Abstract
At the onset of skeletal muscle repetitive contractions, there is a significant delay in the time to achieve oxidative phosphorylation steady state. The purpose of the present study was to examine the factors that limit oxidative phosphorylation at the onset of contractions. NAD(P)H was measured in real time during two contractile periods (2 min each) separated by 5 min of rest in intact single muscle fibres (n = 7) isolated from Xenopus laevis. The fibres were then loaded with the dye tetramethylrhodamine methyl ester perchlorate (TMRM) to evaluate the kinetics of the mitochondrial membrane potential (Δψm) during two further successive contractile periods. At the onset of contractions in the first period, NAD(P)H exhibited a time delay (14.1 ± 1.3 s) before decreasing toward a steady state. In contrast, Δψm decreased immediately after the first contraction and started to be reestablished after 10.7 ± 0.9 s, with restoration to the pre-stimulation values after approximately 32 s. In the second contractile period (5 min after the first), NAD(P)H decreased immediately (i.e. no time delay) after the first contraction and had a significantly shorter time constant compared to the first contractile bout (3.3 ± 0.3 vs. 5.0 ± 0.2 s, P < 0.05). During the second bout, Δψm remained unchanged from pre-stimulation values. These results suggest: (1) that at the onset of contractions, oxidative phosphorylation is primarily limited by the activity of the electron transport chain complexes rather than by a limited level of substrates; and (2) when the muscle is ‘primed’ by previous contractile activity, the faster enhancement of the cellular respiratory rate is due to intrinsic factors within the myofibre.
Key points
During the transition in skeletal muscle from rest to steady state contractions, O2 consumption is limited and the exact mechanisms controlling respiration in intact cells are not completely understood.
Previous contractile activity can ‘prime’ skeletal muscle resulting in a faster enhancement of the O2 consumption during a subsequent bout of contractions.
Here, we showed in intact single muscle fibres that the mitochondrial electron transport chain was activated faster at the onset of contractions when the muscle cells were previously ‘primed’ by contractile activity.
Therefore, factors intrinsic to the muscle cells have a role in the delayed increase of mitochondrial respiration during the onset of contractions. Also, the control of mitochondrial respiration in intact cells is not simply dependent on substrate availability or a simple feedback mechanism but rather on a more complex system.
Introduction
The mechanism of ATP synthesis by oxidative phosphorylation was elucidated by Peter Mitchell's chemiosmotic theory in one of the most important findings in the life sciences of the past century (Huttemann et al. 2008; Nicholls, 2008). Yet the regulation of oxidative phosphorylation is complex and different control mechanisms remain under debate (Balaban, 2009; Ramzan et al. 2010). Mitochondrial interactions with other cellular components, mitochondria to mitochondria interactions, or cell to cell interactions can modulate mitochondrial bioenergetics (Brand & Nicholls, 2011). These complex interactions make the analysis of mitochondrial bioenergetics using intact cell models (vs. isolated mitochondrial models) of great importance. In fact, a recent study (Picard et al. 2011) has demonstrated that mitochondria isolated from skeletal muscle have functional and structural characteristics that differ significantly from intact mitochondria that remain within permeabilized or intact myofibres. Compared to mitochondria that remained within the cellular domain, isolated mitochondria demonstrated alterations in morphology, sensitivity to Ca2+, respiration, and a noticeable increase of the H2O2 generation (Picard et al. 2011). In fact, isolated mitochondrial preparations do not demonstrate any O2 uptake time delay or slowed kinetic response in the adjustment from state IV to state III steady state rate of respiration – which is remarkably different compared to this response in mitochondria within intact systems (Rumsey et al. 1990).
Skeletal muscle contractile activity allows a robust model to study mechanisms controlling oxidative phosphorylation. This model features large variations in the rate of ATP utilization during transitions from rest to contractile activity. In skeletal muscle, anaerobic processes support the increased energy demand during the beginning of a contractile period (Walsh et al. 2008). Muscle oxygen consumption, on the other hand, exhibits a slow and limited increase during the initial seconds that follow the onset of contractile activity (Grassi et al. 1996, 1998a, b; Bangsbo et al. 2000; Gurd et al. 2006), thereby limiting ATP production by oxidative phosphorylation at the onset of exercise. In contracting skeletal muscle, O2 consumption can be limited if O2 delivery is insufficient (Richardson et al. 1999; Grassi et al. 2000; Jones et al. 2006; Goodwin et al. 2012). However, there is substantial evidence that under normal or even under enhanced convective O2 delivery or peripheral O2 diffusion, a delay preceding the increase in O2 consumption still occurs (Grassi et al. 1996, 1998a, b; Bangsbo et al. 2000; Campbell- O'Sullivan et al. 2002), which may result from intrinsic factors limiting O2 consumption during the transition from rest to contractions (Grassi et al. 1996, 1998a, b; Bangsbo et al. 2000; Campbell-O'Sullivan et al. 2002).
Interestingly, it has been demonstrated that the execution of a preceding exercise bout can accelerate pulmonary and muscle O2 consumption on-kinetics during a subsequent period of exercise (Macdonald et al. 1997; Hogan, 2001; Campbell-O'Sullivan et al. 2002; Gurd et al. 2006; Jones et al. 2006; Hernandez et al. 2010; Bowen et al. 2012). However it remains uncertain to whether the faster O2 consumption kinetics seen in ‘primed’ muscle is due to changes in the O2 delivery or diffusion in the myofibres or to intrinsic factors within the myofibre.
The use of an intact single skeletal muscle fibre model permits fluorescence measurement of metabolites and membrane potentials in real time in a fully functioning cell, and also avoids complicating factors such fibre-type uncertainty, and inhomogeneity of blood flow and oxygen delivery. Our laboratory has investigated the changes in intracellular  (
( ) in contracting single intact Xenopus muscle fibres and found that a time delay occurs before the fall in
) in contracting single intact Xenopus muscle fibres and found that a time delay occurs before the fall in 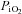 after the initiation of contractions (Hogan, 2001; Howlett & Hogan, 2003). In this model, O2 availability to mitochondria is determined only by diffusive factors and changes in
after the initiation of contractions (Hogan, 2001; Howlett & Hogan, 2003). In this model, O2 availability to mitochondria is determined only by diffusive factors and changes in  reflect changes in
reflect changes in  (Howlett & Hogan, 2003). This observation suggests that oxygen availability does not limit mitochondrial respiration during the initial phase of a contractile period in single fibres (Hogan, 2001). Following a short rest period (5 min),
(Howlett & Hogan, 2003). This observation suggests that oxygen availability does not limit mitochondrial respiration during the initial phase of a contractile period in single fibres (Hogan, 2001). Following a short rest period (5 min),  decreased faster in these same fibres during the initial moments of a subsequent contractile period (Hogan, 2001). Because O2 availability was the same in both contractile periods, these results suggests that the faster up-regulation of oxidative phosphorylation during the second contractile activity was caused by changes in intracellular factors other than O2 availability (Hogan, 2001).
decreased faster in these same fibres during the initial moments of a subsequent contractile period (Hogan, 2001). Because O2 availability was the same in both contractile periods, these results suggests that the faster up-regulation of oxidative phosphorylation during the second contractile activity was caused by changes in intracellular factors other than O2 availability (Hogan, 2001).
The driving force of oxidative phosphorylation is the proton motive force (Δp). Δp is composed of chemical potential (ΔpH) and an electrical potential (Δψm, the mitochondrial membrane potential) across the mitochondrial inner membrane, which are established by the electron transport chain (ETC), which pumps protons from the mitochondrial matrix to the intermembrane space (Nicholls & Ferguson, 2002). Proton pumping by the ETC is dependent on the oxidation of reducing equivalents (e.g. NADH) and the availability of O2, the final acceptor of the electrons, and can be limited by high values of Δψm, which is the major determinant of Δp (Nicholls & Ferguson, 2002). Consequently, the link between NADH oxidation in mitochondria and Δψm constitutes factors that are suitable indicators of the activity of the respiratory chain complexes. Thus, the analysis of the NADH and Δψm kinetics during the transition from rest to contractions can be used to address questions concerning limiting factors and the control of oxidative phosphorylation at the onset of contractions. In addition, measurements of NADH and Δψm kinetics may help to explain how a prior contractile activity results in a faster up-regulation of oxidative phosphorylation during a subsequent contractile period.
The aim of the present study was to determine the kinetic response and the factors limiting oxidative phosphorylation at the onset of contractions, and to investigate mitochondrial bioenergetics onset kinetics in ‘primed’ skeletal muscle. To address these issues, NAD(P)H autofluorescence and the Δψm kinetics were measured during two identical contractile periods, separated by 5 min of rest, in highly oxidative fatigue resistant intact single skeletal muscle fibres. We hypothesized that after a ‘priming’ contractile period, the enhancement in the oxidative phosphorylation rate would be faster during the transition from rest to contractile activity and that this would result in faster kinetic responses of the NAD(P)H and the Δψm.
Methods
Animals and ethical approval
All procedures were approved by the University of California, San Diego institutional animal care and use committee and conform to the guidelines of the American Physiological Society. Adult female Xenopus laevis were double pithed and decapitated, and the lumbrical muscles (II–IV) were removed. Intact single muscle fibres were microdissected under dark field illumination and only highly oxidative, fatigue resistant fibres were chosen as described previously (Stary et al. 2004). Dissections and experiments were performed in Ringer solution (116.5 mm NaCl, 2 mm KCl, 1.9 mm CaCl2, 2 mm NaH2PO4, 0.1 mm EGTA, pH 7.0) at room temperature (20–22°C).
After dissection, platinum clips were attached to the fibre tendons and mounted in a single muscle strip myograph (model 920CS, Danish Myo Technology (DMT), Aarhus, Denmark) and placed on the stage of an inverted microscope. Tetanic contractions were evoked by end to end electrical stimulation (70 Hz, 250 ms train duration, 2 ms monophasic pulses, 8 V) using a Grass S48 stimulator (Quincy, MA, USA). Tension development was measured with a force transducer system (model KG4, 0–50 mN, DMT). A Biopac System MP100WSW (Santa Barbara, CA, USA) A-D converter was used to convert the analog signal to digital, and the data were analysed with AcqKnowledgeIII 3.2.6 software (Biopac Systems). Fibre length was adjusted to achieve the maximal isometric tetanic tension (L0, at 70 Hz). After setting L0, fibres were allowed to rest for 40 min. The average diameter of the fibres was 92.1 ± 19.5 μm (n = 7) while the average tetanic tension at 70 Hz was 234 ± 28 kPa.
Experimental protocol
Single fibres were stimulated for two identical 2 min periods of repetitive tetanic contractions (70 Hz, 250 ms train, 2 ms pulses, one contraction every 2 s) separated by 5 min of rest. A previous study demonstrated that Xenopus single fibres contracting under the same stimulation protocol reach close to a maximal O2 consumption rate (Howlett & Hogan, 2001). In these two contractile periods, NAD(P)H autofluorescence was measured as described below in detail. In order to confirm that the fibre type was fatigue resistant, a fatiguing repetitive contractile period (fatigue protocol) was carried out according to a previously established criterion (Stary et al. 2004). The fatigue protocol consisted of a series of repeated tetanic contractions (70 Hz, 250 ms train, 2 ms pulses, 8 V) with increments in train frequency every 2 min (0.25, 0.33, 0.5 and 1 contractions per sec) as exemplified in Fig. 1A. The fatigue protocol was carried out either 60 min before or 60 min after the first two contractile bouts. No differences in the NADH traces were observed among these fibres, confirming that 60 min was enough time to reestablish a pre-stimulation state (see Fig. 3F).
Figure 1. Representative isometric tension development recordings during a fatigue protocol (A) and during the four contractile periods (0.5 contractions per second) performed while measuring NAD(P)H and TMRM fluorescence (B).
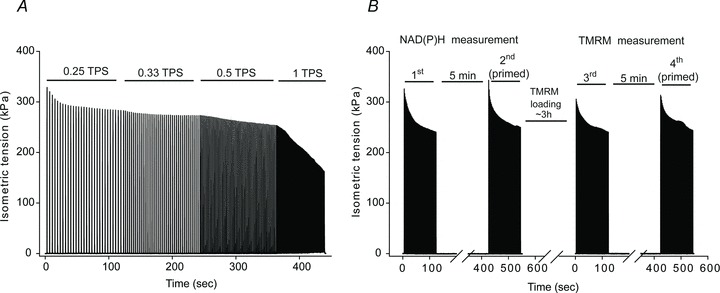
The time between the NADH and TMRM measurement was approximately 210 min.
Figure 3. NAD(P)H and TMRM fluorescence of single Xenopus muscle fibres in response to mitochondrial membrane depolarization, low Po2, inhibition of complex IV and V and the ‘priming’ effect resulted from previous contractile activity.

A and B, NAD(P)H autofluorescence variations after partial mitochondrial uncoupling by the addition of CCCP (A) or during perfusion at low Po2 (∼4 Torr) (B). C–E, TMRM whole cell fluorescence changes (C) after inhibition of ATP synthase by oligomycin, after Δψm collapse by the addition of CCCP (D); and during contractions when the respiratory chain was inhibited by the addition of sodium cyanide (○) and then washed out (•) (n = 3) (E). F, NAD(P)H fluorescence changes in one fibre during contractile bouts under non-primed and primed conditions, performed 5 min apart (non-primed 1 (•); primed 1 (○)) and after losing the priming effect after 60 min of rest (non-primed 2 (▪)) and again under a primed condition, 5 min after the previous bout (primed 2 (□)). NAD(P)H fluorescence was measured every 3 s.
After the first two contractile periods, the fibres were rested for approximately 3.5 h while tetramethylrhodamine methyl ester perchlorate (TMRM) was loaded in order to measure the Δψm, as will be further detailed. After TMRM loading, the fibres were stimulated in the same manner as for the first two contractile periods (2 × 2 min at 70 Hz, 250 ms train, 2 ms pulses, 0.5 contractions per second, separated by 5 min of rest) as shown in Fig. 1B.
Analysis of the NAD(P)H and Δψm by fluorescence spectroscopy
NAD(P)H and Δψm kinetics were obtained by fluorescence spectroscopy using a Photon Technology International (Birmingham, NJ, USA) illumination and detection system (DeltaScan model), integrated with a Nikon inverted microscope with a 40× Fluor objective. All reagents were from Sigma-Aldrich (St Louis, MO, USA) unless specified. When dimethyl sulphoxide (DMSO) was used to solubilize the reagents, the final concentration of DMSO in Ringer solution was always less than 0.1%. NAD(P)H, and TMRM fluorescence readings were collected continuously during contractions. In order to eliminate the movement artifact resulting from the fibre contraction, only the fluorescence points during the time frame between the contractions were analysed.
Analysis of the NAD(P)H kinetics during contractions
To monitor NAD(P)H in the first two contractile periods, the fibres were illuminated with an excitation wavelength of 350 nm and the fluorescence emission signal at 450 nm was recorded. The relative contribution of NADH and NADPH to the blue autofluorescence is unknown and so the signal can be a combination of both (Mayevsky & Rogatsky, 2007). Nevertheless, the specificity of the autofluorescence to mitochondrial NAD(P)H content in skeletal muscle is well characterized (Hogan et al. 2005; Mayevsky & Rogatsky, 2007).
To evaluate the NAD(P)H kinetics, a monoexponential curve fit model incorporating a time delay was used (eqn (1)).
| (1) |
Parameter values (A and y0) were fixed and obtained from the unfitted data. In eqn (1), y0 indicates the value of the lowest value attained during the monoexponential decay; A, the amplitude between the y0 attained and the value before the start of the decrease; td, the time delay; and τ the time constant of the function for the fundamental component.
In order to demonstrate that the autofluorescence in our system corresponded to changes in the mitochondrial NAD(P)H, fluorescence was measured under control conditions. When mitochondria are partially uncoupled, such as by the addition of a small amount of a proton ionophore, NADH oxidation by complex I is enhanced in order to avoid the disruption of the Δψm and consequently NAD(P)H fluorescence should decrease. For this, mitochondria were partially uncoupled by the addition of 20 nm of carbonyl cyanide 3-chlorophenylhydrazone (CCCP). Conversely, when the electron transport chain is inhibited, such as by the absence of O2, NADH oxidation rate by complex I is decreased and the NAD(P)H fluorescence is expected to increase. We superfused the fibre with Ringer solution pre-equilibrated with 100% N2, which reduced the extracellular  in the experimental chamber to approximately 4 Torr (
in the experimental chamber to approximately 4 Torr ( was measured using a fibre optic oxygen sensor, Oxymicro; World Precision Instruments, Sarasota, FL, USA). These control experiments were conducted in a separate group of fibres.
was measured using a fibre optic oxygen sensor, Oxymicro; World Precision Instruments, Sarasota, FL, USA). These control experiments were conducted in a separate group of fibres.
Analysis of Δψm kinetics during contractions
To monitor changes in Δψm during the last two contractile periods, fibres were equilibrated with 150 nm of the fluorescent probe tetramethylrhodamine methyl ester perchlorate (TMRM; Invitrogen, Carlsbad, CA, USA). TMRM is a lipophilic cationic dye that preferentially accumulates in the mitochondrial matrix according to the Nernst equation (Ward et al. 2000; Nicholls et al. 2003; Ward, 2010). At the concentration used in the present study, TMRM fluorescence in the mitochondrial matrix is self-quenched (Ward et al. 2000; Nicholls et al. 2003; Ward, 2010). Under this condition when Δψm is decreased (i.e. depolarized), TMRM moves from the mitochondrial matrix to the cytosol increasing the whole cell fluorescence (Ward, 2010). When the Δψm is repolarized, TMRM returns to the matrix, decreasing the whole cell fluorescence emission (Ward, 2010). TMRM loading was carried out at room temperature in the dark. The TMRM signal was obtained by illuminating the fibres at 550 nm and collecting the emission at 580 nm. While loading the fibre with TMRM, fluorescence emission was followed for 2 s every 10 min up to a plateau, indicating that an equilibrium was attained between the bath and the cytosol, which occurred in approximately 3.5 h. All fluorescence measurements during contractions were carried out in the presence of 150 nm TMRM in the experimental solution. Due to the different patterns observed for the Δψm kinetics during the two successive contractile periods, the kinetic parameters of the change in the TMRM fluorescence were obtained directly from the data points with a resolution of 2 s.
The reliability of TMRM fluorescence as an indicator of Δψm was confirmed by experiments in a separate group of fibres with the addition of either oligomycin (2 μg ml−1), CCCP (1 μm) or sodium cyanide (1 mm). In the presence of sodium cyanide, fibres were electrically stimulated at 0.5 trains per second. Following contractions in the presence of this compound, fibres were washed with Ringer solution and rested for 1 h. Then these fibres were stimulated to contract again in control conditions.
Data analysis
Values are given as means ± standard error of the mean. To analyse the changes occurring at different time points during a same contractile period, one-way ANOVA followed by Tukey's test was used. To analyse the differences occurring during different contractile periods over time, two-way ANOVA followed by the Bonferroni test was used. All the analyses were carried out using GraphPad Prism v. 4.00 for Windows (GraphPad Software, San Diego, CA, USA) and P < 0.05 was considered statistically significant.
Results
Changes in tension generation during the repetitive contractions
The changes in tension developed by the fibres (n = 7) throughout the four contractile bouts performed while measuring NAD(P)H and TMRM fluorescence are illustrated in Fig. 2. While the initial force of the second bout was 14.2 ± 2.5% higher than for the first contraction of the first bout (P < 0.05, Fig. 2), this difference was smaller when the first five contractions were compared (approximately 7% higher during the 2nd compared to the 1st bout; P < 0.05) and no differences in force development between the first and the third and the fourth bouts were observed. For the four contractile bouts, the final force was reduced approximately 22% compared to the initial force development during each contractile period at the end of the 120 s.
Figure 2. Change in relative isometric tetanic force development during the 120 s contractile periods.

All 4 contractile bouts are normalized by the initial contraction from the first contractile bout, which NAD(P)H was measured. *P < 0.05 vs. 1st bout and vs. 3rd bout (two-way ANOVA).
Characterization of the NAD(P)H and Δψm fluorescent signals
The addition of a small amount of CCCP causes a partial loss of the electrochemical gradient and consequently enhances the oxidation of NADH. NAD(P)H autofluorescence decreased after the mitochondria were partially uncoupled by the addition of 20 nm CCCP (Fig. 3A). In contrast, under a limited O2 availability, oxidation of NADH is expected to decrease. At low extracellular  NAD(P)H fluorescence increased (Fig. 3B). These results demonstrate how the NAD(P)H signal varies under different oxidative phosphorylation rates and confirms the integrity of our fluorescent NAD(P)H signal.
NAD(P)H fluorescence increased (Fig. 3B). These results demonstrate how the NAD(P)H signal varies under different oxidative phosphorylation rates and confirms the integrity of our fluorescent NAD(P)H signal.
As expected, the addition of the ATP synthase inhibitor oligomycin, resulted in a decrease of the TMRM fluorescence indicating an increase of Δψm (Fig. 3C) (Nicholls et al. 2003). Collapsing the Δψm with CCCP caused a steep increase of the TMRM as previously reported (Fig. 3D) (Ward et al. 2000; Nicholls et al. 2003; Ward, 2010). The spike of the TMRM signal was followed by a very slow decay of the fluorescence resulting from the restoration of the Nernst equilibrium of TMRM between the cytosol and the bath (Ward et al. 2000; Nicholls et al. 2003; Ward, 2010).
The dependence of the whole cell TMRM fluorescence on Δψm was confirmed when fibres were stimulated in the presence of cyanide, a complex IV inhibitor. In the presence of cyanide, the TMRM fluorescence increased continuously during the contractile bout (Fig. 3E), indicating a progressive loss of Δψm. After washing out the cyanide, the fluorescence transiently increased during the first contractions and then was subsequently restored towards baseline (Fig. 3E), as normally observed in control conditions for all fibres analysed in the present study. These results confirm the integrity of our Δψm signal.
NAD(P)H and Δψm responses during repetitive contractions
Figure 4A shows the NAD(P)H fluorescence response from fibres (n = 7) during the time course of the contractile bouts. During the first contractile bout, NAD(P)H fluorescence decreased exponentially after an initial time delay of 14.5 ± 1.3 s and was significantly less than the value observed at rest from the 18 s time point through the end of stimulation (P < 0.05). Unlike the first contractile bout, the kinetics of the NAD(P)H signal during the second contractile bout did not demonstrate a time delay (td = 0.3 ± 0.3 s). During the second bout, the NAD(P)H signal was significantly less immediately after the first contraction (P < 0.001 vs. resting values). The time constant (τ) of the decrease in the NAD(P)H was significantly smaller during the second bout compared to the first bout (3.3 ± 0.3 vs. 5.0 ± 0.2 s respectively, P < 0.05). Thus, during the transition from rest to contractions, NAD(P)H oxidation was enhanced when the fibres were ‘primed’ by previous activity. When the NAD(P)H signals between the first and second bouts were compared in a time matched manner (two-way ANOVA), no significant differences were observed during rest. During the first 16 s of stimulation the NAD(P)H signal was higher in the first bout compared to the second (P < 0.05), and from this time point through the end of stimulation, no differences were observed. Since the NAD(P)H autofluorescence signal in muscle fibres reflects the reductive state of the mitochondrial NAD(P)H/NAD pool, after the initial nine contractions (18 s of stimulation), the mitochondrial reductive state was not different in the first and second bouts during stimulation.
Figure 4. Relative changes in NAD(P)H autofluorescence (A) and whole cell TMRM fluorescence (B) in intact Xenopus muscle fibres (n = 7) during two identical contractile periods separated by 5 min of rest.

The 1st and 3rd contractile bouts are represented by filled circles and the 2nd and 4th bouts are represented by open circles.
After resting periods of 1 h, the priming effect of a previous contractile activity over the NAD(P)H kinetics at onset of contractions was no longer observed (Fig. 3F). Therefore we can assume that the fibres were in a same ‘unprimed’ condition during the first and third contractile bout, which was performed after a resting period lasting more than 3 h.
Figure 4B illustrates the time course of the Δψm responses as indicated by the whole cell TMRM fluorescence changes during the repeated contractile bouts in the single fibres. Changes in the TMRM fluorescence represent changes in the Δψm occurring between contractions as described in Methods. During the third bout, the fluorescence increased immediately after the first contraction (P < 0.05 vs. resting values) attaining a peak value approximately 9.7 ± 0.9 s after the onset of contractions with an amplitude of 6.2 ± 1.0% (Fig. 4B). This increased TMRM fluorescence reading indicates that Δψm was decreasing. Whole cell TMRM fluorescence was restored to the pre-stimulation values by 32 s after the onset of contractions (P > 0.05 vs. resting values). From this point until the end of stimulations, TMRM fluorescence was not different from pre-stimulation values. However, during the fourth bout, TMRM fluorescence did not change significantly from pre-stimulation values until the 60th second after initiation of the stimulation when compared to the pre-stimulation values (P < 0.05, Fig. 4B). Comparing the TMRM fluorescence responses between the third and the fourth bouts, the signal was significantly smaller in the fourth bout between the second (after the first contraction) and 30th second (after 15 contractions) after the initiation of contractions (P < 0.05).
Discussion
In the present study we demonstrated that in intact single skeletal muscle cells, during the transition from rest to contractile activity: (1) a fall in the NAD(P)H autofluorescence was preceded by a significant time delay; (2) the mitochondrial membrane potential fell immediately after the first contraction and reestablished pre-stimulation levels during the contractile period; and (3) when the fibres were ‘primed’ by previous contractile activity, NAD(P)H autofluorescence decreased immediately after the first contraction while the mitochondrial membrane potential was not altered compared to resting values.
Time delay in the up-regulation of the electron transport chain activity
The complexes of the electron transport chain (ETC) oxidize reducing equivalents in order to establish a proton motive force (Δp), which has as a principle component the mitochondrial membrane potential (Δψm) (Nicholls & Ferguson, 2002). The Δp will be used by ATP synthase to generate ATP during mitochondrial respiration (Nicholls & Ferguson, 2002). The up-regulation of mitochondrial respiration requires O2 and reducing equivalents (e.g. NADH) availability. Changes in the cell NAD(P)H fluorescence reflect the changes in the rates of reduction and/or oxidation of the mitochondrial pool of NAD(P)H and has been considered a reliable indicator of mitochondrial energetic status and O2 availability in isolated cells or tissues (Hogan et al. 2005; Mayevsky & Chance, 2007; Balaban, 2009). In the present study, during the initial contractile period (1st bout), we observed a time delay before the fall in the NAD(P)H fluorescence after the onset of contractions. This delay could result from either a similar increase in the rate of oxidation and reduction of NAD(P)H during the first contractions, or a delayed increase in NAD(P)H oxidation rate. The latter is likely to be the main cause of this delay of the NAD(P)H autofluorescence kinetics since previous studies from our laboratory have suggested that the O2 consumption in contracting single fibres also demonstrates a time delay (Hogan, 2001; Howlett & Hogan, 2003). Thus, factors other than the abundance of O2 and NADH (e.g. the activity of different mitochondrial protein complexes) are likely to be limiting oxidative phosphorylation at the onset of contractions in our single fibre preparation; otherwise we would expect to observe an immediate decrease of these variables (Hogan, 2001; Hogan et al. 2005). In fact, our laboratory has previously shown that when O2 availability was restricted by a low  of the bath (0–2 Torr), during the transition from rest to contractions, the NAD(P)H signal was increased in contrast to a decrease of the signal at physiological
of the bath (0–2 Torr), during the transition from rest to contractions, the NAD(P)H signal was increased in contrast to a decrease of the signal at physiological  (30 Torr) (Hogan et al. 2005). This confirms that during the transition from rest to contractions at physiological
(30 Torr) (Hogan et al. 2005). This confirms that during the transition from rest to contractions at physiological 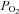 , oxidative phosphorylation is not limited by the O2 availability in isolated intact muscle fibres. Furthermore, a decreased rate of reduction of NAD(P)H after the initial contractions is unlikely because the cytosolic and mitochondrial NADH generation are expected to be enhanced due to an increased glycolytic rate and enhanced tricarboxylic acid cycle flux (Howlett & Hogan, 2003; Walsh et al. 2008).
, oxidative phosphorylation is not limited by the O2 availability in isolated intact muscle fibres. Furthermore, a decreased rate of reduction of NAD(P)H after the initial contractions is unlikely because the cytosolic and mitochondrial NADH generation are expected to be enhanced due to an increased glycolytic rate and enhanced tricarboxylic acid cycle flux (Howlett & Hogan, 2003; Walsh et al. 2008).
The present study demonstrated that in single muscle fibres contracting at a rate that elicits near maximal O2 consumption (Howlett & Hogan, 2001), Δψm decreased immediately after the first contraction and was later reestablished during the contractile bout. Δψm can be diminished due to an increased backflow of protons to the mitochondrial matrix (i.e. via ATP synthase and proton leak) or by the exchange of charged molecules, such as Ca2+, ADP and Pi between the cytoplasm and the matrix (Nicholls & Ferguson, 2002). We could not distinguish how much of the Δψm changes during the contractile period were caused either by a decrease in the proton gradient or by an increased transport of charged molecules across the inner mitochondrial membrane. Nevertheless, during stimulation, a decrease in the Δψm represents a decrease in the Δp, while the reestablishment of Δψm likely results from an increased activity of the ETC. The maximal changes in the TMRM fluorescence during contractions (∼6%, Fig. 4B) were much smaller than the changes observed when the proton electrochemical gradient is completely disrupted by a proton ionophore (up to ∼200%, Fig. 3D). Thus it is likely that that the proton motive force was never diminished to levels that could compromise ATP generation capacity by mitochondria.
A decrease in the NADH levels and an increase in the Δψm are processes that can result from an enhancement in the activity of the ETC. Interestingly, comparing both of the initial contractile periods (1st and 3rd), the lag phase required for Δψm to begin to be reestablished (∼11 s after initiation of stimulation) resembles the delay that preceded the decrease in the NAD(P)H fluorescence (∼14 s). The decline in the intracellular  (
( ) was previously reported to start at a similar time frame (12–13 s) in single fibres under the same stimulation parameters of the current investigation (Hogan, 2001; Howlett & Hogan, 2003). In addition, the lag phase required for Δψm to be completely reestablished to the pre-stimulation levels (∼32 s) was similar to the time which NAD(P)H fluorescence reached its lowest value (∼28 s, see Fig. 4). These results suggest that during the contractile period, the transient decrease of the Δψm between contractions and the restoration of the Δψm to pre-stimulation levels seemed to be dependent on the rate of NAD(P)H oxidation by the ETC. In support of this, when we added cyanide (complex IV inhibitor) to inhibit the ETC activity and stimulated the fibres to contract, during the first contractions the Δψm decreased in a similar manner as when cyanide was absent (Fig. 3E). Thereafter in the presence of cyanide, Δψm continued to depolarize, while in the control condition, Δψm tended to return to the resting values. Thus, during the transition from rest to contractions in an ‘unprimed’ state, oxidative phosphorylation may be controlled at the level of the respiratory chain as demonstrated by metabolic control analysis in isolated mitochondria from rat skeletal muscle in state 3 (i.e. ADP-stimulated respiration in the presence of excess substrate) (Rossignol et al. 2000).
) was previously reported to start at a similar time frame (12–13 s) in single fibres under the same stimulation parameters of the current investigation (Hogan, 2001; Howlett & Hogan, 2003). In addition, the lag phase required for Δψm to be completely reestablished to the pre-stimulation levels (∼32 s) was similar to the time which NAD(P)H fluorescence reached its lowest value (∼28 s, see Fig. 4). These results suggest that during the contractile period, the transient decrease of the Δψm between contractions and the restoration of the Δψm to pre-stimulation levels seemed to be dependent on the rate of NAD(P)H oxidation by the ETC. In support of this, when we added cyanide (complex IV inhibitor) to inhibit the ETC activity and stimulated the fibres to contract, during the first contractions the Δψm decreased in a similar manner as when cyanide was absent (Fig. 3E). Thereafter in the presence of cyanide, Δψm continued to depolarize, while in the control condition, Δψm tended to return to the resting values. Thus, during the transition from rest to contractions in an ‘unprimed’ state, oxidative phosphorylation may be controlled at the level of the respiratory chain as demonstrated by metabolic control analysis in isolated mitochondria from rat skeletal muscle in state 3 (i.e. ADP-stimulated respiration in the presence of excess substrate) (Rossignol et al. 2000).
Together these results confirm that during the initial phase of a contractile period in single skeletal muscle fibres, there is a delay in the increase of the oxidative phosphorylation rate that seems to be caused by a delayed activation of the ETC complexes.
Effect of a prior contractile bout on mitochondrial respiration at onset of contractions
When fibres were stimulated to contract 5 min after a previous contractile period, NAD(P)H fluorescence decreased immediately after the first contraction and at a significantly faster rate than during the first contractile period, while Δψm was unchanged in between contractions compared to resting levels (Fig. 4). Under ‘primed’ conditions, the activity of the ETC seemed to be enhanced faster at the onset of contractions than under ‘unprimed’ conditions, resulting in an immediate and faster increase of the rate of NAD(P)H oxidation and the coupled proton pumping by the ETC complexes, thus avoiding the observation of changes in the Δψm during the contractile period. This is in line with previous findings that showed that the time delay for the fall of 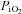 in Xenopus single fibres during two successive contractile periods was significantly shortened during a second contractile bout (Hogan, 2001).
in Xenopus single fibres during two successive contractile periods was significantly shortened during a second contractile bout (Hogan, 2001).
It has been suggested that an enhanced pyruvate dehydrogenase (PDH) activity results in higher levels of substrates available to be oxidized in the early moments of exercise, which could also ‘push’ the respiratory chain to higher activity levels (Timmons et al. 1998; Gurd et al. 2006). A previous study using intact single muscle fibres verified that the activation of PDH by dichloroacetate (DCA) indeed resulted in a faster fall in the  during contractions (Howlett & Hogan, 2003). However, the time delay of the
during contractions (Howlett & Hogan, 2003). However, the time delay of the  kinetics in the single fibres during the first seconds of the contractile bout was not different between control and DCA treated fibres (Howlett & Hogan, 2003), and other studies with human and dog muscle have also failed to show differences in the O2 consumption kinetics with DCA (Bangsbo et al. 2002; Grassi et al. 2002). In the present study, the NAD(P)H pre-stimulation levels were not different between the first and second bouts. Thus, the NAD(P)H abundance does not seem to be the major or the only mechanism regulating oxidative phosphorylation during the beginning of a contractile period in these single muscle fibres.
kinetics in the single fibres during the first seconds of the contractile bout was not different between control and DCA treated fibres (Howlett & Hogan, 2003), and other studies with human and dog muscle have also failed to show differences in the O2 consumption kinetics with DCA (Bangsbo et al. 2002; Grassi et al. 2002). In the present study, the NAD(P)H pre-stimulation levels were not different between the first and second bouts. Thus, the NAD(P)H abundance does not seem to be the major or the only mechanism regulating oxidative phosphorylation during the beginning of a contractile period in these single muscle fibres.
The classical regulatory mechanism of oxidative phosphorylation is based on a first order feedback control exerted by ADP (Chance & Williams, 1955; Chance et al. 2006). A single rate-determining step rarely can be applied in metabolic sequences, such as oxidative phosphorylation, in which many steps are expected to control the overall flux through the metabolic pathway (Nicholls & Ferguson, 2002). In this regard, evidence indicates that a first order control exerted by ADP is not the single factor controlling oxidative phosphorylation in muscle cells (Balaban, 2009; Wust et al. 2011). A parallel and/or an allosteric regulation of oxidative phosphorylation in skeletal muscle at the onset of contractions has been proposed based on studies with computer models of oxidative phosphorylation (Korzeniewski & Zoladz, 2006; Korzeniewski, 2007) and has recently been supported by experimental results (Wust et al. 2011). One proposed mechanism for this is that increases in the cytosolic Ca2+ concentrations that occur in parallel to the enhanced ATP demand during contractions can increase the mitochondrial Ca2+ influx and ultimately regulate oxidative phosphorylation by the activation of mitochondrial dehydrogenases and ATP synthase (Balaban, 2009). In line with a parallel model of regulation of oxidative phosphorylation, different studies confirmed that the proteins of the respiratory chain can undergo reversible phosphorylation which could be regulating oxidative phosphorylation in a process that might be induced by Ca2+ as well (Hopper et al. 2006; Huttemann et al. 2007, 2008; Balaban, 2009; Phillips et al. 2011). The delayed activation of the respiratory chain during the ‘unprimed’ contractile periods (i.e. 1st and 3rd) may be related to the time necessary for a parallel/allosteric regulation of mitochondrial protein complexes to occur. In mitochondria from muscle fibres previously ‘primed’, the regulation of the respiratory chain complexes, perhaps enhanced by elevated mitochondrial Ca2+ levels or altered phosphorylation state, could persist from the previous contractile period. This agrees with a study of Jouaville et al. (1999) which demonstrated that Ca2+ accumulation in mitochondria of HeLa cells resulted in an increased ATP synthesis for periods longer than the rise of mitochondrial calcium (Jouaville et al. 1999). Also, Lannergren et al. (2001) showed that in intact frog fibres, mitochondrial Ca2+ remained elevated for approximately 60 min after cessation of a contractile period inducing fatigue (Lannergren et al. 2001). Interestingly, in permeabilized skeletal muscle fibre bundles, Ca2+-independent spontaneous contractile activity was shown to increase the sensitivity of mitochondrial respiration to ADP, suggesting that contractile activity itself can modulate oxidative phosphorylation (Perry et al. 2011). A restricted ADP diffusion rate to mitochondria in a ‘unprimed’ condition can also explain the observed NAD(P)H kinetics. The voltage-dependent anion channel (VDAC) is the major pathway of ADP and ATP through the mitochondrial outer membrane. Tubulin can induce a reversible blockage of VDAC and since tubulin is a cytoskeleton protein, VDAC–tubulin interaction could be suppressed by a rearrangement of the cytoskeleton caused by contractile activity (Perry et al. 2011; Sheldon et al. 2011).
On the basis of Mitchell's chemiosmotic theory, the fundamental factor controlling mitochondria respiration is the disequilibrium between the redox potential across the ETC complexes and the proton motive force (Δp) (Nicholls & Ferguson, 2002). When ‘unprimed’, NAD(P)H oxidation rate during the initial contractions was unaltered despite a significant decrease of Δp (i.e. Δψm), confirming that ETC activity was limited under this condition. When ‘primed’, mitochondria appear to be able to avoid disequilibrium between the ETC redox potential (i.e. NAD(P)H) and the Δp (i.e. Δψm) during the initial contractions. Conceivably minimal changes in Δψm triggered an immediate increase in the NAD(P)H oxidation rate. Our intact single cell model clearly demonstrates that intrinsic factors modulate oxidative phosphorylation during the transition from rest to contractions when repeated contractile periods are performed, in a manner independent of substrate availability to the myofibre (i.e. O2 and NADH). It is important to observe that in models where the O2 delivery systems to the muscle are present (e.g. contracting isolated dog muscle in situ) O2 availability to the muscle may limit the rate of O2 consumption kinetics during contractions at high intensities (i.e. close to maximal O2 consumption) (Grassi et al. 2000). Thus, a plausible hypothesis is that under unprimed conditions an allosteric/parallel mechanism may be exerting a strong control over oxidative phosphorylation, whereas under a primed condition, a feedback control mechanism may be the major mechanism controlling mitochondrial bioenergetics. The implication of this hypothesis is that in intact live myofibres during rest, a lower activity of the ETC would avoid an inefficient and unnecessary level of oxidative phosphorylation and a consequent hyper-polarization of the Δψm and an associated reactive oxygen species generation (Kemp, 2011; Wüst et al. 2011). During and after a contractile period, the ETC would be maximally activated and able to enhance the oxidative phosphorylation rate in a situation where ATP utilization is increased. Our results indicate that differences in the kinetics of mitochondrial bioenergetics, as analysed by different parameters, seen in previous studies may be due to experimental differences in whether the muscle being examined had preceding contractile activity or not. Additionally, it is possible that in primed mitochondria, smaller changes in the Δp are required to induce an enhanced proton extrusion by the respiratory chain. As suggested by Huttemann et al. (2008), structural alterations could be occurring in the respiratory chain complexes in a way that a smaller decrease of Δψm may be sufficient to up-regulate the respiratory chain activity.
Furthermore, the NAD(P)H and Δψm tended to attain steady state levels during contractile stimulation patterns that were not different between bouts, while force generation was progressively decreasing (Fig. 1 and Fig. 2). If a decreased force generation is associated with a lower ATP turnover rate, a steady state level of NAD(P)H could be a result of a tight regulation between the rates of NADH generation and oxidation exerted by a feedback mechanism. If the ATP turnover and the oxidative phosphorylation rates were constant while force generation was progressively decreasing, mitochondrial ATP generation per force unit would be enhanced throughout the contractile period. An increase in muscle O2 consumption was shown to occur simultaneously to the development of fatigue during high intensity contractions in maximally activated dog muscle (Zoladz et al. 2008) and suggests that intrinsic factors in the myofibres may have some role in the ‘slow-component-like’ increase in oxygen consumption observed during constant load heavy intensity exercise.
Technical limitations
It is fair to assume that Xenopus isolated single fibres respect Hill's oxygen diffusion principles such as a radial oxygen diffusion direction, a homogeneous distribution of oxygen consumption along the cell, an absence of myoglobin facilitated diffusion, and a homogeneous field of O2 encompassing the myofibre (Stary & Hogan, 1999; van der Laarse et al. 2005). Even though Xenopus myofibres lack myoglobin and are likely to possess a radial O2 gradient, it has been previously shown that under extracellular ambient  tension, the
tension, the 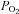 at the core of the cell is well above of that at which oxidative phosphorylation should be limited (Stary & Hogan, 1999). This can be somehow different in working muscles in situ where fibres will have a much smaller surface area in contact with the microcirculation and have other complicating factors that might restrict O2 delivery to fibres at the onset of contractions. Additionally, in the present study we analysed fatigue-resistant fibres, which may have discernible differences in the control of oxidative phosphorylation compared to other fibre types. For example, it has been shown in permeabilized fibres that different fibre types show significant differences in the regulation of oxidative phosphorylation kinetics by ADP and Pi (Kuznetsov et al. 1996; Scheibye-Knudsen & Quistorff, 2009). In respect to the fluorescent measurements conducted here, it worth mentioning that loading the cells with high TMRM concentration, which results in a quenched condition of the fluorophore in the mitochondrial matrix (see Methods), is more suitable for qualitative analysis of changes in the Δψm but not absolute values in millivolts. However this quenched condition is extremely sensitive and certainly adequate to monitor real time whole cell kinetics of Δψm in fully functional and contracting single fibres (Nicholls & Ferguson, 2002; Ward, 2010).
at the core of the cell is well above of that at which oxidative phosphorylation should be limited (Stary & Hogan, 1999). This can be somehow different in working muscles in situ where fibres will have a much smaller surface area in contact with the microcirculation and have other complicating factors that might restrict O2 delivery to fibres at the onset of contractions. Additionally, in the present study we analysed fatigue-resistant fibres, which may have discernible differences in the control of oxidative phosphorylation compared to other fibre types. For example, it has been shown in permeabilized fibres that different fibre types show significant differences in the regulation of oxidative phosphorylation kinetics by ADP and Pi (Kuznetsov et al. 1996; Scheibye-Knudsen & Quistorff, 2009). In respect to the fluorescent measurements conducted here, it worth mentioning that loading the cells with high TMRM concentration, which results in a quenched condition of the fluorophore in the mitochondrial matrix (see Methods), is more suitable for qualitative analysis of changes in the Δψm but not absolute values in millivolts. However this quenched condition is extremely sensitive and certainly adequate to monitor real time whole cell kinetics of Δψm in fully functional and contracting single fibres (Nicholls & Ferguson, 2002; Ward, 2010).
Summary
In these fatigue resistant single muscle fibres, at the beginning of an unprimed contraction period, there was a significant time delay preceding a decrease in the NAD(P)H levels while Δψm decreased immediately at the onset of contractions. Thus, the NAD(P)H level was not a limiting factor for mitochondrial respiration. After a short rest period, during a subsequent contractile bout, NAD(P)H decreased immediately after the first contraction and at a faster rate compared to the initial contractile bout while Δψm was unchanged from resting values. Therefore, when skeletal muscle has been previously primed by contractile activity, ATP generation by oxidative phosphorylation is immediately enhanced during the transition from rest to contractile activity. Our results suggest that in skeletal muscle, mitochondrial respiration is limited at some point of the electron transport chain and that mitochondria require an initial or a minimal parallel/allosteric regulation in order to respond without a delay to the classical regulatory signals of oxidative phosphorylation (i.e. ADP, Pi).
Acknowledgments
This study was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant AR-040155.
Glossary
- CCCP
carbonyl cyanide 3-chlorophenylhydrazone
- Δψm
mitochondrial membrane potential
- DCA
dichloroacetate
- ETC
electron transport chain

intracellular

- PDH
pyruvate dehydrogenase
- Δp
proton motive force
- ΔpH
chemical potential
- TMRM
tetramethylrhodamine methyl ester perchlorate
- VDAC
voltage-dependent anion channel
Author contributions
The work was done at UCSD. All authors participated in the conception and design of the study, interpretation of the data and drafting of the manuscript. Data collection and analysis was performed by P.G.G. The final version of the manuscript was approved by all authors. No conflicts of interests, financial or otherwise, are declared by the authors.
References
- Balaban RS. Domestication of the cardiac mitochondrion for energy conversion. J Mol Cell Cardiol. 2009;46:832–841. doi: 10.1016/j.yjmcc.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangsbo J, Gibala MJ, Krustrup P, Gonzalez-Alonso J, Saltin B. Enhanced pyruvate dehydrogenase activity does not affect muscle O2 uptake at onset of intense exercise in humans. Am J Physiol Regul Integr Comp Physiol. 2002;282:R273–280. doi: 10.1152/ajpregu.2002.282.1.R273. [DOI] [PubMed] [Google Scholar]
- Bangsbo J, Krustrup P, Gonzalez-Alonso J, Boushel R, Saltin B. Muscle oxygen kinetics at onset of intense dynamic exercise in humans. Am J Physiol Regul Integr Comp Physiol. 2000;279:R899–906. doi: 10.1152/ajpregu.2000.279.3.R899. [DOI] [PubMed] [Google Scholar]
- Bowen TS, Cannon DT, Murgatroyd SR, Birch KM, Witte KK, Rossiter HB. The intramuscular contribution to the slow oxygen uptake kinetics during exercise in chronic heart failure is related to the severity of the condition. J Appl Physiol. 2012;112:378–387. doi: 10.1152/japplphysiol.00779.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand MD, Nicholls DG. Assessing mitochondrial dysfunction in cells. Biochem J. 2011;435:297–312. doi: 10.1042/BJ20110162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell-O'Sullivan SP, Constantin-Teodosiu D, Peirce N, Greenhaff PL. Low intensity exercise in humans accelerates mitochondrial ATP production and pulmonary oxygen kinetics during subsequent more intense exercise. J Physiol. 2002;538:931–939. doi: 10.1113/jphysiol.2001.013238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance B, Im J, Nioka S, Kushmerick M. Skeletal muscle energetics with PNMR: personal views and historic perspectives. NMR Biomed. 2006;19:904–926. doi: 10.1002/nbm.1109. [DOI] [PubMed] [Google Scholar]
- Chance B, Williams GR. Respiratory enzymes in oxidative phosphorylation. I. Kinetics of oxygen utilization. J Biol Chem. 1955;217:383–393. [PubMed] [Google Scholar]
- Goodwin ML, Hernandez A, Lai N, Cabrera ME, Gladden LB. VO2 on-kinetics in isolated canine muscle in situ during slowed convective O2 delivery. J Appl Physiol. 2012;112:9–19. doi: 10.1152/japplphysiol.01480.2010. [DOI] [PubMed] [Google Scholar]
- Grassi B, Poole DC, Richardson RS, Knight DR, Erickson BK, Wagner PD. Muscle O2 uptake kinetics in humans: implications for metabolic control. J Appl Physiol. 1996;80:988–998. doi: 10.1152/jappl.1996.80.3.988. [DOI] [PubMed] [Google Scholar]
- Grassi B, Gladden LB, Samaja M, Stary CM, Hogan MC. Faster adjustment of O2 delivery does not affect VO2 on-kinetics in isolated in situ canine muscle. J Appl Physiol. 1998a;85:1394–1403. doi: 10.1152/jappl.1998.85.4.1394. [DOI] [PubMed] [Google Scholar]
- Grassi B, Gladden LB, Stary CM, Wagner PD, Hogan MC. Peripheral O2 diffusion does not affect VO2 on-kinetics in isolated insitu canine muscle. J Appl Physiol. 1998b;85:1404–1412. doi: 10.1152/jappl.1998.85.4.1404. [DOI] [PubMed] [Google Scholar]
- Grassi B, Hogan MC, Greenhaff PL, Hamann JJ, Kelley KM, Aschenbach WG, Constantin-Teodosiu D, Gladden LB. Oxygen uptake on-kinetics in dog gastrocnemius in situ following activation of pyruvate dehydrogenase by dichloroacetate. J Physiol. 2002;538:195–207. doi: 10.1113/jphysiol.2001.012984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassi B, Hogan MC, Kelley KM, Aschenbach WG, Hamann JJ, Evans RK, Patillo RE, Gladden LB. Role of convective O2 delivery in determining VO2 on-kinetics in canine muscle contracting at peak VO2. J Appl Physiol. 2000;89:1293–1301. doi: 10.1152/jappl.2000.89.4.1293. [DOI] [PubMed] [Google Scholar]
- Gurd BJ, Peters SJ, Heigenhauser GJ, LeBlanc PJ, Doherty TJ, Paterson DH, Kowalchuk JM. Prior heavy exercise elevates pyruvate dehydrogenase activity and speeds O2 uptake kinetics during subsequent moderate-intensity exercise in healthy young adults. J Physiol. 2006;577:985–996. doi: 10.1113/jphysiol.2006.112706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez A, McDonald JR, Lai N, Gladden LB. A prior bout of contractions speeds VO2 and blood flow on-kinetics and reduces the VO2 slow-component amplitude in canine skeletal muscle contracting in situ. J Appl Physiol. 2010;108:1169–1176. doi: 10.1152/japplphysiol.01318.2009. [DOI] [PubMed] [Google Scholar]
- Hogan MC. Fall in intracellular PO2 at the onset of contractions in Xenopus single skeletal muscle fibers. J Appl Physiol. 2001;90:1871–1876. doi: 10.1152/jappl.2001.90.5.1871. [DOI] [PubMed] [Google Scholar]
- Hogan MC, Stary CM, Balaban RS, Combs CA. NAD(P)H fluorescence imaging of mitochondrial metabolism in contracting Xenopus skeletal muscle fibers: effect of oxygen availability. J Appl Physiol. 2005;98:1420–1426. doi: 10.1152/japplphysiol.00849.2004. [DOI] [PubMed] [Google Scholar]
- Hopper RK, Carroll S, Aponte AM, Johnson DT, French S, Shen RF, Witzmann FA, Harris RA, Balaban RS. Mitochondrial matrix phosphoproteome: effect of extra mitochondrial calcium. Biochemistry. 2006;45:2524–2536. doi: 10.1021/bi052475e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett RA, Hogan MC. Intracellular PO2 decreases with increasing stimulation frequency in contracting single Xenopus muscle fibers. J Appl Physiol. 2001;91:632–636. doi: 10.1152/jappl.2001.91.2.632. [DOI] [PubMed] [Google Scholar]
- Howlett RA, Hogan MC. Dichloroacetate accelerates the fall in intracellular PO2 at onset of contractions in Xenopus single muscle fibers. Am J Physiol Regul Integr Comp Physiol. 2003;284:R481–485. doi: 10.1152/ajpregu.00078.2002. [DOI] [PubMed] [Google Scholar]
- Huttemann M, Lee I, Pecinova A, Pecina P, Przyklenk K, Doan JW. Regulation of oxidative phosphorylation, the mitochondrial membrane potential, and their role in human disease. J Bioenerg Biomembr. 2008;40:445–456. doi: 10.1007/s10863-008-9169-3. [DOI] [PubMed] [Google Scholar]
- Huttemann M, Lee I, Samavati L, Yu H, Doan JW. Regulation of mitochondrial oxidative phosphorylation through cell signaling. Biochim Biophys Acta. 2007;1773:1701–1720. doi: 10.1016/j.bbamcr.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Jones AM, Berger NJ, Wilkerson DP, Roberts CL. Effects of “priming” exercise on pulmonary O2 uptake and muscle deoxygenation kinetics during heavy-intensity cycle exercise in the supine and upright positions. J Appl Physiol. 2006;101:1432–1441. doi: 10.1152/japplphysiol.00436.2006. [DOI] [PubMed] [Google Scholar]
- Jouaville LS, Pinton P, Bastianutto C, Rutter GA, Rizzuto R. Regulation of mitochondrial ATP synthesis by calcium: evidence for a long-term metabolic priming. Proc Natl Acad Sci U S A. 1999;96:13807–13812. doi: 10.1073/pnas.96.24.13807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp G. Implications of rapid early oxygen consumption in exercising skeletal muscle. J Physiol. 2011;589:6243–6244. doi: 10.1113/jphysiol.2011.218933. author reply 6245–6246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korzeniewski B. Regulation of oxidative phosphorylation through parallel activation. Biophys Chem. 2007;129:93–110. doi: 10.1016/j.bpc.2007.05.013. [DOI] [PubMed] [Google Scholar]
- Korzeniewski B, Zoladz JA. Biochemical background of the VO2 on-kinetics in skeletal muscles. J Physiol Sci. 2006;56:1–12. doi: 10.2170/physiolsci.m93. [DOI] [PubMed] [Google Scholar]
- Kuznetsov AV, Tiivel T, Sikk P, Kaambre T, Kay L, Daneshrad Z, Rossi A, Kadaja L, Peet N, Seppet E, Saks VA. Striking differences between the kinetics of regulation of respiration by ADP in slow-twitch and fast-twitch muscles in vivo. Eur J Biochem. 1996;241:909–915. doi: 10.1111/j.1432-1033.1996.00909.x. [DOI] [PubMed] [Google Scholar]
- Lannergren J, Westerblad H, Bruton JD. Changes in mitochondrial Ca2+ detected with Rhod-2 in single frog and mouse skeletal muscle fibres during and after repeated tetanic contractions. J Muscle Res Cell Motil. 2001;22:265–275. doi: 10.1023/a:1012227009544. [DOI] [PubMed] [Google Scholar]
- Macdonald M, Pedersen PK, Hughson RL. Acceleration of VO2 kinetics in heavy submaximal exercise by hyperoxia and prior high-intensity exercise. J Appl Physiol. 1997;83:1318–1325. doi: 10.1152/jappl.1997.83.4.1318. [DOI] [PubMed] [Google Scholar]
- Mayevsky A, Chance B. Oxidation-reduction states of NADH in vivo: from animals to clinical use. Mitochondrion. 2007;7:330–339. doi: 10.1016/j.mito.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Mayevsky A, Rogatsky GG. Mitochondrial function in vivo evaluated by NADH fluorescence: from animal models to human studies. Am J Physiol Cell Physiol. 2007;292:C615–640. doi: 10.1152/ajpcell.00249.2006. [DOI] [PubMed] [Google Scholar]
- Nicholls DG. Forty years of Mitchell's proton circuit: From little grey books to little grey cells. Biochim Biophys Acta. 2008;1777:550–556. doi: 10.1016/j.bbabio.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls DG, Ferguson SJ. Bioenergetics 3. San Diego: Academic Press; 2002. [Google Scholar]
- Nicholls DG, Vesce S, Kirk L, Chalmers S. Interactions between mitochondrial bioenergetics and cytoplasmic calcium in cultured cerebellar granule cells. Cell Calcium. 2003;34:407–424. doi: 10.1016/s0143-4160(03)00144-1. [DOI] [PubMed] [Google Scholar]
- Perry CG, Kane DA, Lin CT, Kozy R, Cathey BL, Lark DS, Kane CL, Brophy PM, Gavin TP, Anderson EJ, Neufer PD. Inhibiting myosin-ATPase reveals a dynamic range of mitochondrial respiratory control in skeletal muscle. Biochem J. 2011;437:215–222. doi: 10.1042/BJ20110366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips D, Aponte AM, Covian R, Balaban RS. Intrinsic protein kinase activity in mitochondrial oxidative phosphorylation complexes. Biochemistry. 2011;50:2515–2529. doi: 10.1021/bi101434x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard M, Taivassalo T, Ritchie D, Wright KJ, Thomas MM, Romestaing C, Hepple RT. Mitochondrial structure and function are disrupted by standard isolation methods. PLoS One. 2011;6:e18317. doi: 10.1371/journal.pone.0018317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramzan R, Staniek K, Kadenbach B, Vogt S. Mitochondrial respiration and membrane potential are regulated by the allosteric ATP-inhibition of cytochrome c oxidase. Biochim Biophys Acta. 2010;1797:1672–1680. doi: 10.1016/j.bbabio.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Richardson RS, Grassi B, Gavin TP, Haseler LJ, Tagore K, Roca J, Wagner PD. Evidence of O2 supply-dependent VO2 max in the exercise-trained human quadriceps. J Appl Physiol. 1999;86:1048–1053. doi: 10.1152/jappl.1999.86.3.1048. [DOI] [PubMed] [Google Scholar]
- Rossignol R, Letellier T, Malgat M, Rocher C, Mazat JP. Tissue variation in the control of oxidative phosphorylation: implication for mitochondrial diseases. Biochem J. 2000;347:45–53. [PMC free article] [PubMed] [Google Scholar]
- Rumsey WL, Schlosser C, Nuutinen EM, Robiolio M, Wilson DF. Cellular energetics and the oxygen dependence of respiration in cardiac myocytes isolated from adult rat. J Biol Chem. 1990;265:15392–15402. [PubMed] [Google Scholar]
- Scheibye-Knudsen M, Quistorff B. Regulation of mitochondrial respiration by inorganic phosphate; comparing permeabilized muscle fibers and isolated mitochondria prepared from type-1 and type-2 rat skeletal muscle. Eur J Appl Physiol. 2009;105:279–287. doi: 10.1007/s00421-008-0901-9. [DOI] [PubMed] [Google Scholar]
- Sheldon KL, Maldonado EN, Lemasters JJ, Rostovtseva TK, Bezrukov SM. Phosphorylation of voltage-dependent anion channel by serine/threonine kinases governs its interaction with tubulin. PLoS One. 2011;6:e25539. doi: 10.1371/journal.pone.0025539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stary CM, Hogan MC. Effect of varied extracellular PO2 on muscle performance in Xenopus single skeletal muscle fibers. J Appl Physiol. 1999;86:1812–1816. doi: 10.1152/jappl.1999.86.6.1812. [DOI] [PubMed] [Google Scholar]
- Stary CM, Mathieu-Costello O, Hogan MC. Resistance to fatigue of individual Xenopus single skeletal muscle fibres is correlated with mitochondrial volume density. Exp Physiol. 2004;89:617–621. doi: 10.1113/expphysiol.2004.027763. [DOI] [PubMed] [Google Scholar]
- Timmons JA, Gustafsson T, Sundberg CJ, Jansson E, Hultman E, Kaijser L, Chwalbinska-Moneta J, Constantin-Teodosiu D, Macdonald IA, Greenhaff PL. Substrate availability limits human skeletal muscle oxidative ATP regeneration at the onset of ischemic exercise. J Clin Invest. 1998;101:79–85. doi: 10.1172/JCI1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Laarse WJ, des Tombe AL, van Beek-Harmsen BJ, Lee-de Groot MB, Jaspers RT. Krogh's diffusion coefficient for oxygen in isolated Xenopus skeletal muscle fibers and rat myocardial trabeculae at maximum rates of oxygen consumption. J Appl Physiol. 2005;99:2173–2180. doi: 10.1152/japplphysiol.00470.2005. [DOI] [PubMed] [Google Scholar]
- Walsh B, Stary CM, Howlett RA, Kelley KM, Hogan MC. Glycolytic activation at the onset of contractions in isolated Xenopus laevis single myofibres. Exp Physiol. 2008;93:1076–1084. doi: 10.1113/expphysiol.2008.042440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward MW. Quantitative analysis of membrane potentials. Methods Mol Biol. 2010;591:335–351. doi: 10.1007/978-1-60761-404-3_20. [DOI] [PubMed] [Google Scholar]
- Ward MW, Rego AC, Frenguelli BG, Nicholls DG. Mitochondrial membrane potential and glutamate excitotoxicity in cultured cerebellar granule cells. J Neurosci. 2000;20:7208–7219. doi: 10.1523/JNEUROSCI.20-19-07208.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wust RC, Grassi B, Hogan MC, Howlett RA, Gladden LB, Rossiter HB. Kinetic control of oxygen consumption during contractions in self-perfused skeletal muscle. J Physiol. 2011;589:3995–4009. doi: 10.1113/jphysiol.2010.203422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wüst RCI, Grassi B, Hogan MC, Howlett RA, Gladden LB, Rossiter HB. Implications of rapid early oxygen consumption in exercising skeletal muscle: The empirical, the theoretical and the rational. J Physiol. 2011;589:6245–6246. [Google Scholar]
- Zoladz JA, Gladden LB, Hogan MC, Nieckarz Z, Grassi B. Progressive recruitment of muscle fibers is not necessary for the slow component of VO2 kinetics. J Appl Physiol. 2008;105:575–580. doi: 10.1152/japplphysiol.01129.2007. [DOI] [PubMed] [Google Scholar]


