzsche M, Stockmar C, Hepp P, Josten C, Wellner M, Schering S, Buchwalow IB. Nitric oxide synthase in human skeletal muscle related to defined fibre types. Histochem Cell Biol. 2006;125:567–573. doi: 10.1007/s00418-005-0108-7. [DOI] [PubMed] [Google Scholar]
- Slawinska U, Kasicki S. Altered electromyographic activity pattern of rat soleus muscle transposed into the bed of antagonist muscle. J Neurosci. 2002;22:5808–5812. doi: 10.1523/JNEUROSCI.22-14-05808.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe T, Beam KG, Adams BA, Niidome T, Numa S. Regions of the skeletal muscle dihydropyridine receptor critical for excitation-contraction coupling. Nature. 1990;346:567–569. doi: 10.1038/346567a0. [DOI] [PubMed] [Google Scholar]
- Vanhatalo A, Bailey SJ, Blackwell JR, DiMenna FJ, Pavey TG, Wilkerson DP, Benjamin N, Winyard PG, Jones AM. Acute and chronic effects of dietary nitrate supplementation on blood pressure and the physiological responses to moderate-intensity and incremental exercise. Am J Physiol Regul Integr Comp Physiol. 2010;299:R1121–1131. doi: 10.1152/ajpregu.00206.2010. [DOI] [PubMed] [Google Scholar]
- Weitzberg E, Hezel M, Lundberg JO. Nitrate-nitrite-nitric oxide pathway: implications for anesthsiology and intensive care. Anesthesiology. 2010;113:1460–1475. doi: 10.1097/ALN.0b013e3181fcf3cc. [DOI] [PubMed] [Google Scholar]
- Westerblad H, Allen DG. The role of sarcoplasmic reticulum in relaxation of mouse muscle; effects of 2,5-di(tert-butyl)-1,4-benzohydroquinone. J Physiol. 1994;474:291–301. doi: 10.1113/jphysiol.1994.sp020022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H, Duty S, Allen DG. Intracellular calcium concentration during low-frequency fatigue in isolated single fibers of mouse skeletal muscle. J Appl Physiol. 1993;75:382–388. doi: 10.1152/jappl.1993.75.1.382. [DOI] [PubMed] [Google Scholar]


