Abstract
Background:
Diabetes is an important disease. This disease is a metabolic disorder characterized by hyperglycemia resulting from perturbation in insulin secretion, insulin action or both. Honey bee venom contains a wide range of polypeptide agents. The principle components of bee venom are mellitin and phospholipase A2. These components increase insulin secretion from the β-cells of pancreas. This study was conducted to show the hypoglycemic effect of honey bee venom on alloxan induced diabetic male rats.
Methods:
Eighteen adult male rats weighting 200±20 g were placed into 3 randomly groups: control, alloxan monohydrate-induced diabetic rat and treated group that received honey bee venom daily before their nutrition for four months. Forty eight hours after the last injection, blood was collected from their heart, serum was dissented and blood glucose, insulin, triglyceride and total cholesterol were determined.
Results:
Glucose serum, triglyceride and total cholesterol level in treated group in comparison with diabetic group was significantly decreased (P< 0.01). On the other hand, using bee venom causes increase in insulin serum in comparison with diabetic group (P< 0.05).
Conclusion:
Honeybee venom (apitoxin) can be used as therapeutic option to lower blood glucose and lipids in diabetic rats.
Keywords: Alloxan, Glucose, Honeybee Venom, Rat
Introduction
Diabetes type 1 is mainly results from auto-immune beta cell destruction, while viral infections and chemical agents seem to be the triggers of the disease. The well-documented effect of insulin is to mediate carbohydrates, proteins and lipids storage. Therefore diabetes is considered as a defect of lipids, proteins and carbohydrates metabolism in which all the body systems and organs are affected (Williams and Pickup 2000). The effect of insulin deficiency mainly results in elevation of cholesterol, phospholipids and free fatty acid (Williams and Pickup 2000). Natural toxins have been traditionally used to heal diseases and honeybee venom (apitoxin) is of a great importance in this regard.
The venom is composed of varieties of peptides (mellitin, apamin, secapin, tertiapin, ado lapin, and MCD peptid), enzymes (phospholipase A2, hyaluronidase, acid phosphomonoesterase, lysophospholipase), active amines (histamine, dopamine, norepinephrine, serotonin) and many other substances (Son et al. 2007). Bee venom and Bee sting had significant effect on quality of life (Wesselius et al. 2005). Mellitin and phospholipase A2 are the most important ingredients and play a great role in irritation and allergic responses leading to anti-inflammatory and analgesic effects (Mazzanti et al. 2007). Mellitin as the most important ingredient in bee venom is a strong phospholipase A2 activator which is composed of 26 amino acids and makes up 50% of the dried venom. Those bee venom ingredients that contain a high molecular weight, like hyaluronidase and phospholipase A2, cause the immune reactions (Bomalaski et al. 1989, Zalat et al. 1999). Apamin, mellitin and phospholipase A2 contained in bee venom, are strong immunoregulators but their effects on diabetes have not been investigated (Habermann 1972, Gauldie et al. 1976). BV apamin is known as a neurotoxin from central nervus system hyperexcitability through inhibition of the axonal potassium channels (Son et al. 2007, Pedarzani et al. 2008). Melittin,the major constituent of bee venom, has an anti-inflammatory effect through its functionality on the anterior pituitary gland, which in turns may result in stimulation of cortisol production from the adrenal gland (Son et al. 2007). Part of anti-inflammatory effect of melittin may attribute to its interaction with cell surface moieties which seems to render the cytotoxic effects of melittin on cancer cells (Mirshafiey 2007, Son et al. 2007).
With regard to the side effects and high prices of blood sugar lowering chemicals, achieving new therapeutic agents with low side effects seems to be necessary. Nowadays diabetic’s population is growing rapidly while encountering various life-threatening disease conditions that require researchers to evaluate related therapeutic, alleviative and preventive treatments. The main purpose of the study was to evaluate the effects of Iranian honeybee venom (apitoxin) on blood levels of glucose and insulin in alloxan induced diabetic rat.
Materials and Methods
Honeybee venom preparation procedure
Bee venom samples were collected from beehives using an electric shocker, on February 2010 in Khuzestan, Iran. The electric shocker is composed of two components: one component as shocker and the other to collect the venom and concomitant material. The collecting unit is wooden and composed of a network of wires with small gaps between them. A glass plane is inserted under the network. The shocker was supplied by a transformer to produce a light electric shock. The shocker is designed to produce a light electric shock once every few seconds. The collector panel was first located on bottom of the beehives and then on the top, to collect the desired amount of the bee venom. When the shocker was turned on, the honeybees stroked the wires and received a light electric shock and were stimulated them to sting and discharged their bee venom. Alarm pheromone produced by the exited bees to crowd and discharge toxin. The shocker was turned off after 25 minutes and the collecting panel was removed from the beehive, and the dried bee venom material scratched and transferred to a proper container. To evaluate the toxin production efficiency after collecting the samples, the crystallized bee venom material weighed using a sensitive scale.
Animals
Healthy adult male Lewwis rats weighting 200±20 g were maintained in Darou Pakhsh Pharmaceutical Mfg Co, Tehran (Iran). All animals left to acclimatize for one week before the experiment. The animal room was maintained under a constant 12-h light: 12-h dark cycle and temperature of 23±3 °C and relative humidity of 70±10% throughout the experimental period. The rats were given free access to standard pellets and water. For bees to be adapted to the new environment condition, all the experiments were carried out 10 days after their first residence.
The research was approved by Ethical Committee of the university.
Experimental design
Eighteen rats were placed randomly into three groups (n= 6):
Control group: normal saline 0/9% injected intraperitoneally.
Diabetic group: this group became diabetic by injection Alloxan monohydrate at 150 mg/kg intraperitoneally.
Bee venom-treated group: at first received Alloxan monohydrate to induce diabetes and Iranian bee venom (apitoxin) at 0/5 mg/kg (the best dose chosen after pretest) after diabetes confirmation intraperitoneally at fasting condition every day for four consecutive weeks (Kim et al. 1999, Dong et al. 2007).
Blood sugar test was performed in all groups. Blood glucose level was measured using Acua Check (Germany). To measure the blood glucose level, a small incision was made on the animals’ tail using a lancet and a drop of fresh blood was extracted and used for glucometery. These samples were collected in fasting condition and expressed in mg/dl.
Alloxan monohydrate (Sigma-Aldrich Germany) was used to induce diabetes in rats. The drug was administrated intraperitoneally at the rate of 150 mg/kg (Viana et al. 2004, Antia et al. 2005). The drug administration leads to pancreas β -cells apoptosis and necrosis. The method is preferred to induce diabetes in many other animals (Soto et al. 2001). Following the administration a condition of hyperglycemia like diabetes type 1 appeared in rats (Byung-Hyun and Jin-Woo 2001). Seventy two hours after the administration, blood glucose level was measured using Acua Check to determine diabetic condition. In this study, blood glucose level elevation over 280 mg/dl is supposed to reveal diabetic condition (Zhang and Tan 2003). Usual signs of diabetes including polydipsia, polyuria and weight loss were observed 6–7 days following the administration (Nuraliev et al. 1992, El- demerdash et al. 2005).
To measure glucose level, fasting blood samples were collected 2 weeks following bee venom administration using “Stone” method. In this method, venous blood was collected from infraorbital sinus using a hematocrit tube. The animal is kept between forefinger and thumb while the tube is inserted to the orbital foramen with a rotational movement. The capillaries are usually very sensitive and fragile here and burst following a light pressure. When a few almost large drops of blood were collected, the hematocrit tube is removed. The method is suitable for repetitive blood sampling especially for primary analysis following 2 weeks of administration. The blood glucose level was determined using enzymatic kits.
Biochemical analysis
The animals were anesthetized with ether after 16 h of fasting to collect blood for analysis. Forty eight hours after the last injection, blood samples were collected from the heart and centrifuged (3000 rpm for 15 min at 4 °C) for separating the serum. The serum was then frozen at −70 °C for the biochemical analysis. Then the amount of blood glucose, insulin, cholesterol and triglyceride in blood serum weredetermined.Serum glucose level was measured by kinetic (enzymatic) and colorimetric methods using Glucose Estimation Kit (Pars Azmoon, Iran). Theserum insulin levels were assayed with an ELISA, IRMA (Biosource Europe SA), serum glucose, triglyceride and total cholesterol levels were determinedusingcommercial kits and enzymatic assays.
Statistical analysis
The values were expressed as mean ±S.E.M Statistical analyses were performed by one way analysis of variance (ANOVA) followed by Tukey multiple comparison test. P< 0.05 were considered as significant.
Results
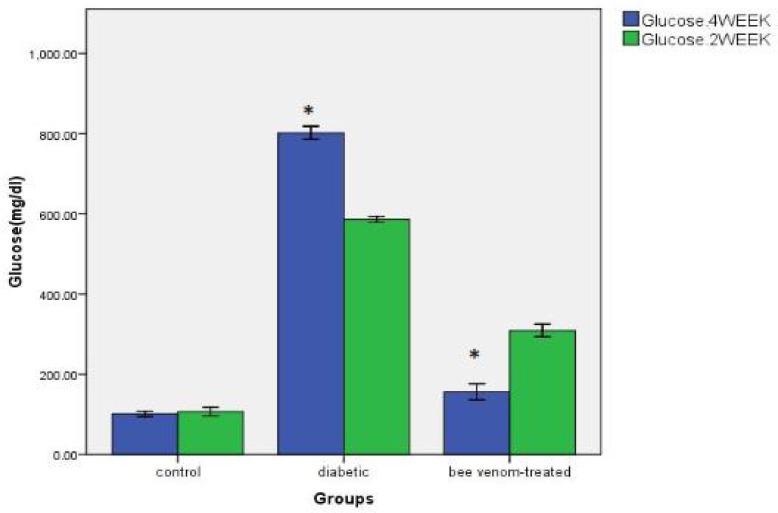
Bee venom preparation results: the bee venom samples were collected and panel scratched using a sterile blade and kept in clean dark vials. Average amount of venom collected from three beehives (each containing estimated 10000 bees) was 147.7 mg. The average for each beehive was calculated 49.56 mg. Alloxan administration result showed a significant increase in blood glucose level in (diabetic) group compared with control group after 2 and 4 weeks (P< 0.05). In addition a significant increase was observed in blood glucose level in diabetic group after 4 weeks compared with 2-week period (P< 0.05). There was a significant decline in blood glucose level in bee venom- treated group compared with diabetic group (P< 0.05). Also there was a significant decline in blood glucose level in diabetic treated group after 4 weeks compared with 2-week period (P< 0.05) (Fig. 1).
Fig. 1.
Changes of blood glucose level after injection of bee venom in diabetic rats
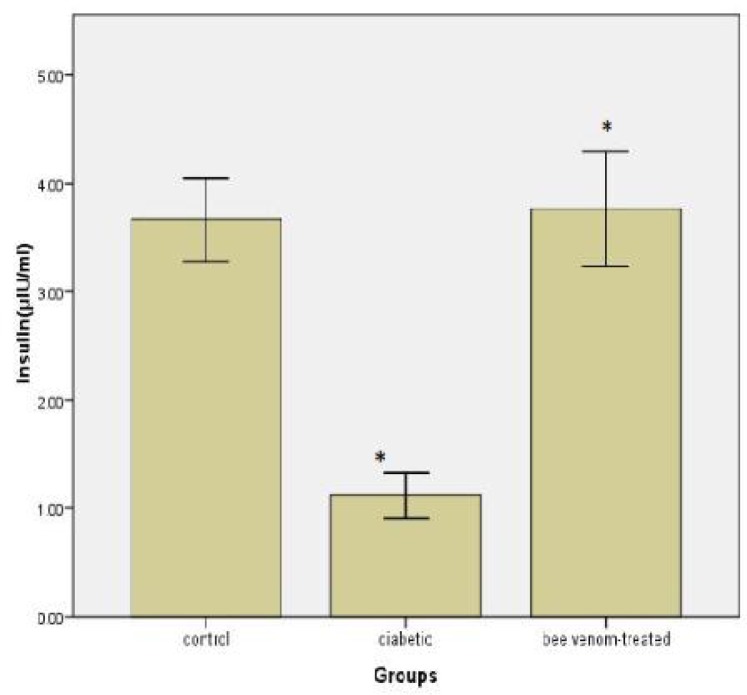
The effect of bee venom administration on serum insulin level is illustrated in Figure 2. Our results showed that serum insulin level significantly decreased in diabetic group compared with controls (P< 0.05). Also there was a significant increase in bee venom-treated group compared with controls (P< 0.05) (Fig. 2).
Fig. 2.
Serum insulin levels after injection of bee venom in diabetic rats. Difference between control and treated group are significant with *P< 0.05.
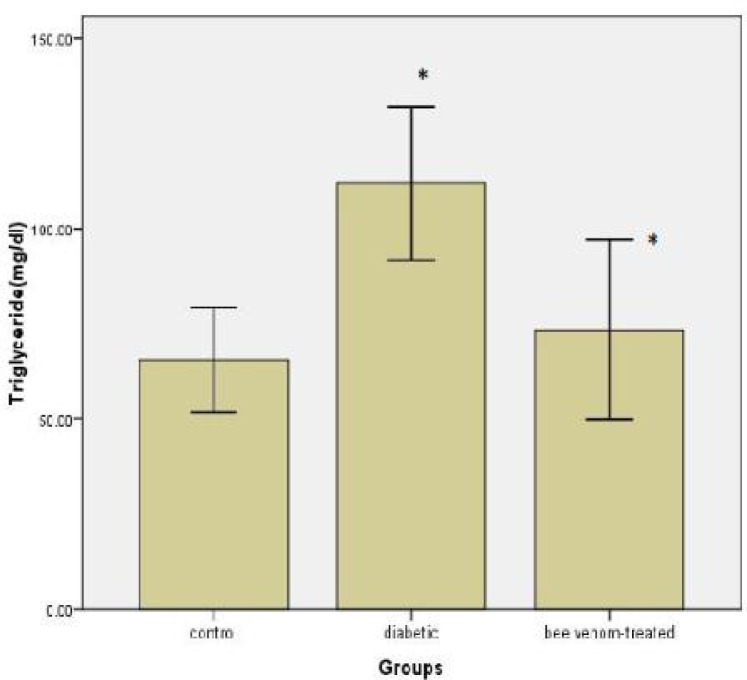
The effect of bee venom administration on serum triglyceride (TG) content is illustrated in Figure 3. There was a significant increase in serum TG content in diabetic group compared with controls (P< 0.05). A significant decline was observed in bee venom-treated group compared with diabetic animals (P< 0.05). But no significant difference was observed in serum TG content in bee venom-treated group compared with control (Fig. 3).
Fig. 3.
Triglyceride levels after injection of bee venom in diabetic rats. Difference between control and treated group are significant with *P< 0.05.
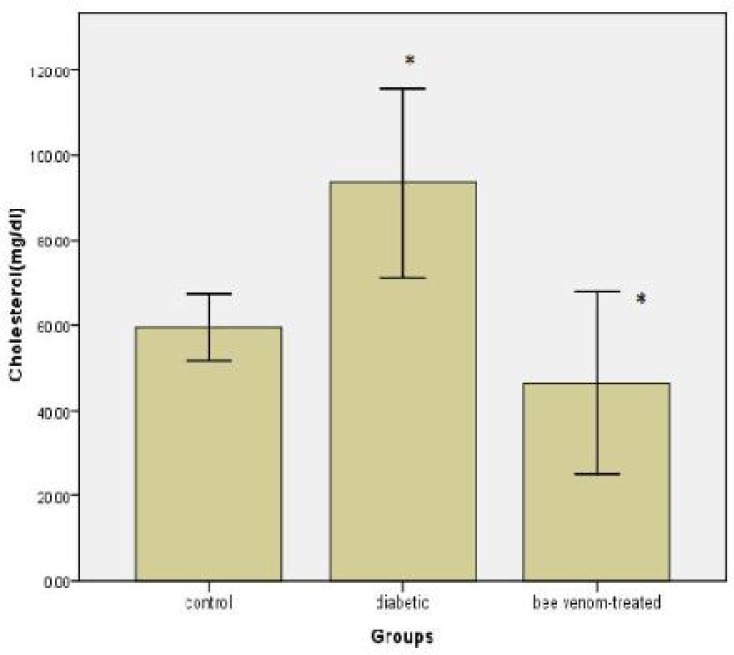
A significant increase was observed in serum total cholesterol in diabetic group compared with controls (P< 0.05). There was a significant decline in bee venom-treated group compared with diabetic animals (P< 0.05). But no significant difference was observed in cholesterol content in bee venom-treated group compared with control group (P= 0.552) (Fig. 4).
Fig. 4.
Cholesterol levels after injection of bee venom in diabetic rats. Difference between control and treated group are significant with *P< 0.05.
Discussion
In our study, blood glucose level increased following alloxan monohydrate administration which led to pancreas B-cells destruction (Byung-Hyun and Jin-Woo 2001). Blood glucose level decreased following bee venom treatment. This may be contributed to substances like mellitin and phospholipase A2 contained in the venom. They may play a role in diminishing inflammation of Islets of Langerhans and thus elevating blood insulin level. With regard to the fact that insulin regulates blood glucose level, bee venom could decrease glucose content via increasing insulin secretion (Morgan and Montague 1984, Fujimoto and Metz 1987, Kim et al. 1999, Simonson et al. 2000). Following alloxan administration in diabetic rats, blood glucose level and triglyceride (TG) content were elevated, which indicate insulin role in regulating lipid metabolism (Zhang and Tan 2003). Insulin activates the enzyme lipoprotein lipase and hydrolysis triglycerides (Frayn 1993). According to the obtained results, bee venom decreased blood TG content. One could explain the observed decline as follows: bee venom improves glycemic control and decreases blood glucose level. Also glucose consumption is increased instead of lipids. Acetyl coA derived from pyrovic acid enters Krebs cycle which finally leads to glucose metabolism,however Acetyl coA can enter TG synthesis pathway in usual condition (Zhang and Tan 2003). Blood cholesterol level increased following Alloxan administration. (Yadav et al. 2004). A decline was observed in cholesterol content of bee venom -treated group (Kim et al. 1999). Probably cholesterol lowering effect is largely due to inhibition of its absorption in small intestine and promoting its hepatic release. The liver plays a critical role in discharging cholesterol via bile secretion (Reinner et al. 1989).
Alloxan administration led to destruction of Islets of Langerhans and diminished insulin secretion in diabetic rats. Treating the rats with honeybee venom (apitoxin) increased insulin secretion up to control levels. According to the published reports, mellitin polypeptide and phospholipase A2, which are two main component of the venom, promote insulin secretion. According to the literature, the observed effect is mediated by extracellular calcium and calcium channels. When these channels are opened large amounts of calcium enters the β-cells and excite them to secret insulin (Morgan and Montague 1984, Fujimoto and Metz 1987, Kim et al. 1999, Simonson et al. 2000). In a study on the effects of bee venom on Islets of Langerhans inflammation and onset of insulin dependent diabetes, Kim et al. (1999) showed that intensity of inflammation and onset of diabetes declined following bee venom treatment. They also found that insulin, TG and cholesterol levels decreased in diabetic rats compared with non-diabetic animals (Kim et al. 1999). According to the experiments of Morgan et al. (1984), mellitin polypeptide promotes insulin secretion from Islets of Langerhans in vitro. The obtained results suggest that mellitin as a valuable candidate for further studies on β-cells plasma membrane role in regulating insulin secretion. The findings also indicate that mellitin can depolarize plasma membranes of β-cells and acts as a calcium transporter in the cell, which in turn promotes insulin granules secretion. The effect of mellitin on insulin secretion depends on extracellular calcium (Morgan and Montague 1984). Simonson et al. (2000) found that mellitin may promote insulin secretion via activating phospholipase A2 in Islets of Langerhans. Their results indicate that phospholipase A2 activation plays a role in compensating insulin resistance response in Islets of Langerhans (Simonson et al. 2000). Treatment with exogenous phospholipase A2 or mellitin promotes arachidonic acid and lysophospholipids production and insulin secretion. Produced arachidonic acid and lysophospholipids may corporate in two-step insulin production mechanism. Arachidonic acid produced by phospholipase A2 induction may act as a calcium transporter in to β -cells and promote insulin secretion (Fujimoto and Metz 1987).
Conclusion
In this study, our results indicate that honeybee venom (apitoxin) can be used as therapeutic option to lower blood glucose and lipids in diabetic rats, however further biochemical and pharmacological studies are necessary to provide more detailed understanding of the issue.
Acknowledgments
This study was supported by the Darou Pakhsh pharmaceutical Mfg Co, Tehran (Iran). The authors declare that there is no conflict of interests.
References
- Antia BS, Okokon JE, Okon PA. Hypoglycemic effect of aqueous leaf extract of Persea Americana Mill on alloxan induced diabetic rats. Indian J Pharmacol. 2005;37:325–326. [Google Scholar]
- Bomalaski JS, Baker D, Resurreccion NV, Clark MA. Rheumatoid arthritis synovial fluid phospholipase A2 activating protein (PLAP) stimulates human neutrophil degranulation and superoxide ion production. J Agents Actions. 1989;27(3–4):425–427. doi: 10.1007/BF01972841. [DOI] [PubMed] [Google Scholar]
- Byung-Hyun P, Jin-Woo P. The protective effect of Amomum xanthides extracts against alloxan-induced diabetic rats through the suppression of NF kB activation. J Exp Med. 2001;33:64–68. doi: 10.1038/emm.2001.12. [DOI] [PubMed] [Google Scholar]
- Dong JS, Jae Woong L, Young HL, Ho Sueb S, Chong KL, Jin TH. Therapeutic application of anti-arthritis, pain-releasing, and anti-cancer effects of bee venom and its constituent compounds. Pharmacol Ther. 2007;115:246–270. doi: 10.1016/j.pharmthera.2007.04.004. [DOI] [PubMed] [Google Scholar]
- El-Demerdash FM, Yousef MI, Abou El, Naga NI. Biochemical study on the hypoglycemic effects of onion and garlic in alloxan- induced diabetic rats. Food Chem Toxicol. 2005;43:57–63. doi: 10.1016/j.fct.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Frayn KN. Insulin resistance and lipid metabolism. Curr Opin Lipidol. 1993;4:197–204. [Google Scholar]
- Fujimoto WY, Metz SA. Phasic effects of glucose, phospholipase A2, and lysophospholipids on insulin secretion. J Endocrinol. 1987;120(5):1750. doi: 10.1210/endo-120-5-1750. [DOI] [PubMed] [Google Scholar]
- Gauldie J, Hanson JM, Rumjanek FD, Shipolini RA, Vernon ChA. The peptide components of beevenom. Eur J Biochem. 1976;61:369–3761. doi: 10.1111/j.1432-1033.1976.tb10030.x. [DOI] [PubMed] [Google Scholar]
- Habermann E. Science. 3. Vol. 177. Washington DC; 1972. Bee and wasp venoms; pp. 14–322. [DOI] [PubMed] [Google Scholar]
- Kim Jong-Yeon, Cho Song-Hyun, Kim Yong-Woon, Jang Eung-Chan, Park So-Yung, Kim Eun- Jung, Lee Suck-Kang. Effect of BCG,Lymphotoxin and Bee Venom on insulities and Development of IDDM in Non-Obese Diabetic Mice. J Korean Med Sci. 1999;14:648–652. doi: 10.3346/jkms.1999.14.6.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JF. Antidiabetic drugs. J Med Lib. 2003;5:5–6. [Google Scholar]
- Kim JY, cho SH, kim YW, Jang EC, Park SY, Kim EJ, Lee SK. Effects of BCG, lymphotoxin and bee venom on insulitis and development of IDDM in non-obese diabetic mice. J Korean Med Sci. 1999;14(6):648–652. doi: 10.3346/jkms.1999.14.6.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JD, Park HJ, Chae Y, Lin S. An overview of Bee Venom acupuncture in the treatment of arthritis. Evid Based Complement Alternat Med. 2005;2:79–84. doi: 10.1093/ecam/neh070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzanti C, Spanevello R, Ahmed M, Schmatz R, Mazzanti A, Salbego F, Grac D, Sallis E, Morsch V, Schetinger M. Cyclosporine inhibits acetyl cholinesterase activity in rats experimentally demyelinated with ethidium bromide. Int J Devl Neuroscience. 2007;25:259–264. doi: 10.1016/j.ijdevneu.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Mirshafiey A. Venom therapy in multiple sclerosis. Neuropharmacology. 2007;53:353–361. doi: 10.1016/j.neuropharm.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Morgan NG, Montague w. Stimulation of insulin secretion from isolated rat islets of Langerhans by melittin. J Biosci Rep. 1984;4(8):665–671. doi: 10.1007/BF01121020. [DOI] [PubMed] [Google Scholar]
- Nuraliev IN, Avezov GA. The efficacy ofquercetin in alloxan diabetes. Eksp Klin Farmakol. 1992;55:42–44. [PubMed] [Google Scholar]
- Pedarzani P, Stocker M. Molecular and cellular basis of small and intermediate conductance,calcium activated potassium channel function in the brain. Cell Mol Sci. 2008;65:3196–3217. doi: 10.1007/s00018-008-8216-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyed Khoei N, Atashpaz S, Ghabili K, Seyed Khoei N, Omidi Y. Melittin and hyaluronidase compound derived from Bee venom for the treatment of multiple sclerosis. Iran J Med Hypotheses Ideas. 2009;9(3):24. [Google Scholar]
- Simonsson E, Karlsson S, Ahren B. Islet phospholipase A(2) activation is potentiated in insulin resistant mice. Biochem Biophys Res Communi. 2000;272(2):539–543. doi: 10.1006/bbrc.2000.2820. [DOI] [PubMed] [Google Scholar]
- Son DJ, Lee JW, Lee YH, Song HS, Lee CK, Hong JT. Therapeutic application of anti-arthrities, pain-releasing, and anti-cancer effect of Bee Venom and its compounds. Pharmacol Ther. 2007;115:246–270. doi: 10.1016/j.pharmthera.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Soto C, del Razo LM, Neri L. Alloxan decreases intracellular potassium content of the iolated frog skin epithelium. Comp Biochem Physiol C Toxico Pharmacol. 2001;130(1):19–27. doi: 10.1016/s1532-0456(01)00213-7. [DOI] [PubMed] [Google Scholar]
- Reinner E, Bjorkhem I, Angelin B, Ewerth S, Einarsson K. Bile acid synthesis in humans: regulation of hepatic microsomal cholesterol 7 alpha-hydroxylase activity. J Gastroenterol. 1989;97:1498–1505. doi: 10.1016/0016-5085(89)90395-8. [DOI] [PubMed] [Google Scholar]
- Viana GS, Medeiros AC, Lacerda AM, Leal LK, Vale TG, Matos FJ. Hypoglycemic and anti-lipidemic effects of the aqueous extract of Cissus si-cyoides. J BMC Pharmacol. 2004;8:4–9. doi: 10.1186/1471-2210-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesselius T, Heersema DJ, Mostert JP, Heerings M, Admiraai-Behloul F, Talebian A, Van Buchen MA, De Keyser J. A randomized crossover study of Bee sting therapy for multiple sclerosis. Neurology. 2005;65:1764–1768. doi: 10.1212/01.wnl.0000184442.02551.4b. [DOI] [PubMed] [Google Scholar]
- Williams G, Pickup JC. Handbook of Diabetes. 2nd edition. Blackwell Science; Oxford: 2000. [Google Scholar]
- Yadav UC, Moorthy K, Baquer NZ. Effects of sodium orthovanadate and Trigonella foenum-graecum seeds on hepatic and renal lipogenic enzymes and lipid profile during alloxan diabetes. J Biosci. 2004;29(1):81–91. doi: 10.1007/BF02702565. [DOI] [PubMed] [Google Scholar]
- Zalat S, Nabil Z, Hussein A, Rakha M. Biochemical and haematological studies of some solitary and social bee venoms. Egypt J Biology. 1999;1:57–7. [Google Scholar]
- Zhang XF, Tan BKH. Effects of an ethanolic extract of Gynura procumbens on serum glucose, cholesterol and triglyceride levels in normal and streptozotocin-induced diabetic rats. Singapore Med J. 2003;41(1):1–6. [PubMed] [Google Scholar]