Abstract
Brainstem vago-vagal neurocircuits modulate upper gastrointestinal functions. Derangement of these sensory-motor circuits is implicated in several pathophysiological states, such as gastroesophageal reflux disease (GERD), functional dyspepsia and, possibly, pancreatitis. While vagal circuits controlling the stomach have received more attention, the organization of brainstem pancreatic neurocircuits is still largely unknown. We aimed to investigate the in vitro and in vivo modulation of brainstem vagal circuits controlling pancreatic secretion. Using patch clamp techniques on identified vagal pancreas-projecting neurones, we studied the effects of metabotropic glutamate receptor (mGluR) agents in relation to the effects of exendin-4, a glucagon-like peptide 1 analogue, cholecystokinin (CCK) and pancreatic polypeptide (PP). An in vivo anaesthetized rat preparation was used to measure pancreatic exocrine secretion (PES) and plasma insulin following microinjection of metabotropic glutamate receptor (mGluR) agonists and exendin-4 in the brainstem. Group II and III mGluR agonists (2R,4R-4-aminopyrrolidine-2,4-dicarboxylate (APDC) and l(+)-2-amino-4-phosphonobutyric acid (l-AP4), respectively) decreased the frequency of miniature inhibitory and excitatory postsynaptic currents (mIPSCs and mEPSCs, respectively) in the majority of the neurones tested. All neurones responsive to l-AP4 were also responsive to APDC, but not vice versa. Further, in neurones where l-AP4 decreased mIPSC frequency, exendin-4 increased, while PP had no effect upon, mIPSC frequency. Brainstem microinjection of APDC or l-AP4 decreased plasma insulin secretion, whereas only APDC microinjections increased PES. Exendin-4 microinjections increased plasma insulin. Our results indicate a discrete organization of vagal circuits, which opens up promising avenues of research aimed at investigating the physiology of homeostatic autonomic neurocircuits.
Key points
The pancreas consists of two functional parts, exocrine, which releases digestive enzymes, and endocrine, which releases hormones, such as insulin.
Both parts are under neural regulatory control by the vagus nerve. Vago-vagal neurocircuits integrate the sensory information, chemical or mechanical, from the gastrointestinal tract with the motor output back to the gastrointestinal system, including the pancreas.
Both excitatory and inhibitory vago-vagal neural circuits are regulated by many neurotransmitters, including glutamate acting on different types of metabotropic glutamate receptors.
In this study, we show that different subtypes of metabotropic glutamate receptors regulate differentially exocrine and endocrine pancreatic functions by affecting different neurocircuits.
The present study provides the physiological basis to develop pharmacological strategies aimed to provide a better understanding of pathophysiological conditions, such as pancreatitis or diabetes, that affect selectively the exocrine or endocrine pancreas.
Introduction
Brainstem vagal neurocircuits are the central components of the sensory-motor vago-vagal reflexes that modulate upper gastrointestinal functions. These brainstem vagal neurocircuits integrate sensory inputs carried by vagal afferent fibres, which synapse on second order neurones of nucleus tractus solitarii (NTS). NTS neurones assimilate this sensory information and transmit the integrated signal to higher CNS centres as well as to the efferent preganglionic neurones of the dorsal motor nucleus of the vagus (DMV; Travagli et al. 2006).
Several substantial lines of evidence indicate that derangement of vagal sensory-motor functions may play a prominent role in many gastrointestinal-related pathologies, such as functional dyspepsia, gastroparesis, gastrooesophageal reflux disease (GERD) and, possibly, pancreatitis (Schmulson & Mayer, 1998; Van Oudendove et al. 2004; Frisby et al. 2005; Kindt & Tack, 2006; van Westerloo et al. 2006; Liu et al. 2008; Chandra & Liddle, 2009).
Increasing evidence suggests that different vagally mediated gastrointestinal (GI) and pancreatic functions are controlled by segregated neural circuits (Browning et al. 1999, 2005a; Mussa & Verberne, 2008; Babic et al. 2011; Mussa et al. 2011). These vagal circuits are modulated selectively by a range of neurotransmitters, including glutamate acting on metabotropic glutamate receptors (mGluRs). In fact, group II and group III mGluRs have been shown to inhibit the sensitivity of gastrooesophageal vagal afferents and to decrease synaptic transmission to NTS neurones (Glaum & Miller, 1993; Chen et al. 2002; Jin et al. 2004; Page et al. 2005; Chen & Bonham, 2005). We have shown recently that group II and group III mGluRs, whose activation decreases cAMP levels (Cartmell & Schoepp, 2000), modulate excitatory and inhibitory synaptic transmission to identified gastric-projecting DMV neurones (Browning & Travagli, 2007). We have also shown that the GABAergic terminals impinging upon DMV neurones contain tonically active group II mGluR, whereas glutamatergic terminals contain both group II and group III mGluRs, although they do not receive tonic glutamatergic inputs (Browning & Travagli, 2007). We further demonstrated that the modulation of the levels of cAMP within the dorsal vagal complex (DVC; i.e. DMV, NTS and area postrema) plays a major role in regulating the vagal motor output to the stomach (Browning & Travagli, 2001, 2009, 2010; Browning et al. 2004). These observations highlight the relevance of mGluRs in the DVC and suggest that their organization is highly specific in vagal gastric-projecting circuits. The organization of mGluRs in vagal pancreas-projecting circuits, however, is unknown.
The first aim of this study was to investigate the organization of mGluRs on synapses impinging onto pancreas-projecting DMV neurones.
Both in vivo and in vitro studies have demonstrated that sensory-motor vagal neurocircuits exert critical control over pancreatic exocrine secretion (PES) and insulin secretion, by integrating neural and humoral signals and adjusting pancreatic secretions to respond to on demand physiological needs (Ionescu et al. 1983; Singer et al. 1986; Inui et al. 1986; Rinaman & Miselis, 1987; Ami et al. 1993; Gicquel et al. 1994; Siaud et al. 1995; Mussa et al. 2011). In fact, in vivo studies have shown that microinjection of either the GABAA receptor antagonist bicuculline, or CCK into the DMV increases, whereas microinjection of pancreatic polypeptide (PP) or somatostatin decreases, PES (Okumura et al. 1995; Liao et al. 2005; Viard et al. 2007; Mussa & Verberne, 2008). In vitro studies have shown that GLP-1, PP and CCK alter the activity of identified pancreas-projecting DMV neurones (Browning et al. 2005b; Wan et al. 2007a,b,c). Furthermore, pancreas-projecting DMV neurones have been shown to respond to either PP or GLP-1, but not both peptides (Wan et al. 2007c). These in vitro findings suggest that separate populations of DMV neurones may be devoted to the regulation of different pancreatic functions. For example, DMV neurones devoted to the modulation of endocrine functions may respond to GLP-1 but not to CCK or PP while DMV neurones devoted to the modulation of exocrine functions respond to CCK or PP but not to GLP-1. The effects of mGluRs and CCK, PP or GLP-1 on the same neurone have not been studied, however; nor have the effects of microinjections of mGluR in the DMV on PES and plasma insulin.
The second aim of this study was to determine, using in vitro and in vivo techniques, whether pancreatic insulin and exocrine secretions are under the modulatory control of distinct subpopulations of vagal motoneurones in the rat.
Methods
Ethical approval
All experiments were approved by the Penn State University Institutional Animal Care and Use Committee.
Retrograde tracing
Pancreas-projecting DMV neurones were labelled by application of the fluorescent neuronal tracer DiI to the pancreas as described previously (Browning et al. 2005a; Wan et al. 2007a). Briefly, during isofluorane (2.5% with air, 600 ml min−1) anaesthesia, the abdominal area of juvenile rats of either sex was cleaned and the pancreas exposed following a laparotomy. The pancreas was isolated and DiI crystals were affixed to the pancreas surface using a fast-hardening epoxy resin. The abdomen was closed and rats were allowed to recover for 7–15 days before experimentation.
Electrophysiological recording
Rat brainstem slices were prepared as described previously (Browning et al. 1999). Briefly, rats were anaesthetized and the brainstem was removed and placed in chilled, oxygenated Krebs solution (in mm: 126 NaCl, 25 NaHCO3, 2.5 KCl, 1.2 MgCl2, 2.4 CaCl2, 1.2 NaH2PO4, and 11 glucose, maintained at pH 7.4 by bubbling with 95% O2–5% CO2). Five to six coronal slices through the rostro-caudal extent of the DVC were cut at 300 μm and incubated in Krebs solution at 30°C for at least 90 min before recording. A single slice was transferred to a custom-made perfusion chamber, kept in place with a nylon mesh and maintained at 30°C by perfusion with warm Krebs solution at a rate of 2.5–3.0 ml min−1. Pancreas-projecting neurones were identified using a Nikon E600FN microscope equipped with epifluorescence filters; typically, following labelling of the pancreas, an average of one to two unequivocally labelled neurones were observed in each brainstem slice; electrophysiological recordings were made under bright-field illumination using Nomarski optics.
Whole-cell recordings were made with patch pipettes of resistance 2–5 MΩ when filled with intracellular solution. Potassium gluconate (in mm: 128 potassium gluconate, 10 KCl, 0.3 CaCl2, 1 MgCl2, 10 Hepes, 1 EGTA, 2 ATP-Na, and 0.25 GTP-Na, adjusted to pH7.35 with KOH) was used to study excitatory postsynaptic currents (EPSCs), while KCl (in mm: 140 KCl, 1 CaCl2, 1 MgCl2, 10 Hepes, 10 EGTA, 2 ATP-Na, and 0.25 GTP-Na, adjusted to pH7.35 with KOH) was used to study inhibitory postsynaptic currents (IPSCs). Data were acquired using a Axopatch 1D amplifier (Molecular Devices, Sunnyvale, CA, USA) at a rate of 10 kHz, filtered at 2 kHz, digitized via a Digidata 1320 interface (Molecular Devices) before being analysed on a personal computer using MiniAnalysis software (Jaejin Software, Leonia, NJ, USA). Only recordings with a series resistance <15 MΩ were considered acceptable.
The experiments were done in the presence of tetrodotoxin (TTX; 1 μm) and the GABAA receptor antagonist bicuculline (50 μm) to isolate miniature EPSCs (mEPSCs) or in the presence of TTX and kynurenic acid (1 mm) to isolate miniature IPSCs (mIPSCs).
Drugs were applied via perfusion through a series of manually operated valves at concentrations demonstrated previously to be effective (Browning & Travagli, 2007; Wan et al. 2007b; Holmes et al. 2009). Agonists were applied for periods of time sufficient for the response to reach plateau and neurones were allowed to recover fully between drug additions. Antagonists were perfused for at least 5 min before assessment of their effects. Each neurone served as its own control; a minimum variation in frequency of 50% from baseline was considered as effective. Student's t test was used to compare results of different treatments. Statistical significance was defined as P < 0.05. Results are expressed as means ± SEM.
Pancreatic duct cannulation and plasma collection
Sprague–Dawley rats of either sex (200–500 g; n = 53) were fasted overnight (water ad libitum) and anaesthetized with Inactin (135 mg kg−1, i.p.) before performing a midline laparotomy. The common bile-pancreatic duct was cannulated to collect PES (n = 35) in 10 min intervals. The total volume was measured before protein content was assessed in 5 μl samples using a BCA protein assay kit (Pierce, Rockford, IL, USA) and expressed as μg per 10 min. Baseline and drug-induced protein secretion were measured over a 30 min equilibration period before and 90 min after drug treatment, respectively.
Whole blood samples (100–200 μl; n = 18) were collected from the femoral vein before (×2) and after (×4 at 5 min intervals) microinjection. Plasma was separated and processed for insulin concentration using ELISA kit (Crystal Chem, Downers Grove, IL, USA). Data are expressed as ng (ml plasma)−1.
Microinjection in the DVC
Following cannulation of the pancreatic duct (for exocrine secretion studies) or the femoral artery (for plasma insulin studies), rats were placed in a stereotaxic frame and the lower medulla was exposed. A glass micropipette (30–40 μm tip diameter) was directed into the DVC under microscopic guidance (from calamus scriptorius, mm: +0.2–0.5 rostro-caudal, 0.1–0.3 medio-lateral and −0.5 dorso-ventral) for drug delivery. Vehicle (0.9% saline), 2R,4R-4-aminopyrrolidine-2,4-dicarboxylate (APDC; 0.18–1.04 nmol), (2S)-α-ethylglutamic acid (EGLU; 15 nmol), l(+)-2-amino-4-phosphonobutyric acid (l-AP4; 0.6–1.04 nmol) or exendin-4 (0.45 nmol) were applied in 60 nl volumes by pressure ejection over a 1 min period. Fluorescent microspheres (Fluoresbrite carboxy NYO; Polysciences, Warrington, PA, USA) were included in the injectate for the post hoc verification of the injection site.
Statistical analysis
Data are expressed as mean ± SEM. Student's paired or grouped t tests were used to compare the results. Significance was defined as P < 0.05.
Verification of injection site
At the end of the experiment, the rat was perfused transcardially with saline followed by fixative (4% paraformaldehyde in 0.1 m phosphate buffered saline). The brainstem was removed and stored in fixative with 20% sucrose at 4°C for at least 48 h before cutting 50 μm-thick coronal slices. A Nikon E400 equipped with TRITC filters and Element software was used to visualize and record the injection site.
Drugs and chemicals
APDC, EGLU, l-AP4 and (RS)-α-methylserine- O-phosphate (MSOP) were purchased from Tocris (Ellisville, MO, USA); tetrodotoxin was purchased from Alomone Labs (Jerusalem, Israel). All other chemicals were purchased from Sigma-Aldrich (St Louis, MO, USA).
Results
Whole-cell patch-clamp recordings were made from a total of 116 pancreas-projecting neurones from 55 rats.
In control conditions the amplitude of miniature excitatory and inhibitory postsynaptic currents was 30 ± 1.4 and 59 ± 4.7 pA, respectively (mEPSC: n = 37; mIPSC: n = 39); none of the pharmacological procedures described below changed the amplitude significantly (data not shown).
Group II mGluRs decrease inhibitory synaptic transmission
In 17 of 24 neurones, perfusion with APDC decreased the frequency of mIPSCs (from 1.5 ± 0.39 to 0.4 ± 0.13 events s−1; P < 0.05; Fig. 1). Perfusion with EGLU had no effect on the frequency (1.3 ± 0.4 to 1.1 ± 0.3 events s−1; P > 0.05) of mIPSCs in any of five neurones tested (Fig. 2) but blocked the APDC-induced decrease in mIPSC frequency (0.79 ± 0.43 before and 0.57 ± 0.3 events s−1 after APDC; n = 4).
Figure 1. The group II mGluR agonist APDC decreases the frequency but not amplitude of mIPSCs and mEPSCs.
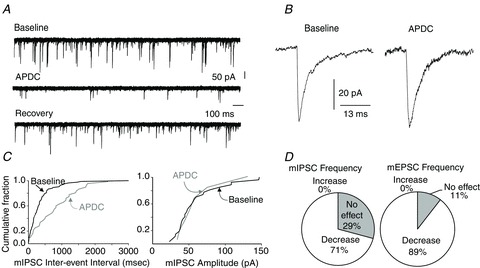
A, representative traces from a pancreas-projecting DMV neurone showing mIPSCs in baseline conditions (top), APDC (middle) and during recovery (bottom). Holding potential = −60 mV. B, average mIPSC trace before (left) and during (right) APDC perfusion. C, cumulative-fraction graphs showing the APDC-induced decrease in mIPSC frequency (left), but not amplitude (right). D, pie charts showing the percentage of pancreas-projecting DMV neurones in which APDC decreased the frequency of mIPSC (left) and mEPSC (right).
Figure 2. Different effects of mGluRs on miniature currents.
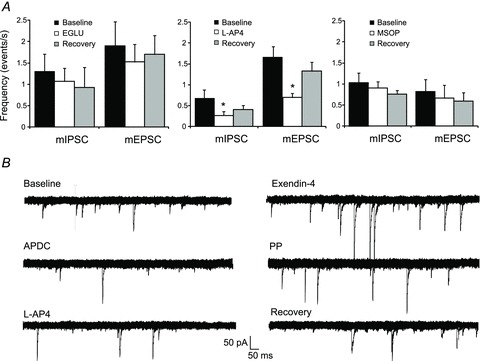
A, bar graphs showing the effects of the group II mGluR antagonist EGLU, the group III mGluR agonist l-AP4 and the group III mGluR antagonist MSOP on miniature currents. *P < 0.05. B, representative traces from a pancreas-projecting DMV neurone showing that perfusion with APDC and l-AP4 decreased, exendin-4 increased and PP had no effect upon mIPSCs frequency. All traces are from the same neurone; each agonist was perfused at approximately 15 min intervals during which mIPSCs frequency returned to baseline levels (not shown).
These data indicate that presynaptic group II mGluR modulate inhibitory synaptic transmission to pancreas-projecting DMV neurones, but are not tonically active.
Group II mGluRs decrease excitatory synaptic transmission
Perfusion with APDC decreased the frequency of mEPSCs from 1.7 ± 0.3 to 0.6 ± 0.1 events s−1 (P < 0.05) in 17 of 19 neurones tested (Fig. 1). APDC had no effect on the two remaining neurones. Perfusion of the slice with EGLU had no effect on the frequency (1.9 ± 0.57 before and 1.5 ± 0.41 events s−1 during EGLU; P > 0.05) of mEPSCs (n = 9; Fig. 2), but blocked the APDC-induced decrease in mEPSC frequency (2.85 ± 0.95 before and 2.0 ± 0.78 events s−1 after APDC; n = 2).
These data indicate that presynaptic group II mGluRs modulate excitatory synaptic transmission to pancreas-projecting DMV neurones, but are not tonically active.
Group III mGluRs decrease inhibitory synaptic transmission
Application of l-AP4 decreased mIPSC frequency from 0.7 ± 0.21 to 0.3 ± 0.11 events s−1 in 11 of 19 neurones (P < 0.05; Fig. 2). Application of MSOP alone had no effect on mIPSC frequency in any of the neurones tested (n = 11; Fig. 2), but blocked the l-AP4 induced inhibition of mIPSC frequency (0.33 ± 0.04 before and 0.23 ± 0.04 events s−1 after l-AP4; n = 5).
These data suggest that group III mGluRs are present, though not tonically active, on a subpopulation of inhibitory synaptic terminals impinging on pancreas-projecting DMV neurones.
Group III mGluRs decrease excitatory synaptic transmission
l-AP4 (100 μm) decreased the mEPSC frequency from 1.6 ± 0.27 to 0.7 ± 0.10 events s−1 in 17 of 26 neurones tested (P < 0.05). The group III mGluR antagonist MSOP (500 μm; n = 8) had no effect on the frequency of mEPSCs (Fig. 2), but blocked the effects of l-AP4 to decrease mEPSC frequency (0.99 ± 0.27 before and 0.89 ± 0.31 events s−1 after l-AP4; n = 5).
These data suggest that group III mGluRs are present on excitatory synapses impinging on pancreas-projecting DMV neurones and that these receptors are not tonically active.
Differential responses of pancreas-projecting DMV neurones to group II and group III mGluR agonists
To determine whether group II and group III mGluRs are present on inputs impinging upon the same population of DMV neurones, the effects of both APDC and l-AP4 were studied. APDC decreased the frequency of mIPSCs from 1.3 ± 0.5 to 0.5 ± 0.2 events s−1 in 11 out of 15 neurones (P < 0.05). Following washout and recovery, application of l-AP4 decreased mIPSC frequency from 1.2 ± 0.35 to 0.6 ± 0.2 events s−1 (n = 5 of 11 neurones tested; Fig. 2).
Perfusion with APDC decreased mEPSC frequency from 1.6 ± 0.30 to 0.6 ± 0.11 events s−1 in 12 of 12 neurones tested (P < 0.05). Following washout and recovery, application of l-AP4 decreased mEPSC frequency from 1.6 ± 0.41 to 0.71 ± 0.17 events s−1 (n = 11 of 12 neurones tested).
These data demonstrate that group III mGluRs are present on the majority of the excitatory, but on a smaller proportion of inhibitory, synaptic terminals that also express group II mGluRs.
To determine whether group II and group III mGluRs are present on specific populations of pancreas-projecting DMV neurones that control either exocrine or endocrine pancreatic functions, we tested the effects of the GLP-1 analogue exendin-4 (presumed to work on endocrine pancreatic circuits selectively), PP and CCK (presumed to work on exocrine pancreatic circuits selectively) on neurones that responded to group II or III mGluR agonists.
Responses to exendin-4, PP and CCK were tested in seven neurones in which APDC decreased mIPSC frequency from 1.03 ± 0.21 to 0.4 ± 0.07 events s−1 (P < 0.05). Four of seven neurones responded to exendin-4 with an increase in mIPSC frequency from 0.79 ± 0.12 to 1.27 ± 0.25 events s−1 (P < 0.05), whereas none of the seven neurones responded to PP (Fig. 2) and none of three neurones tested responded to CCK.
These data indicate that group II mGluRs are present on inhibitory synaptic terminals responsive to exendin-4, but not to CCK and/or PP, suggesting that the GABAergic synapses may control endocrine pancreatic secretion selectively.
In neurones in which APDC decreased mEPSC frequency from 2.1 ± 0.53 to 0.85 ± 0.21 events s−1 (P < 0.05), PP (100 nm) decreased the frequency in 2 of 7 neurones (from 0.97 to 0.5 and from 2.73 to 0.99 events s−1), CCK (100 nm) increased the frequency in 1 of 7 from 0.76 to 1.2 events s−1, while exendin-4 (100 nm) increased the frequency in 4 of 7 neurones tested from 2.83 ± 0.82 to 4.88 ± 1.79 events s−1.
These data indicate that of the excitatory synaptic terminals responsive to group II mGluRs a small subgroup is responsive to exendin-4, CCK and/or PP, suggesting that these glutamatergic synapses may control both exocrine and endocrine pancreatic secretions.
None of three neurones in which l-AP4 decreased mIPSC frequency (from 1.6 ± 0.46 to 0.78 ± 0.26 events s−1) responded to PP, whereas all three responded to exendin-4 with an increased frequency (Fig. 2).
Twelve neurones in which l-AP4 decreased mEPSC frequency (from 1.72 ± 0.37 to 0.70 ± 0.13 events s−1; P < 0.05) were examined for responses to other peptides. One of nine neurones tested responded to PP with a decrease in mEPSC frequency from 1.16 to 0.65 events s−1, 1 of 12 responded to CCK with an increase in mEPSC frequency from 0.76 to 1.2 events s−1, whereas 7 of 11 responded to exendin-4 with an increased frequency (from 2 ± 0.53 to 3.69 ± 1.08 events s−1; P < 0.05).
These data show that, in contrast to group II mGluRs, group III mGluRs are present in the majority of exendin-4 responsive neurones, suggesting that these receptors may control pancreatic endocrine secretion mainly.
We then conducted a series of in vivo experiments to test whether the results of the electrophysiological experiments translated into the predicted secretory responses of the pancreas, i.e. APDC would modulate both PES and insulin secretion, while l-AP4 would modulate insulin secretion only.
Activation of group II mGluR increased, whereas activation of group III mGluR had no effect on, pancreatic exocrine secretion (PES)
Baseline volume of PES was 216 ± 11.4 μl (10 min)−1 and none of the pharmacological procedures described below changed it significantly; injections of vehicle into the DVC had no effect on PES (n = 3).
Microinjection in the DVC of APDC (0.18–1.04 nmol) increased PES in a dose-dependent manner (Table 1). Peak secretion was observed 10–20 min after microinjection and returned to baseline within 30 min. While microinjection of the highest dose of APDC (1.04 nmol) decreased PES volume, it also induced respiratory distress and was therefore not used for analysis. In contrast, microinjection of 15 nmol EGLU in the DVC had no effect on PES (341 ± 81.4 before and 320 ± 81.5 μg (10 min)−1 after EGLU injections; n = 6; P > 0.05; Fig. 3).
Table 1.
Brainstem microinjection of group II and group III mGluR agonists alter pancreatic exocrine and endocrine secretion
| PES (μg protein (10 min)−1) | Insulin (ng ml−1) | |
|---|---|---|
| APDC | ||
| Baseline | 269 ± 38.8 | 0.31 ± 0.05 |
| 0.18 nmol | 277 ± 29.7 | — |
| 0.4 nmol | 387 + 56.6 | — |
| 0.6 nmol | 449 ± 81.9* | 0.16 ± 0.03* |
| l-AP4 | ||
| Baseline | 179 ± 23.8 | 0.33 ± 0.08 |
| 0.6 nmol | 176 ± 20.9 | 0.20 ± 0.07* |
| 1.04 nmol | 211 ± 31.2 | — |
P < 0.05.
Figure 3. Microinjection of APDC into the DVC increases PES, whereas microinjection of EGLU has no effect.
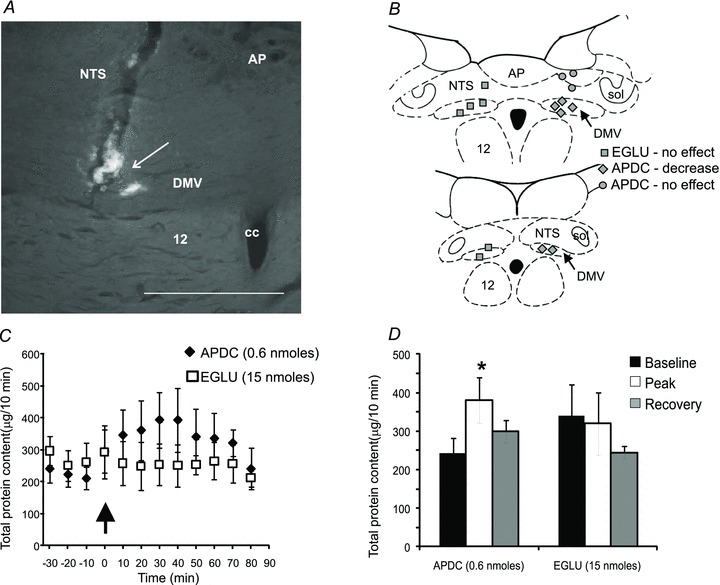
A, photomicrograph of a coronal section through the DVC showing the localization of APDC microinjection (arrow). Scale bar = 0.3 mm. B, schematic drawing of the DVC showing the location of APDC injection sites. Each dot represents one injection site shown on separate sides for clarity. C, graph showing the time course of the effect of APDC and EGLU microinjections into the DVC on the total protein output, measured at 10 min intervals. Microinjections were made at time 0 (arrow). D, bar graph showing the peak effect of APDC and EGLU on the total protein output.
Injections of APDC into regions on the dorsal surface of the DVC, probably outside the DMV (n = 6), did not alter protein secretion, suggesting that effects of APDC on PES are specific to actions at the DMV (Fig. 3). Microinjection of l-AP4 (0.6 and 1.04 nmol) had no effect upon PES (Table 1; n = 4 each; P > 0.05; data not shown).
These data suggest that activation of brainstem group II, but not group III, mGluRs modulates vagally mediated PES.
Activation of group II and group III mGluRs in the DVC decreased whereas exendin-4 increased insulin secretion
Baseline plasma insulin concentration was 0.28 ± 0.03 ng ml−1 and was unaffected by saline microinjection (97 ± 3.5% of control).
Microinjection of APDC or l-AP4 decreased plasma insulin secretion (Table 1) with the peak response occurring within 5–10 min and lasting 15–20 min (Fig. 4).
Figure 4. Microinjection of APDC or l-AP4 decreases while exendin-4 increases plasma insulin.
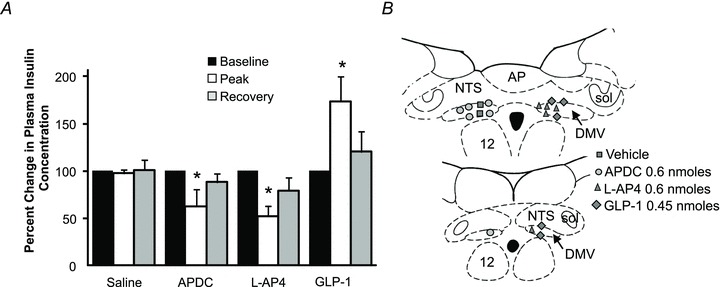
A, bar graph showing the normalized effects of APDC, l-AP-4 and GLP-1 on plasma insulin. B, schematic drawing of the DVC showing the location of the injection sites. Each dot represents one injection site shown on separate sides for clarity. *P < 0.05.
Following microinjection into the DVC of 0.45 nmol GLP-1, plasma insulin concentration increased from 0.25 ± 0.05 to 0.39 ± 0.07 ng ml−1, peaked 5 min after the injection and returned to baseline (0.28 ± 0.07 ng ml−1) 20 min later (Fig. 4).
These data suggest that activation of both group II and group III mGluRs in the DVC decrease insulin secretion. In addition, these data show that GLP-1-mediated activation of DVC neurons increases insulin secretion.
Discussion
Our study reports four novel findings: (1) group II and III mGluRs are present on both excitatory and inhibitory synapses impinging on pancreas-projecting DMV neurons, and these receptors are not tonically active; (2) neurons responding to group II mGluR agonists are likely to respond to CCK, PP and/or exendin-4, while the majority of neurones responding to l-AP4 respond to exendin-4 also; (3) activation of group II, but not group III, mGluRs in the DVC increases PES; (4) activation of both group II and III mGluRs in the DVC reduces, while exendin-4 increases, plasma insulin.
Recent reports have demonstrated a non-uniform role of mGluRs in the regulation of gastric vagal reflexes. Indeed, both group II and group III mGluRs inhibit mechanical sensitivity of vagal gastrooesophageal afferents in ferrets (Page et al. 2005); conversely, mice deficient in mGluR4 (a group III mGluR), have increased sensitivity of gastric mucosal receptors compared to wild-types, but similar sensitivity of tension receptors (Blackshaw et al. 2011). Furthermore, in gastric-projecting DMV neurons, group II mGluRs are activated tonically on inhibitory but not excitatory synapses, while group III mGluRs, although not activated tonically, are present on excitatory, but not inhibitory, synapses (Browning & Travagli, 2007).
The organization of mGluRs on pancreas-projecting neurones highlighted in the present study differs from that reported previously for gastric-projecting neurones (Browning & Travagli, 2007) and provides further support to the concept that separate sensory-motor vagal pathways modulate the diverse functions of organs along the upper GI tract. This discrete organization will allow further studies modelling pathophysiologies that affect vagal sensory-motor derangements, such as GERD, functional dyspepsia, gastroparesis and pancreatic exocrine or endocrine dysfunctions.
In pancreas-projecting DMV neurones, both group II and III are present presynaptically, although not activated tonically, on excitatory and inhibitory synapses. Group II mGluRs modulate vagal output controlling both PES and insulin secretion while group III mGluRs modulate vagal output to insulin secreting cells only. These data suggest a large degree of specificity of mGluRs on brainstem vagal circuits regulating pancreatic functions. Indeed, when combined with speculations from previous electrophysiological reports (Wan et al. 2007a,b,c; Viard et al. 2007), the electrophysiological and in vivo data presented here confirm that separate vagal circuits regulate PES or insulin secretion, and indicate that pharmacological treatments can be used to affect these circuits differentially. Such differences in the organization of neural circuits between gastric- and pancreatic-projecting neurones may reflect differences in afferent vagal inputs that regulate their activity.
Brainstem vagal gastric neurocircuits are under tonic influence of inhibitory γ-aminobutyric acid (GABA) inputs (Sivarao et al. 1998; Babic et al. 2011), while glutamatergic synapses do not appear to have a relevant role in controlling baseline gastric motility (Sivarao et al. 1998). Similarly, PES and insulin secretion are under tonic GABAergic control (Mussa & Verberne, 2008; Mussa et al. 2011); it is not known, however, whether ionotropic glutamatergic inputs within the brainstem neurocircuitry play a relevant role in their regulation.
We reported recently that the ongoing release of low levels of glutamate in the NTS results in tonic activation of mGluRs on GABAergic ‘gastric’ synapses in the DMV, which, by modulating the levels of cAMP (Cartmell & Schoepp, 2000), traffics receptors negatively coupled to adenylate cyclase to and from the presynaptic terminals. Consequently, mGluRs can differentially regulate the vagal motor output to the stomach (Browning & Travagli, 2001, 2009, 2010; Browning et al. 2004). In contrast, the lack of tonic activation of mGluRs on ‘pancreatic’ synapses highlights the potential for anatomical differences in synaptic inputs from vagal afferent fibres and suggests that vagal afferent fibres in pancreatic vago-vagal loops require a more robust activation to convey sensory information or may convey information via hormonal rather than vagal afferent pathways. This observation is further supported by recent data demonstrating the lack of vagal mechano- and chemoreceptors in pancreatic tissues (Schloithe et al. 2008).
Results of the present study suggest that mGluRs, independently of the neurocircuit activated, play a major role in modulating brainstem vagal synaptic terminals involved in regulating pancreatic secretion.
PES was increased by microinjection of the group II mGluR agonist, APDC. Since activation of group II mGluRs in the DMV had the same physiological outcome on PES as antagonism of GABAA receptors (Mussa & Verberne, 2008), it is possible that activation of group II mGluRs results in a decrease of GABA release onto pancreas-projecting DMV neurones. The subsequent disinhibition (i.e. excitation) of cholinergic excitatory neurones in intrapancreatic ganglia results in increased PES. This scenario implies that the effect on glutamatergic terminals from NTS to DMV may have a secondary role under baseline conditions. In fact, if a decreased release of glutamate was the predominant mechanism by which group II mGluRs affected PES, one would expect that microinjections of APDC into the DVC would have the opposite effect of that elicited by antagonism of brainstem GABAA receptors, i.e decrease in PES.
Both group II and III mGluR agonists microinjected into the DVC decreased, while exendin-4 increased, plasma insulin secretion. Since mGluR agonists decreased the frequency of miniature currents and exendin-4, instead, increased their frequency, one would reasonably surmise that distinct GABAergic and glutamatergic inputs to DMV neurons are involved in both non-adrenergic–non-cholinergic and cholinergic pathways to pancreatic β cells, in line with recent work from Verberne's group (Mussa et al. 2011).
In addition to being activated by vagal afferents from the viscera, NTS neurones also show an increase in activity under numerous physiological conditions, including increased plasma osmolarity, decreased plasma volume or stress (Crane et al. 2005; Geerling & Loewy, 2007; Goebel et al. 2009). Under these conditions, activation of NTS neurones could result in an increased release of GABA and/or glutamate onto DMV neurones regulating pancreatic secretions. By decreasing the release of GABA and glutamate onto DMV neurons, mGluRs may provide a means to stabilize the vagal output to the pancreas, thus preventing sudden changes in the activity of these neurones under different physiological conditions.
Another possibility is that pancreas-projecting DMV neurones do not receive tonic glutamatergic inputs under baseline conditions. This suggestion is supported by the data showing that synaptic terminals impinging on pancreas-projecting DMV neurones do not contain tonically active mGluRs and antagonism of these receptors had no effect on PES. However, a technical consideration that needs to be acknowledged is that in vivo experiments were done in fasted animals. Since PES and insulin secretions are stimulated by duodenal macronutrients and duodenal peptides (Li & Owyang, 1994; Li et al. 2000, 2001), the lack of response to l-AP4 may be due to the fact that the baseline PES is too low to detect additional decreases in pancreatic output.
Several lines of evidence have demonstrated that separate vagal pathways control exocrine and endocrine pancreatic secretions. Different stimulation frequencies have divergent effects on vagally stimulated gastric acid versus insulin and glucagon secretion (Berthoud & Powley, 1987), while electrical stimulation of gastric and hepatic branches of the vagus increase insulin secretion in an independent and additive manner (Berthoud & Powley, 1990; Berthoud et al. 1990), suggesting that different vagal branches may control different populations of pancreatic islet cells. Moreover, different neurotransmitters regulate pancreatic exocrine and endocrine secretions. Microinjections of PP into the DVC affect PES without affecting basal insulin secretion (Ahren et al. 1995; Krowicki & Hornby, 1995; Okumura et al. 1995; Putnam et al. 1989). In contrast, GLP-1 stimulates insulin secretion via direct actions on β-cells as well as via vagally mediated mechanisms (this paper and Drucker, 2006; Larsen & Holst, 2005). In addition, previous studies from our laboratory showed that pancreas-projecting DMV neurones respond to either GLP-1 or PP, but not both peptides (Wan et al. 2007c). These studies further support the concept that different neurocircuits regulate separate pancreatic functions.
In conclusion, in this study we report the first description of the discrete organization of mGluRs on brainstem vagal neurocircuits controlling pancreatic functions. We show that group II mGluRs are present on both excitatory and inhibitory synapses on neurones controlling insulin and PES. Conversely, group III mGluRs and GLP-1 receptors are present on excitatory and inhibitory synapses controlling insulin secretion only. The differences between brainstem vagal pancreatic- and gastric-controlling neurocircuits opens up promising avenues of research aimed at investigating models of sensory-motor derangements of the vagus, such as GERD, functional dyspepsia, gastroparesis and pancreatic insulin vs. protein secretion.
Acknowledgments
This work was supported by NSF grant 1049618 and NIH grants DK55530 and DK78364. We thank Cesare M. and Zoraide Travagli and W. Nairn Browning for support and encouragement.
Glossary
- CCK
cholecystokinin
- DMV
dorsal motor nucleus of the vagus
- DVC
dorsal vagal complex
- GABA
γ-aminobutyric acid
- GERD
gastroesophageal reflux disease
- GI
gastrointestinal
- GLP-1
glucagon-like peptide 1
- mEPSC
miniature excitatory postsynaptic current
- mIPSC
miniature inhibitory postsynaptic current
- mGluR
metabotropic glutamate receptor
- NTS
nucleus tractus solitarii
- PES
pancreatic exocrine secretion
- PP
pancreatic polypeptide
- TTX
tetrodotoxin
Author contributions
Conception and design of the experiments: T.B., K.B. and R.A.T. Collection, analysis and interpretation of data: T.B., X.T., Y.K. and R.A.T. Drafting the article or revising it critically for important intellectual content: T.B., K.N.B. and R.A.T. The authors have no disclosures.
References
- Ahren B, Gingerich RL, Havel PJ. Effects of cholecystokinin and glucagon-like peptide 1 on the secretion of pancreatic polypeptide in mice. Regul Pept. 1995;59:297–302. doi: 10.1016/0167-0115(95)00081-l. [DOI] [PubMed] [Google Scholar]
- Ami M, Doi R, Inoue K, Chowdhury P, Rayford PL. The influence of vagotomy on basal and postprandial pancreatic secretion and plasma levels of gastrointestinal hormones in conscious rats. Surg Gynecol Obstet. 1993;177:577–582. [PubMed] [Google Scholar]
- Babic T, Browning KN, Travagli RA. Differential organization of excitatory and inhibitory synapses within the rat dorsal vagal complex. Am J Physiol Gastrointest Liver Physiol. 2011;300:G21–G32. doi: 10.1152/ajpgi.00363.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthoud HR, Fox EA, Powley TL. Localization of vagal preganglionics that stimulate insulin and glucagon secretion. Am J Physiol Regul Integr Comp Physiol. 1990;258:R160–R168. doi: 10.1152/ajpregu.1990.258.1.R160. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Powley TL. Characteristics of gastric and pancreatic responses to vagal stimulation with varied frequencies: evidence for different fiber calibers? J Auton Nerv Syst. 1987;19:77–84. doi: 10.1016/0165-1838(87)90147-0. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Powley TL. Identification of vagal preganglionics that mediate cephalic phase insulin response. Am J Physiol Regul Integr Comp Physiol. 1990;258:R523–R530. doi: 10.1152/ajpregu.1990.258.2.R523. [DOI] [PubMed] [Google Scholar]
- Blackshaw LA, Page AJ, Young RL. Metabotropic glutamate receptors as novel therapeutic targets on visceral sensory pathways. Front Neurosci. 2011;5:40. doi: 10.3389/fnins.2011.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning KN, Coleman FH, Travagli RA. Characterization of pancreas-projecting rat dorsal motor nucleus of the vagus neurons. Am J Physiol Gastrointest Liver Physiol. 2005a;288:G950–G955. doi: 10.1152/ajpgi.00549.2004. [DOI] [PubMed] [Google Scholar]
- Browning KN, Coleman FH, Travagli RA. Effects of pancreatic polypeptide on pancreas-projecting rat dorsal motor nucleus of the vagus neurons. Am J Physiol Gastrointest Liver Physiol. 2005b;289:G209–G219. doi: 10.1152/ajpgi.00560.2004. [DOI] [PubMed] [Google Scholar]
- Browning KN, Kalyuzhny AE, Travagli RA. μ-Opioid receptor trafficking on inhibitory synapses in the rat brainstem. J Neurosci. 2004;24:9344–9352. doi: 10.1523/JNEUROSCI.1676-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning KN, Renehan WE, Travagli RA. Electrophysiological and morphological heterogeneity of rat dorsal vagal neurones which project to specific areas of the gastrointestinal tract. J Physiol. 1999;517:521–532. doi: 10.1111/j.1469-7793.1999.0521t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning KN, Travagli RA. The peptide TRH uncovers the presence of presynaptic 5-HT1A receptors via activation of a second messenger pathway in the rat dorsal vagal complex. J Physiol. 2001;531:425–435. doi: 10.1111/j.1469-7793.2001.0425i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning KN, Travagli RA. Functional organization of presynaptic metabotropic glutamate receptors in vagal brainstem circuits. J Neurosci. 2007;27:8979–8988. doi: 10.1523/JNEUROSCI.1105-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning KN, Travagli RA. Modulation of inhibitory neurotransmission in brainstem vagal circuits by NPY and PYY is controlled by cAMP levels. Neurogastroenterol Motil. 2009;21:1309–e126. doi: 10.1111/j.1365-2982.2009.01367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning KN, Travagli RA. Plasticity of vagal brainstem circuits in the control of gastric function. Neurogastroenterol Motil. 2010;22:1154–1163. doi: 10.1111/j.1365-2982.2010.01592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartmell J, Schoepp DD. Regulation of neurotransmitter release by metabotropic glutamate receptors. J Neurochem. 2000;75:889–907. doi: 10.1046/j.1471-4159.2000.0750889.x. [DOI] [PubMed] [Google Scholar]
- Chandra R, Liddle RA. Neural and hormonal regulation of pancreatic secretion. Curr Opin Gastroenterol. 2009;25:441–446. doi: 10.1097/MOG.0b013e32832e9c41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Bonham AC. Glutamate suppresses GABA release via presynaptic metabotropic glutamate receptors at baroreceptor neurones in rats. J Physiol. 2005;562:535–551. doi: 10.1113/jphysiol.2004.076885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Ling Eh EH, Horowitz JM, Bonham AC. Synaptic transmission in nucleus tractus solitarius is depressed by Group II and III but not Group I presynaptic metabotropic glutamate receptors in rats. J Physiol. 2002;538:773–786. doi: 10.1113/jphysiol.2001.012948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane JW, French KR, Buller KM. Patterns of neuronal activation in the rat brain and spinal cord in response to increasing durations of restraint stress. Stress. 2005;8:199–211. doi: 10.1080/10253890500333817. [DOI] [PubMed] [Google Scholar]
- Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3:153–165. doi: 10.1016/j.cmet.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Frisby CL, Mattsson JP, Jensen JM, Lehmann A, Dent J, Blackshaw LA. Inhibition of transient lower esophageal sphincter relaxation and gastroesophageal reflux by metabotropic glutamate receptor ligands. Gastroenterol. 2005;129:995–1004. doi: 10.1053/j.gastro.2005.06.069. [DOI] [PubMed] [Google Scholar]
- Geerling JC, Loewy AD. Sodium depletion activates the aldosterone-sensitive neurons in the NTS independently of thirst. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1338–R1348. doi: 10.1152/ajpregu.00391.2006. [DOI] [PubMed] [Google Scholar]
- Gicquel N, Nagain C, Chariot J, Tsocas A, Levenez F, Corring T, Roze C. Modulation of pancreatic secretion by capsaicin-sensitive sensory neurons in the rat. Pancreas. 1994;9:203–211. doi: 10.1097/00006676-199403000-00010. [DOI] [PubMed] [Google Scholar]
- Glaum SR, Miller RJ. Metabotropic glutamate receptors depress afferent excitatory transmission in the rat nucleus tractus solitarii. J Neurophysiol. 1993;70:2669–2672. doi: 10.1152/jn.1993.70.6.2669. [DOI] [PubMed] [Google Scholar]
- Goebel M, Stengel A, Wang L, Tache Y. Restraint stress activates nesfatin-1-immunoreactive brain nuclei in rats. Brain Res. 2009;1300:114–124. doi: 10.1016/j.brainres.2009.08.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes GM, Browning KN, Tong M, Qualls-Creekmore E, Travagli RA. Vagally mediated effects of glucagon-like peptide 1: in vitro and in vivo gastric actions. J Physiol. 2009;587:4749–4759. doi: 10.1113/jphysiol.2009.175067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inui A, Mizuno N, Oya M, Morioka H, Ogawa T, Ishida M, Baba S. Effects of amino acids on pancreatic polypeptide before and after vagotomy in the dog. Diabetologia. 1986;29:262–264. doi: 10.1007/BF00454888. [DOI] [PubMed] [Google Scholar]
- Ionescu E, Rohner-jeanrenaud F, Berthoud HR, Jeanrenaud B. Increases in plasma insulin levels in response to electrical stimulation of the dorsal motor nucleus of the vagus nerve. Endocrinology. 1983;112:904–910. doi: 10.1210/endo-112-3-904. [DOI] [PubMed] [Google Scholar]
- Jin YH, Bailey TW, Andresen MC. Cranial afferent glutamate heterosynaptically modulates GABA release onto second-order neurons via distinctly segregated metabotropic glutamate receptors. J Neurosci. 2004;24:9332–9340. doi: 10.1523/JNEUROSCI.1991-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindt S, Tack J. Impaired gastric accommodation and its role in dyspepsia. Gut. 2006;55:1685–1691. doi: 10.1136/gut.2005.085365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krowicki ZK, Hornby PJ. Pancreatic polypeptide, microinjected into the dorsal vagal complex, potentiates glucose-stimulated insulin secretion in the rat. Reg Pept. 1995;60:185–192. doi: 10.1016/0167-0115(95)00130-1. [DOI] [PubMed] [Google Scholar]
- Larsen PJ, Holst JJ. Glucagon-related peptide 1 (GLP-1): hormone and neurotransmitter. Regul Pept. 2005;128:97–107. doi: 10.1016/j.regpep.2004.08.026. [DOI] [PubMed] [Google Scholar]
- Li Y, Hao Y, Zhu J, Owyang C. Serotonin released from intrestinal enterochromaffin cells mediates luminal non-cholecystokinin-stimulated pancreatic secretion in rats. Gastroenterol. 2000;118:1197–1207. doi: 10.1016/s0016-5085(00)70373-8. [DOI] [PubMed] [Google Scholar]
- Li Y, Owyang C. Endogenous cholecystokinin stimulates pancreatic enzyme secretion via vagal afferent pathway in rats. Gastroenterol. 1994;107:525–531. doi: 10.1016/0016-5085(94)90180-5. [DOI] [PubMed] [Google Scholar]
- Li Y, Wu XY, Zhu JX, Owyang C. Intestinal serotonin acts as paracrine substance to mediate pancreatic secretion stimulated by luminal factors. Am J Physiol Gastrointest Liver Physiol. 2001;281:G916–G923. doi: 10.1152/ajpgi.2001.281.4.G916. [DOI] [PubMed] [Google Scholar]
- Liao Z, Li ZS, Lu Y, Wang WZ. Glutamate receptors within the nucleus of solitary tract contribute to pancreatic secretion stimulated by intraduodenal hypertonic saline. Auton Neurosci. 2005;120:62–67. doi: 10.1016/j.autneu.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Liu LS, Winston JH, Shenoy MM, Song GQ, Chen JD, Pasricha PJ. A rat model of chronic gastric sensorimotor dysfunction resulting from transient neonatal gastric irritation. Gastroenterol. 2008;134:2070–2079. doi: 10.1053/j.gastro.2008.02.093. [DOI] [PubMed] [Google Scholar]
- Mussa BM, Sartor DM, Rantzau C, Verberne AJ. Effects of nitric oxide synthase blockade on dorsal vagal stimulation-induced pancreatic insulin secretion. Brain Res. 2011;1394:62–70. doi: 10.1016/j.brainres.2011.04.015. [DOI] [PubMed] [Google Scholar]
- Mussa BM, Verberne AJ. Activation of the dorsal vagal nucleus increases pancreatic exocrine secretion in the rat. Neurosci Lett. 2008;433:71–76. doi: 10.1016/j.neulet.2007.12.048. [DOI] [PubMed] [Google Scholar]
- Okumura T, Pappas TN, Taylor IL. Pancreatic polypeptide microinjection into the dorsal motor nucleus inhibits pancreatic secretion in rats. Gastroenterol. 1995;108:1517–1525. doi: 10.1016/0016-5085(95)90702-5. [DOI] [PubMed] [Google Scholar]
- Page AJ, Young RL, Martin CM, Umaerus M, O’donnell TA, Cooper NJ, Coldwell JR, Hulander M, Mattsson JP, Lehmann A, Blackshaw LA. Metabotropic glutamate receptors inhibit mechanosensitivity in vagal sensory neurons. Gastroenterol. 2005;128:402–410. doi: 10.1053/j.gastro.2004.11.062. [DOI] [PubMed] [Google Scholar]
- Putnam WS, Liddle RA, Williams JA. Inhibitory regulation of rat exocrine pancreas by peptide YY and pancreatic polypeptide. Am J Physiol Gastrointest Liver Physiol. 1989;256:G698–G703. doi: 10.1152/ajpgi.1989.256.4.G698. [DOI] [PubMed] [Google Scholar]
- Rinaman L, Miselis RR. The organization of vagal innervation of rat pancreas using cholera toxin-horseradish peroxidase conjugate. J Auton Nerv Syst. 1987;21:109–125. doi: 10.1016/0165-1838(87)90014-2. [DOI] [PubMed] [Google Scholar]
- Schloithe AC, Sutherland K, Woods CM, Blackshaw LA, Davison JS, Toouli J, Saccone GT. A novel preparation to study rat pancreatic spinal and vagal mechanosensitive afferents in vitro. Neurogastroenterol Motil. 2008;20:1060–1069. doi: 10.1111/j.1365-2982.2008.01141.x. [DOI] [PubMed] [Google Scholar]
- Schmulson MJ, Mayer EA. Gastrointestinal sensory abnormalities in functional dyspepsia. Baillieres Clin Gastroenterol. 1998;12:545–556. doi: 10.1016/s0950-3528(98)90023-9. [DOI] [PubMed] [Google Scholar]
- Siaud P, Mekaouche M, Givalois L, Balmefrezol M, Marcilhac A, Ixart G. Effects of pharmacological lesion of adrenergic innervation of the dorsal vagal nucleus on pancreatic insulin secretion in normal and vagotomized rats. Physiol Res. 1995;44:227–231. [PubMed] [Google Scholar]
- Singer MV, Niebel W, Kniesburges S, Hoffmeister D, Goebell H. Action of atropine on the pancreatic secretory response to secretin before and after cutting the extrinsic nerves of the pancreas in dogs. Gastroenterol. 1986;90:355–361. doi: 10.1016/0016-5085(86)90932-7. [DOI] [PubMed] [Google Scholar]
- Sivarao DV, Krowicki ZK, Hornby PJ. Role of GABAA receptors in rat hindbrain nuclei controlling gastric motor function. Neurogastroenterol Motil. 1998;10:305–313. doi: 10.1046/j.1365-2982.1998.00110.x. [DOI] [PubMed] [Google Scholar]
- Travagli RA, Hermann GE, Browning KN, Rogers RC. Brainstem circuits regulating gastric function. Annu Rev Physiol. 2006;68:279–305. doi: 10.1146/annurev.physiol.68.040504.094635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Westerloo DJ, Giebelen IA, Florquin S, Bruno MJ, Larosa GJ, Ulloa L, Tracey KJ, van der PT. The vagus nerve and nicotinic receptors modulate experimental pancreatitis severity in mice. Gastroenterol. 2006;130:1822–1830. doi: 10.1053/j.gastro.2006.02.022. [DOI] [PubMed] [Google Scholar]
- Van Oudendove L, Demyttenaere K, Tack J, Aziz Q. Central nervous system involvement in functional gastrointestinal disorders. Best Pract Res Clin Gastroenterol. 2004;18:663–680. doi: 10.1016/j.bpg.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Viard E, Zheng Z, Wan S, Travagli RA. Vagallymediated, non paracrine effects of cholecystokinin-8s on rat pancreatic exocrine secretion. Am J Physiol Gastrointest Liver Physiol. 2007;293:G494–500. doi: 10.1152/ajpgi.00118.2007. [DOI] [PubMed] [Google Scholar]
- Wan S, Browning KN, Travagli RA. Glucagon-like peptide-1 modulates synaptic transmission to identified pancreas-projecting vagal motoneurons. Peptides. 2007a;28:2184–2191. doi: 10.1016/j.peptides.2007.08.016. [DOI] [PubMed] [Google Scholar]
- Wan S, Coleman FH, Travagli RA. Cholecystokinin-8s excites identified rat pancreatic-projecting vagal motoneurons. Am J Physiol Gastrointest Liver Physiol. 2007b;293:G484–492. doi: 10.1152/ajpgi.00116.2007. [DOI] [PubMed] [Google Scholar]
- Wan S, Coleman FH, Travagli RA. Glucagon-like peptide-1 (GLP-1) excites pancreas-projecting preganglionic vagal motoneurons. Am J Physiol Gastrointest Liver Physiol. 2007c;292:G1474–1482. doi: 10.1152/ajpgi.00562.2006. [DOI] [PubMed] [Google Scholar]


