Abstract
Purpose:
Implant surface treatments that improve early osseointegration may prove useful in long-term survival of uncemented implants. We investigated Acid Etching and Plasma Cleaning on titanium implants.
Methods:
In a randomized, paired animal study, four porous coated Ti implants were inserted into the femurs of each of ten dogs.
PC (Porous Coating; control)
PC+PSHA (Plasma Sprayed Hydroxyapatite; positive control)
PC+ET (Acid Etch)
PC+ET+PLCN (Plasma Cleaning)
After four weeks mechanical fixation was evaluated by push-out test and osseointegration by histomorphometry.
Results:
The PSHA-coated implants were better osseointegrated than the three other groups on outer surface implant porosity (p<0.05) while there was no statistical difference in deep surface implant porosity when compared with nontreated implant. Within the deep surface implant porosity, there was more newly formed bone in the control group compared to the ET and ET+PCLN groups (p<0.05). In all compared groups, there was no statistical difference in any biomechanical parameter.
Conclusions:
In terms of osseointegration on outer surface implant porosity PC+PSHA was superior to the other three groups. Neither the acid etching nor the plasma cleaning offered any advantage in terms of implant osseointegration. There was no statistical difference in any of the biomechanical parameters among all groups in the press-fit model at 4 weeks of evaluation time.
Keywords: Acid etching, canine, osseointegration, plasma cleaning, press-fit, titanium implants.
INTRODUCTION
Worldwide the need of arthroplastic surgery is considerable. Around one million hip replacements are made each year and the number of primary hip replacements is increasing [1]. Implant failure due to aseptic loosening is a very serious, painful and potentially invalidating complication and early initial fixation is essential to ensure long term survival of an implant [2, 3].
If the implant is not stable, micro motion between the implant and the surrounding bone will increase the risk of fibrous encapsulation of the implant [4, 5] which inhibits bone ingrowth and thus increases the risk of loosening of the implant.
Micro-scale topographical changes to the implant surface may affect cellular adhesion and proliferation. Surface modification may improve implant biocompatibility and thereby reduce the risk of long term implant failure.
In this study we attempt to improve early fixation/bone-implant interaction by 1) making micro-scale topographical changes by acid etching and 2) removing surface-adherent pro-inflammatory agents thereby potentially increasing biocompatibility by plasma cleaning.
Acid etching modifies the surface topography on the micro-scale leading to a greater roughness with potential to enhance implant osseointegration [6]. This treatment has been investigated in several studies, both in vitro and in vivo [7-9]. Acid etching creates a surface that enhances cell proliferation and differentiation [10, 11], as well as giving a relatively higher bone-to-implant contact, a better bone ingrowth and better osseointegration of experimental orthopedic titanium implants [12-14].
Plasma cleaning the implant surface could also improve fixation by creating a cleaner more hydrophilic surface relative to the conventional aqueous based processes. This is meant to increase surface wettability which could result in additional fixation benefit [15]. Additional to this primary effect plasma cleaning could yield a positive side effect by removing impurities such as endotoxins. This could potentially increase osseointegration, as endotoxins can induce an inflammatory response, leading to fibrous capsule formation [16]. As already mentioned this inhibits bone ingrowth, and thereby increases the probability of implant loosening [17]. Earlier studies have found promising result using plasma sterilization to increase biocompatibility and thereby osseointegration [18, 19].
The purpose of this canine study is to evaluate the effect of a specific acid etch surface treatment and plasma clean surface treatment on experimental titanium implants. We hypothesize that these surface modifications would improve biomechanical implant fixation and osseointegration.
MATERIALS AND METHODOLOGY
Design
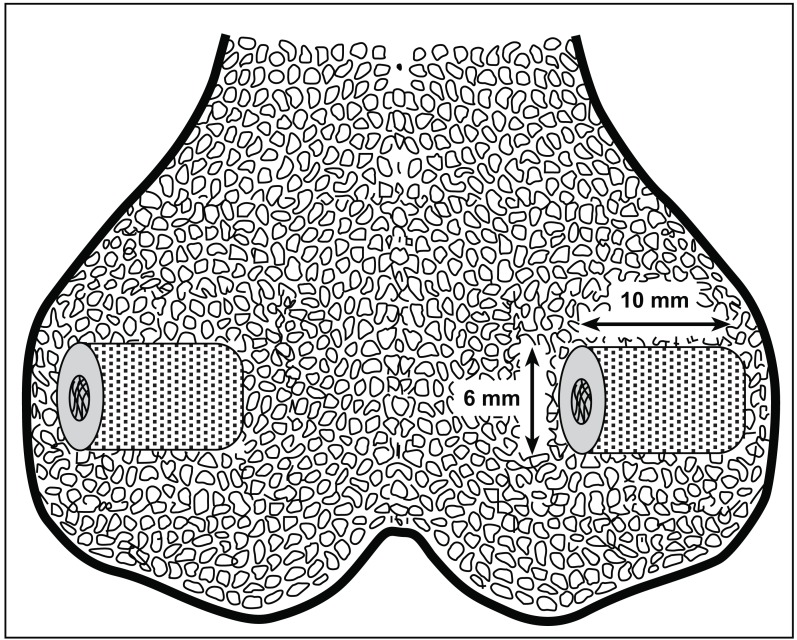
The study was a randomized, paired animal experiment with 10 dogs. Four implants were inserted into each dog: One in each medial and one in each lateral femoral epicondyle (Fig. 1). Bone quality was assumed equal between all four implant locations. The four implants were surface treated in different ways: 1. Porous coating as a negative control (PC), 2. porous coating with Plasma Sprayed Hydroxyapatite as a positive control (PC+PSHA), 3. porous coating with Acid Etching Surface Treatment (PC+ET), and 4. porous coating with Acid Etching and Plasma Cleaning Surface Treatment (PC+ET+PLCN). After an observation period of four weeks, the dogs were terminated and the bones containing the implants were harvested and frozen. Every implant was examined mechanically by push-out-test and microscopically by histomorphometry. The study was approved and monitored by the Danish National Animal Research Inspectorate.
Fig. (1).
Schematic drawing of implants inserted press-fit into femoral epicondyles.
Animals
Ten skeletally mature Labrador canines, with a mean body weight of 34 kg (range 25-39 kg) and an age of 14-15 months. All canines were female and specifically bred for research purpose.
Implants
Custom made cylindrical Titanium alloy core implants (Ti-6A1-4V, Ø = 6 mm, L = 10 mm) with commercially pure titanium porous coating and four different surface treatments were provided by DePuy Inc., Warsaw, IN, USA (Table 1). All Ti-6Al-4V substrates were per ASTM F-136. All Titanium beads were per ASTM F-67. All beads were attached by a sintering process in vacuum furnace. The porous coating had a mean pore diameter of 250 microns and a porosity of 40-50%. To avoid any potential pollution of the implants by hand or instruments when inserted into the bone, the implants were mounted on a threaded rod through a centrally threaded hole.
Table 1.
Implant Specifications
| Implant Type | Coating Material - 1 | Coating Material - 2 | Post Processing | Porosity/Avg. Pore Size |
|---|---|---|---|---|
| Porous Coating | Spherical Ti beads | None | None | 40-50%/250~300 µm |
| Porous Coating + PSHA | Spherical Ti beads | Plasma Sprayed Hydroxyapatite | None | 40-50%/250~300 µm |
| Porous Coating + ET | Spherical Ti beads | None | Acid Etch surface treatment | 40-50%/250~300 µm |
| Porous Coating + ET + PLCN | Spherical Ti beads | None | Acid Etch surface treatment + Plasma Cleaning surface treatment | 40-50%/250~300 µm |
Acid Etch Surface Treatment
The implants were treated at room temperature for six minutes in an acidified NaF solution to form micro‐scale texturing on the bead surface. Then the implants were soaked and rinsed with a detergent containing 1% Alconox and 2% Liquinox (Alconox Inc., White Plains, NY, USA) at 45° C for 30 minutes and then for three consecutive 15 minute treats with RO‐H2O at 45° C. The implants were dried at 60° C.
Evaluating the surface of the Acid Etched implants was done by applying the technology on a polished surface. Average surface roughness was 0.15 μm and XPS (X-ray Photoelectron Spectroscopy) confirmed that none of the chemicals were incorporated in the surface oxide layer.
Plasma Cleaning Surface Treatment
Implants were passivated prior to the cleaning process by the standard validated manufacturing passivation process (ASTM A967-05) used for clinically DePuy Orthopedic implants. Plasma cleaning was done in a plasma chamber (7200 RF Plasma System; PVA TePla America, Inc.) under following conditions: Cycle time of 30 mins, O2-gas flow rate at 250 sccm, chamber pressure of 300 mTorr, and power of 500 Watts.
Plasma Sprayed Hydroxyapatite
HA coating was provided by DePuy (DePuy Inc., Warsaw, IN, USA) using the same validated manufacturing processes used for clinically DePuy Orthopedic implants. All implants were passivated prior to the coating process. Plasma spraying Hydroxyapatite (HA) created implants with following specifications: Coating thickness 40‐60 μm. Crystallinity 78,4%. Calcium/Phosphate-ratio 1,67. Weight-% Tricalciumphosphate 3,29. Weight-% Hydroxyapatite 96,71. Tensile strength 81,0 MPa (Mean).
Surgical Procedure
Under general anesthesia using general sterile conditions, the femoral epicondyles were exposed starting with a 3 cm medial incision. A 2.0 mm guide wire was placed perpendicular to the epicondylar surface, 15 mm from the distal edge of the condyle and 10 mm from the anterior edge of the condyle. A cannulated drill bit of 5.5 mm diameter was then used to create an 11 mm deep drill hole. Drill speed of 2 rotations per second was used to avoid thermal trauma to the bone. Same procedure was repeated for the lateral epicondyle. In each drill hole, the implants were inserted press-fit by light hammer blows with a specially designed implant inserter tool to secure uniform axial placement. Finally, the soft tissues were closed in layers and 10 ml Bupivacaine was given as local infiltration analgesic. The procedure was repeated for the opposite side. Pre‐ and postoperatively, the dogs were given one dose of Cefuroxim, 1.5 g intravenously as antibiotic prophylaxis. Fentanyl transdermal patch (75 μg/h) lasting three days was given as postoperative analgesic treatment. All animals were postoperatively allowed unlimited activity and unrestricted movement. After a four week observation period, the dogs were sedated and euthanized with an overdose of hypersaturated barbiturate. The bones containing the implants were removed from the dogs and kept at -20° C until specimen preparation.
Preparation
The outermost 0.5 mm of the implant-bone specimen was cut off and discarded. The rest of the implant with surrounding bone was divided into two sections perpendicular to the long axis of the implant with a water cooled diamond band saw (Exakt Apparatebau; Norderstedt, Germany). The outermost 3.5 mm was refrozen for use in the mechanical test. The innermost 6.0 mm was stored in 70% alcohol at 5° C for use in histological analysis. The specimens were dehydrated in graded ethanol (70-100%) containing 0.4% basic fuchsine (Merck, Darmstadt, Germany), and embedded in methyl methacrylate (MMA; Merck, Hohenbrunn, Germany). From the MMA block four vertical, uniform, random sections were cut with a hard-tissue microtome (Leiden, KDG-95; MeProTech, Heerhugowaard, The Netherlands) around the centre part of each implant. Before making the sections, the MMA block was rotated randomly around its axis to avoid biased estimates. The 50-μm-thick sections were counterstained with 2% light green (BDH Laboratory Supplies, Poole, UK) and mounted on glass. This provides red staining of non-calcified tissue and green staining of calcified tissues.
Mechanical Testing
Implants were tested to failure on an axial push-out test machine (858 Mini Bionix; MTS, Eden Praire, MN, USA). The specimens were placed on a metal support jig with a 7.4 mm diameter central opening. The implant was centralized over the opening assuring a 0.7 mm distance between the implant and the support jig [20]. The direction of loading was from the cortical surface inward. The implant was pushed through the opening by a 5.0 mm diameter probe with a displacement rate of 5 mm/min on a 10 kN axial load cell. Each specimen length and diameter was measured with a micrometer and used to normalize push-out parameters [21]. Ultimate shear strength (MPa) was determined from the maximal force applied until failure of the bone-implant interface. Apparent stiffness (MPa/mm) was obtained from the slope of the linear section of the curve. Energy absorption (J/m2) was calculated from the area beneath the curve until failure. All push-out parameters were normalized by the cylindrical surface area of the transverse implant section tested.
Histomorphometrical Analysis
Blinded histomorphometrical analysis was done using a stereological software program (CAST grid; Olympus Denmark A/S, Ballerup, Denmark). Fields of vision from a light microscope were captured on a computer monitor and a user-specified grid was superimposed on the microscopic fields. Four vertical sections representative of each implant were analyzed and cumulated. This specimen preparation procedure and the grid system provided highly reliable results with negligible bias [22]. We defined two regions of interest: Zone 0 from implant core surface and 250 µm outwards, and zone 1 from a line between the core implant and “the outermost bead” and 750 µm outwards. In both zones bone-implant contact was defined as the implant surface covered with bone and estimated using sine-weighted lines. In zone 1 bone volume was estimated by point counting. Intraobserver variation was determined as coefficients of variation on double measurements on randomly selected implants (Table 3).
Table 3.
Variation Coefficient (CV %)
| Fibrous Tissue | New Bone | Lamellar Bone | Marrow Space | |||||
|---|---|---|---|---|---|---|---|---|
| Zone 0 (area) | < 1% | < 1% | - | < 1% | ||||
| Zone 1 (area) | - | < 1% | 41% | < 1% | ||||
| Zone 1 (vol.) | - | < 1% | < 1% | < 1% | ||||
Statistics
Intercooled STATA 8.0 software (StataCorp., College Station, TX) was used. All data followed a normal distribution and fulfilled the assumptions for one-way ANOVA. Data analyzed with ANOVA was followed by Students paired t-test. Differences between means were considered statistically significant for p-values less than 0.05.
RESULTS
Surgery
No postoperative complications were seen and all canines were fully weight bearing within three days of surgery. All animals completed the four week observation period. At the implant sites there were no clinical signs of infection.
Biomechanics
None of the four groups were statistical significantly different from others within any of the biomechanical parameters (Table 2).
Table 2.
Biomechanical Push-Out Data (Parametric), Mean (SD)
| Implant | Ultimate Shear Strength (MPa)* | Apparent Stiffness (MPa/mm)** | Total Energy Absorption (MJ/m2)*** | |||
|---|---|---|---|---|---|---|
| PC | 21.72 | (4.70) | 109.73 | (41.50) | 4.23 | (1.14) |
| PC+PSHA | 24.07 | (4.03) | 129.37 | (25.96) | 4.69 | (1.07) |
| PC+ET | 22.71 | (6.56) | 113.25 | (52.21) | 4.85 | (1.64) |
| PC+ET+PLCN | 22.00 | (6.92) | 118.71 | (41.54) | 4.15 | (1.58) |
p = 0.742 (ANOVA).
p = 0.734 (ANOVA).
p = 0.430 (ANOVA).
Histomorphometry
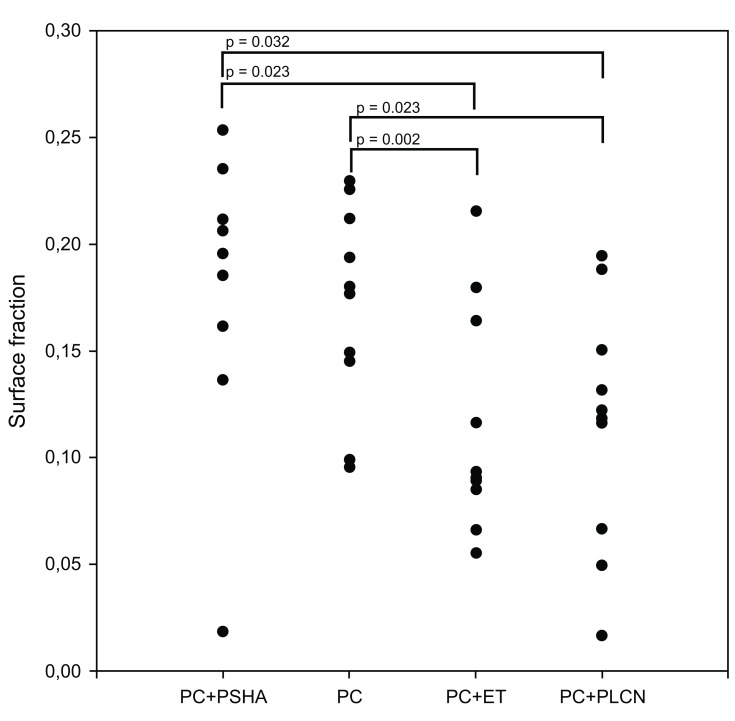
Statistically significant more new bone was observed in zone 0 of PC and PC+PSHA implants compared to the PC+ET and PC+ET+PLCN implants (Fig. 3). No statistical difference in the amount of new bone in zone 0 was observed between PC and PC+PSHA or between PC+ET and PC+ET+PLCN.
Fig. (3).
Histomorphometry; new bone surface fraction in zone 0.
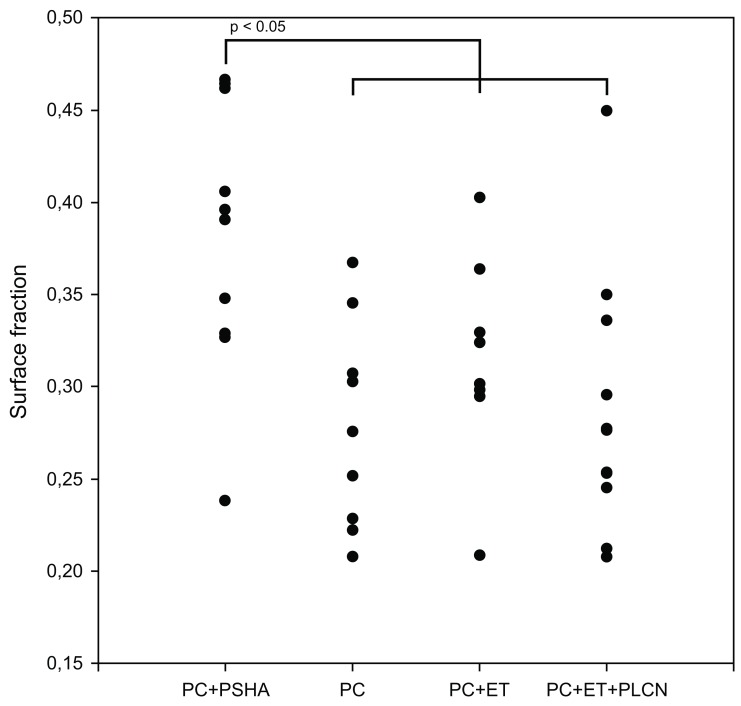
Statistically significant more new bone was observed in zone 1 of PC+PSHA compared to the other three coatings (Fig. 4). No statistically significant differences in volume fractions were found in zone 1.
Fig. (4).
Histomorphometry; new bone surface fraction in zone 1.
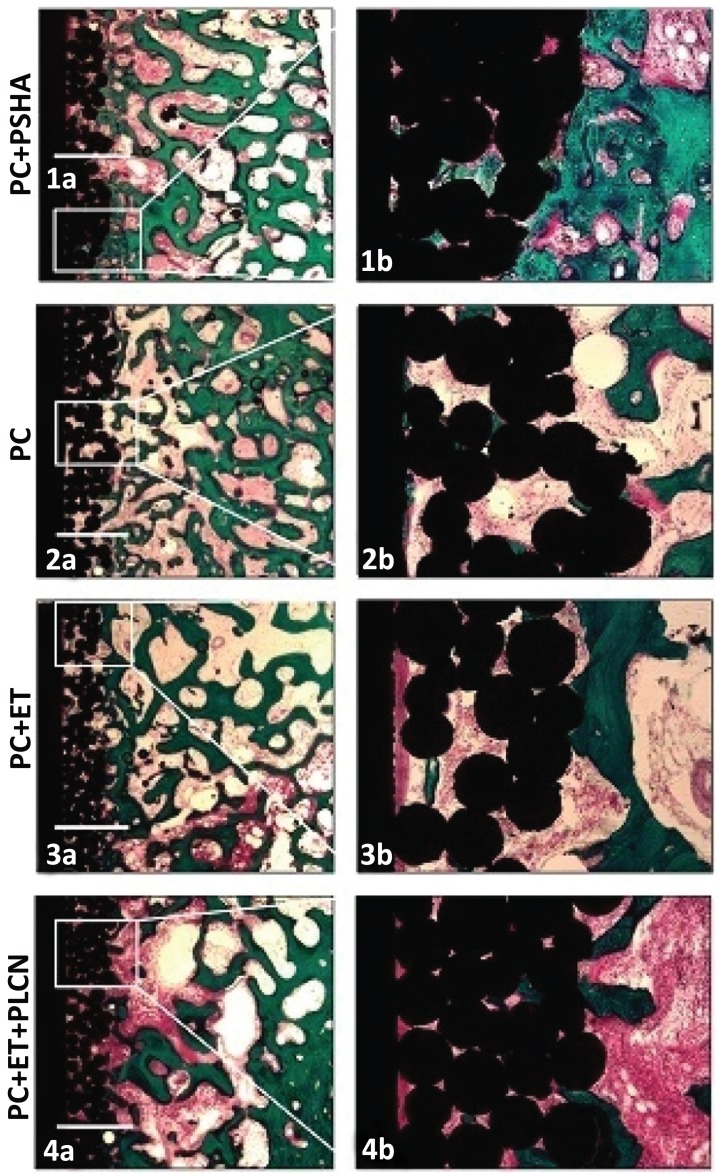
Histological all implants were osseointegrated and lamellar bone was evenly distributed around the drill cavity (Fig. 2). No delamination of the HA coating was observed.”
Fig. (2).
Representative histological sections of the four different treatment groups. More new bone was seen on the PSHA implants in both regions of interest (1a+b) compared to the intervention groups (3a+b and 4a+b). The control group (2a+b) had more new bone in the deep surface implant porosity compared to the intervention groups (3a+b and 4a+b). Both of the etched implant groups showed a distinct rim of fibrous tissue along the core implant (3a+b and 4a+b). Bar = 1 mm.
DISCUSSION
The aim of this study was to investigate a specific Acid Etch Surface Treatment and Plasma Cleaning Surface Treatment on porous coated titanium implants in a well established canine model of osseointegration [21, 23]. We could not identify positive effect of ET and ET+PLCN compared to the control PC group but PSHA-coated implants showed better osseointegration than the other three groups at outer surface implant porosity and that PSHA-coated implants showed better osseointegration than the ET and ET+PLCN groups at deep implant porosity.
This study was designed to investigate effect on early fixation, why conclusion on long term effect should be done with caution. The four weeks observation period was used since this point between healing and remodeling was ideal to measure differences between treatments that affect initial fixation [24, 25]. Previous studies using same model found statistically significant difference in both histological and mechanical parameters [23].
The experimental model used represents the part of cementless joint replacements placed in cancellous bone. We used a non-weight-bearing setup. Compared to a weight-bearing setup it lacks the more clinically relevant conditions as direct load and joint fluid pressure, but it is well standardized and has a high degree of variable control. Although load is not directly applied, the implants are susceptible to load through the biomechanical energy transmission of the bone. Canines were used, as the architecture and composition of canine bone is similar and comparable to human bone [26, 27]. Canine cancellous epiphyseal bone was chosen because the close resemblance with bone where cementless joint replacements are usually implanted. No animal model, however, gives complete information about the effect of a given alloy on human osteogenesis. The four differently treated implants were inserted in each dog, making each dog its own control and thereby preventing interspecies variation. PSHA-coated implants was used as positive control and served as validation of our model by its positive outcome [21]. Young canines were used with assumed high healing potential and in contrast to elderly canines with assumed low healing potential a statistically significant difference is harder to detect, making any difference more clinically relevant [28].
No statistically significant difference was found in the biomechanical data within any of the parameters. The fact that the implants were inserted press-fit and all implants initially were in close contact with bone, could make a potential difference difficult to be detected within the observation period, explaining why we found no correlation between biomechanical and histomorphometrical data as we have previously found using this model [29]. Processing the biomechanical data from the lateral and medial epicondyle separately we find a statistically significant difference. This study is a randomized, paired experiment with systematic rotation of the implants and conclusion should be done with caution as this difference could contribute to an increased variation in the biomechanical analysis but not any bias. Previous studies using same animal experimental model demonstrates a statistically significant difference without differentiation between medial and lateral femoral epicondyle [28].
Within the deep surface implant porosity, we found statistical significantly less newly formed bone in the ET and ET+PLCN groups compared to the PC and PC+PSHA groups, but no statistically significant difference between the PC and PC+PSHA groups. This difference may be due to not rinsed away acidic remnants of the ET or that this specific ET creates a less biocompatible surface. The ET process was evaluated with XPS by applying the treatment on a polished surface, meaning that the surfaces of the ET implants were not directly evaluated. This study cautions robust cleaning of hydrofluoric acid treated deep implant porosity.
The potential positive side effect of plasma cleaning was to remove impurities such as endotoxins. This bio burden can be an overseen problem, when orthopedic implants are inserted in humans [16]. Statistically significant amounts of endotoxins have been found on the surface of commercially available implant components [30]. Endotoxins are aggressive inflammatory agents that may contribute to implant failure by aseptic loosening [31]. New methods of clearing endotoxins from the surface of prostheses have recently been developed [30] and in this study the specific plasma cleaning procedure could remove these impurities as a secondary advantage.
Using this model previous studies found a positive effect of ET on an hydrofluoric treatment of cylindrical non porous surface [32] and now we found no or poorer effect of ET on a porous implant. The technology used in the two different experiments was not similar procedures. They differed with regards to implant surface texture (porous vs non porous) and process parameters (acidic solution, cycle-time, temperature, rinsing and drying), and is therefore not directly comparable. This diversity in outcome calls for further studies on this level of investigation and emphasizes the importance of optimizing the technology before conducting clinical trials. Several other studies in rabbits found a positive effect of acid etching with an enhances early osseointegration [7] and higher strengths of osseointegration of titanium implants [9], with a surface less porous than in our study.
No effect of PLCN was observed on ET implants. The purpose of PLCN was to increase metal surface wettability [15]. The effect could be minimized as the implants post-PLCN was not immersed in isotonic water to keep it from re-contact with air. Furthermore as the potential additional effect of PLCN, acidic passivation can minimize the amount of endotoxins [30, 33], and it is therefore difficult to conclude whether the lack of effect is due to the endotoxin reducing effect of ET or PLCN itself. Interesting perspectives would be intervention groups with PC+PLCN and PC+ET+PLCN+isotonic water. Another interesting perspective would be to insert the implants in a gap model, making it potentially easier to detect a small difference in new bone formation.
CONCLUSION
This study indicates that the hydrofluoric acid etched implants may cause reduced biocompatibility at deep implant porosity while such difference was not observed on the outer surface implant porosity, cautioning cleaning hydrofluoric acid from deep implant porosity. Furthermore, this study shows that HA coating is superior to non‐HA coated implants with respect to biocompatibility. The lack of positive effect of acid etching compared to previous findings in our group emphasizes the need of preclinical research before using an experimental outcome in a clinical trial.
ACKNOWLEDGEMENTS
We thank Anette Milton and Anna Bay Nielsen from Department of Orthopaedics, Aarhus University Hospital for their skillful technical assistance. DePuy. Inc., Warsaw, IN, USA donated the implants and funded the work. We thank The Korning Foundation for economical support.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1. Lucht U. The danish hip arthroplasty register. Acta Orthop Scand. 2000;71(5):433–9. doi: 10.1080/000164700317381081. [DOI] [PubMed] [Google Scholar]
- 2. Karrholm J, Borssen B, Lowenhielm G, Snorrason F. Does early micromotion of femoral stem prostheses matter? 4-7-year stereoradiographic follow-up of 84 cemented prostheses. J Bone Joint Surg Br. 1994;76(6):912–7. [PubMed] [Google Scholar]
- 3. Ryd L, Albrektsson BE, Carlsson L, et al. Roentgen stereophotogrammetric analysis as a predictor of mechanical loosening of knee prostheses. J Bone Joint Surg Br. 1995;77(3):377–83. [PubMed] [Google Scholar]
- 4. Soballe K, Hansen ES, Rasmussen H, Jorgensen PH, Bunger C. Tissue ingrowth into titanium and hydroxyapatite-coated implants during stable and unstable mechanical conditions. J Orthop Res. 1992;10(2):285–99. doi: 10.1002/jor.1100100216. [DOI] [PubMed] [Google Scholar]
- 5. Aspenberg P, Herbertsson P. Periprosthetic bone resorption. Particles versus movement. J Bone Joint Surg Br. 1996;78(4):641–6. [PubMed] [Google Scholar]
- 6. Fandridis J, Papadopoulos T. Surface characterization of three titanium dental implants. Implant Dent. 2008;17(1):91–9. doi: 10.1097/ID.0b013e318166d9ac. [DOI] [PubMed] [Google Scholar]
- 7. Klokkevold PR, Johnson P, Dadgostari S, Caputo A, Davies JE, Nishimura RD. Early endosseous integration enhanced by dual acid etching of titanium: a torque removal study in the rabbit. Clin Oral Implants Res. 2001;12(4):350–7. doi: 10.1034/j.1600-0501.2001.012004350.x. [DOI] [PubMed] [Google Scholar]
- 8. Att W, Tsukimura N, Suzuki T, Ogawa T. Effect of supramicron roughness characteristics produced by 1- and 2-step acid etching on the osseointegration capability of titanium. Int J Oral Maxillofac Implants. 2007;22(5):719–28. [PubMed] [Google Scholar]
- 9. Cho SA, Park KT. The removal torque of titanium screw inserted in rabbit tibia treated by dual acid etching. Biomaterials. 2003;24(20):3611–7. doi: 10.1016/s0142-9612(03)00218-7. [DOI] [PubMed] [Google Scholar]
- 10. Wei YP, Zhang YM, Zhao YT, Yu ZT, Xu ZX. Studying the biocompatibility of implant titanium alloy after surface treatment by sandblasting and etching. Hua Xi Kou Qiang Yi Xue Za Zhi. 2007;25(6):529–31, 535. [PubMed] [Google Scholar]
- 11. Maekawa K, Yoshida Y, Mine A, van MB, Suzuki K, Kuboki T. Effect of polyphosphoric acid pre-treatment of titanium on attachment, proliferation, and differentiation of osteoblast-like cells (MC3T3-E1) Clin Oral Implants Res. 2008;19(3):320–5. doi: 10.1111/j.1600-0501.2007.01477.x. [DOI] [PubMed] [Google Scholar]
- 12. D'Lima DD, Lemperle SM, Chen PC, Holmes RE, Colwell CW., Jr Bone response to implant surface morphology. J Arthroplasty. 1998;13(8):928–34. doi: 10.1016/s0883-5403(98)90201-7. [DOI] [PubMed] [Google Scholar]
- 13. Cordioli G, Majzoub Z, Piattelli A, Scarano A. Removal torque and histomorphometric investigation of 4 different titanium surfaces: an experimental study in the rabbit tibia. Int J Oral Maxillofac Implants. 2000;15(5):668–74. [PubMed] [Google Scholar]
- 14. Hacking SA, Harvey EJ, Tanzer M, Krygier JJ, Bobyn JD. Acid-etched microtexture for enhancement of bone growth into porous-coated implants. J Bone Joint Surg Br. 2003;85(8):1182–9. doi: 10.1302/0301-620x.85b8.14233. [DOI] [PubMed] [Google Scholar]
- 15. Zhao G, Raines AL, Wieland M, Schwartz Z, Boyan BD. Requirement for both micron- and submicron scale structure for synergistic responses of osteoblasts to substrate surface energy and topography. Biomaterials. 2007;28(18):2821–9. doi: 10.1016/j.biomaterials.2007.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Greenfield EM, Bi Y, Ragab AA, Goldberg VM, Nalepka JL, Seabold JM. Does endotoxin contribute to aseptic loosening of orthopedic implants? J Biomed Mater Res B Appl Biomater. 2005;72(1):179–85. doi: 10.1002/jbm.b.30150. [DOI] [PubMed] [Google Scholar]
- 17. Santavirta S, Xu JW, Hietanen J, Ceponis A, Sorsa T, Kontio R, Konttinen YT. Activation of periprosthetic connective tissue in aseptic loosening of total hip replacements. Clin Orthop Relat Res. 1998;352:16–24. [PubMed] [Google Scholar]
- 18. Tessarolo F, Caola I, Nollo G, Antolini R, Guarrera GM, Caciagli P. Efficiency in endotoxin removal by a reprocessing protocol for electrophysiology catheters based on hydrogen peroxide plasma sterilization. Int J Hyg Environ Health. 2006;209(6):557–65. doi: 10.1016/j.ijheh.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 19. Lerouge S, Wertheimer MR, Yahia LH. Plasma sterilization: a review of parameters, mechanisms, and limitations. Plasmas Polym. 2001;6(3):175–88. [Google Scholar]
- 20. Dhert WJ, Verheyen CC, Braak LH, et al. A finite element analysis of the push-out test: influence of test conditions. J Biomed Mater Res. 1992;26(1):119–30. doi: 10.1002/jbm.820260111. [DOI] [PubMed] [Google Scholar]
- 21.Soballe K. Hydroxyapatite ceramic coating for bone implant fixation. Mechanical and histological studies in dogs. Acta Orthop Scand Suppl. 1993;255:1–58. doi: 10.3109/17453679309155636. [DOI] [PubMed] [Google Scholar]
- 22. Baas J. Adjuvant therapies of bone graft around non-cemented experimental orthopedic implants. Stereological methods and experiments in dogs. Acta Orthop Suppl. 2008;79(330):1–43. [PubMed] [Google Scholar]
- 23. Elmengaard B, Bechtold JE, Soballe K. In vivo study of the effect of RGD treatment on bone ongrowth on press-fit titanium alloy implants. Biomaterials. 2005;26(17):3521–6. doi: 10.1016/j.biomaterials.2004.09.039. [DOI] [PubMed] [Google Scholar]
- 24. Soballe K, Hansen ES, Brockstedt-Rasmussen H, Pedersen CM, Bunger C. Hydroxyapatite coating enhances fixation of porous coated implants. A comparison in dogs between press fit and noninterference fit. Acta Orthop Scand. 1990;61(4):299–306. doi: 10.3109/17453679008993521. [DOI] [PubMed] [Google Scholar]
- 25. Jakobsen T, Kold S, Bechtold JE, Elmengaard B, Soballe K. Local alendronate increases fixation of implants inserted with bone compaction: 12-week canine study. J Orthop Res. 2006;25(4):432–41. doi: 10.1002/jor.20276. [DOI] [PubMed] [Google Scholar]
- 26. Eitel F, Klapp F, Jacobson W, Schweiberer L. Bone regeneration in animals and in man. A contribution to understanding the relative value of animal experiments to human pathophysiology. Arch Orthop Trauma Surg. 1981;99(1):59–64. doi: 10.1007/BF00400911. [DOI] [PubMed] [Google Scholar]
- 27. Aerssens J, Boonen S, Lowet G, Dequeker J. Interspecies differences in bone composition, density, and quality: potential implications for in vivo bone research. Endocrinology. 1998;139(2):663–70. doi: 10.1210/endo.139.2.5751. [DOI] [PubMed] [Google Scholar]
- 28. Baas J, Lamberg A, Jensen TB, Elmengaard B, Soballe K. The bovine bone protein lyophilisate Colloss improves fixation of allografted implants--an experimental study in dogs. Acta Orthop. 2006;77(5):791–8. doi: 10.1080/17453670610013015. [DOI] [PubMed] [Google Scholar]
- 29. Jakobsen T, Kold S, Bechtold JE, Elmengaard B, Soballe K. Effect of topical alendronate treatment on fixation of implants inserted with bone compaction. Clin Orthop Relat Res. 2006;444:229–34. doi: 10.1097/01.blo.0000191273.34786.40. [DOI] [PubMed] [Google Scholar]
- 30. Ragab AA, Van De MR, Lavish SA, et al. Measurement and removal of adherent endotoxin from titanium particles and implant surfaces. J Orthop Res. 1999;17(6):803–9. doi: 10.1002/jor.1100170603. [DOI] [PubMed] [Google Scholar]
- 31. Bi Y, Seabold JM, Kaar SG, Ragab AA, Goldberg VM, Anderson JM, Greenfield EM. Adherent endotoxin on orthopedic wear particles stimulates cytokine production and osteoclast differentiation. J Bone Miner Res. 2001;16(11):2082–91. doi: 10.1359/jbmr.2001.16.11.2082. [DOI] [PubMed] [Google Scholar]
- 32. Daugaard H, Elmengaard B, Bechtold JE, Soballe K. Bone growth enhancement in vivo on press-fit titanium alloy implants with acid etched microtexture. J Biomed Mater Res A. 2008;87(2):434–40. doi: 10.1002/jbm.a.31748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zablotsky MH, Diedrich DL, Meffert RM. Detoxification of endotoxin-contaminated titanium and hydroxyapatite-coated surfaces utilizing various chemotherapeutic and mechanical modalities. Implant Dent. 1992;1(2):154–8. doi: 10.1097/00008505-199205000-00009. [DOI] [PubMed] [Google Scholar]