Abstract
Background:
The present study aimed to demonstrate the seasonal activities of Ixodes ricinus at the pasture level and on the host.
Methods:
A vast pasture in Amol countryside (Mazandaran Province, Iran) which had the potential for a considerable number of cattle and sheep to graze was chosen. Tick sampling from the skin of 130 cattle and 130 sheep were collected every month interval. Simultaneously, the activity of the different stages of I. ricinus on the pasture was considered by dragging method. The collected ticks were placed in jars containing 70% alcohol and sent to the parasitological laboratory for identification.
Results:
The rate of the infestation with adult I. ricinus in cattle and sheep increases gradually with the beginning of fall and reaches its peak in January, February and March while it starts to decline with the beginning of spring as the infestation rate reach to zero in summer months. Accordingly, the highest number of adult I. ricinus existed on the cattle during January, February, and March. In addition, the results of dragging have been revealed that the active tick population in the pasture exists during November, December, January, and March.
Conclusion:
Ixodes ricinus is regarded a common tick species in Amol (Mazandaran). Due to the biological properties of I. ricinus which is active in the cold and humid months of the year, the prevalence of ruminant infestations with I. ricinus in this area increases from November to March but reaches to zero again with the beginning of summer.
Keywords: Ixodes ricinus, seasonal activity, Iran
Introduction
Ixodid tick is one of bloodsucker arthropods belonging to acarina, which cause damage to the host skin and anemia in addition; ticks are also the most important vectors of diseases. In this regard, Ixodes ricinus is the one of the important vectors of highly pathogenic agents affecting both humans and domestic animals worldwide (Kettle 1995). Recently, there has been an increase in the amount of attention paid to ticks as they are vectors for pathogenic agent such as: the spirochete Borrelia burgdorferi etc. Conse quently, numerous researchers in world-wide are engaged in studies on dispersal and seasonal activity of I. ricinus and on the extent of their infestation with B. burgdorferi (Grey et al. 1992, Talleklint et al. 1993, Talleklint et al. 1996, Hubalek et al. 1998, Nilison 1998, O’Connell et al. 1998). There are many studies carried out so far related to the tick fauna in Iran, a number of studies have been conducted in northern part of Iran to investigate the tick diversity.
Razavi et al. (2006) showed that only 86% of the 696 ticks infesting cows in Amol area were I. ricinus, while in Kelardasht area I. ricinus comprised 26.8% of the 798 collected hard ticks (Yossefi et al. 2008). Nabian et al. (2007) showed 2.32% of the 1720 collected ticks were I. ricinus. Razmi et al. (2007) showed that 6.8% of the population of the collected ticks from Mazandaran Province included I. ricinus. The important point related to the studies so far conducted in northern part of Iran is that the seasonal activities of tick fauna has not been perspicuous, although some works has pointed out the tick seasonal activity in Western Azerbaijan (Rahbari et al. 1995, Davudi et al. 2008, Salari et al. 2008) but, it is still a gap in tick ecology.
The present study aimed to demonstrate the seasonal activities of I. ricinus on the host and at the pasture level.
Materials and Methods
A vast pasture in Amol countryside (Mazandaran Province, northern Iran) which had the potential for a considerable number of cattle and sheep to graze was chosen. Tick sampling from the skin of 130 cattle and 130 sheep were collected in every month interval from October 2009 to September 2010. Simultaneously, an area of 250 m2 of the pasture randomly selected for dragging and the activity of the different stages of I. ricinus on the pasture was considered. The collected ticks were placed in jars containing 70% alcohol and were then sent to the laboratory. Tick identification was done according to the morphological structures mentioned by Estrada-Pena et al. (2004). Climatological data were also collected from central station of Amol.
Results
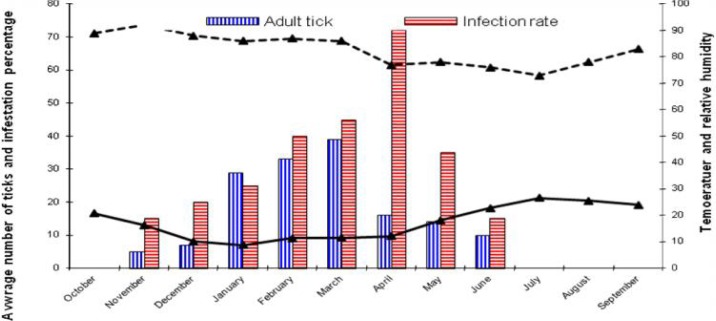
In this study, six species of hard ticks (Ixodid ticks) have been identified including I. ricinus, Boophilus annulatus, Rhipicephalus bursa, Rh. turanicus, Haemaphysalis concinana, Hyalloma deteritium and I. ricinus were allocated 42% of total ticks. Monthly inspection of target animals for I. ricinus tick revealed that the rate of infestation in cattle with adult I. ricinus increased gradually with the beginning of fall and reached its peak in January, February, and March while it started to decline with the beginning of spring as the infestation rate reached to zero in summer. (Fig. 1). Accordingly, the highest number of adult I. ricinus existed on the cattle during winter (Table 1).
Fig. 1.
The seasonal activity of Adult Ixodes ricinus on cattle (October 2009 to September 2010)
Table 1.
The tick intensity of Ixodes ricinus on host and on 10 m2 of the pasture in different seasons
| Season and Annual | Tick intensity on pasture | Tick intensity on hosts | |||
|---|---|---|---|---|---|
|
| |||||
| Larva | Nymph | Adult | Cattle | Sheep | |
| Fall | 4 | 2.3 | 1.7 | 6.08 | 3.4 |
| Winter | 6 | 3.3 | 1.3 | 34.4 | 13.85 |
| Spring | 0 | 0 | 0 | 14.76 | 8.57 |
| Summer | 0 | 0 | 0 | 0 | 0 |
| Annual | 2.5 | 1.4 | 0.75 | 13.81 | 6.45 |
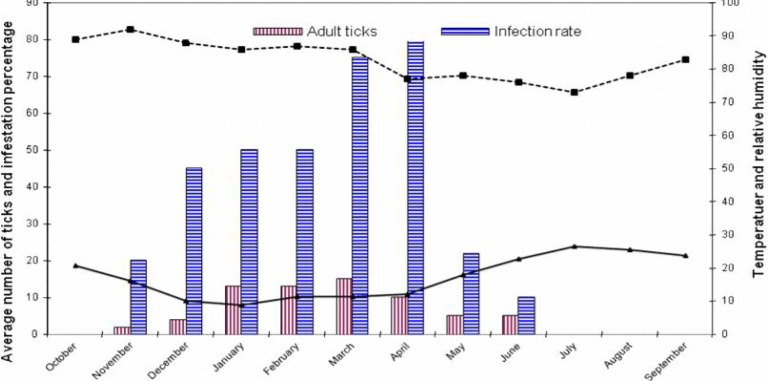
The observations showed that the occurrence of infestation with adult I. ricinus in sheep starts in November and continues to June. In this process, the percentage rate of infestation with adult I. ricinus in sheep increases gradually in fall and reaches its peak in January, February, and March. However, it declines with the beginning of spring in a way that no tick was picked up from sheep in the target area in July, August, and September (Fig. 2), while the greatest average number of adult I. ricinus tick on each animal was found during January, February, and March (Table 1). In addition, the tick intensity on cow and sheep was 13.81 and 6.45 respectively (Table 1).
Fig. 2.
The seasonal activity of adult Ixodes ricinus on sheep (October 2009 to September 2010)
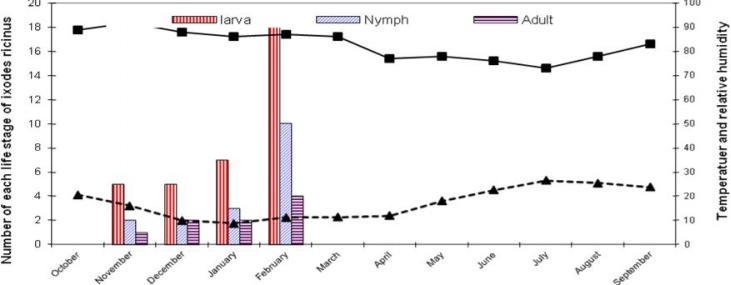
Simultaneous with tick collection from the body of the animals in the target area, the activity of the different stages of I. ricinus on the pasture was carried out by dragging method. The tick population dynamic on the pasture was present during November, December, January, and March while the active population of larvae, nymph, and adult I. ricinus ticks in the area begins in November with a rising trend and reaches its population peak in February. The population balance of the ticks in the pasture was accompanied by increase in nymph, larvae, and adult stages of ticks respectively. However, the population of I. ricinus ticks on the pasture had a different pattern of larva, nymph, and adult intensity in December (Fig. 3).
Fig. 3.
Tick intensity of Ixodes ricinus on 10 m2 of the pastrue (October 2009 to September 2010)
Discussion
The studies which dealing with hard ticks in Iran so far, indicate that I. ricinus tick is only found in the northern provinces of Iran (Razavi et al. 2006, Rahbari et al. 2007, Razmi et al. 2007, Yossefi et al. 2008). I. ricinus is hence regarded a common tick species in Amol. This tick is present as the most common tick in all zoogeographical regions of the world, which have a humid climate, and especially in cold and humid weather (Kott et al. 2004). There is a wide range of hosts for this tick, which mostly feeds on cattle, sheep, horse, deer, wild rabbit, other small mammals, and birds. Since this tick is very sensitive to aridity, its behavior on plants in influenced by its need to maintain and absorb humidity from the environment (Kettle 1995).
Although Rahbari (1995) showed that the largest population of active hard ticks is found on the body of ruminants in Western Azerbaijan Province in spring and summer, it has the lowest frequency in fall. Salim abadi et al. (2008) identified that the largest population of Ixodid tick exist in Yazd Province in summer, the results of this research indicate that the largest number of adult Ixodid ticks on each cow or sheep is found in January, February and March and no adult tick was separated from the body of the ruminants in July, August and September (Fig. 1 and 2). Therefore, we can infer from these results that the presence of adult ticks is more dependent on climatic conditions and its biological properties. These results are the same as those, which was introduced by Tuncer et al. (2004). They determined the seasonal activity of I. ricinus in Turkey, started in December and last until the May of the next year. Also in another work in America, Mackay and Foil (2005) found that the activity of the adult I. scapularis started in November and last until next May. On the other hand the researches conducted by Miroslawa (2004) in Poland, Foldvari et al. (2007) in Hungary and Hrklova et al. (2008) in Slovak, demonstrated two peaks of activity for the adult of I. ricinus, in spring and in summer. According to these authors, the timing of peaks depends on many factors, such as temperature and humidity. The results of the dragging in the Amol area conformed to the results of tick intensity of animal to some extent and showed that the activity of adult I. tick started in November and continues until February (Fig. 3). The relative humidity was slow down from May to September, which was less that 80%. Hence, adult tick was observed neither in pasture nor on the body of the animals especially after July (Fig. 1 and 2). The rate of infestation with adult I. ricinus in cattle and sheep has been increased gradually during fall and reached its peak (75% in cattle and 80% in sheep) in January, February, and May. However, it started to decline with the beginning of spring and reached zero in summer times. In Western Azerbaijan, the rate of the infestation with hard ticks was 55%, 57% and 62% in sheep, goat and cattle respectively Rahbari (1995). The infestation rate with I. ricinus in target animals increased with the decrease in temperature and rise in humidity but decreased with the rise in temperature and decrease in humidity hence, it can be concluded that the activity of I. ricinus was highly dependent to the cold and humid months of the year.
The results of dragging on the pasture showed that the activity of the nymph stage of I. ricinus tick started in November and last until February, which showed a rising trend. This activity stopped in spring and summer (Fig. 3). In Denmark through dragging method, it was found that the nymphal stage of I. ricinus has a dramatic decline in its activity from May to September but slightly increased in September (Kalsbeek and Frandsen 1996). In Ireland, the nymphal stage of I. ricinus activity started in March and reached to zero after a slight increase. Then, it indicated a limited rise in activity from August to November (Gray 1980). Their activities showed bimodal but the comparison between these results with those achieved in Amol, revealed that the main reason for this difference could be the different geographical and climatic conditions of the two regions.
The results of dragging on the pasture show that the active population of I. ricinus in different stages in the target area has a maximum activity curve during the year (Fig. 3). In other words, it has a unimodel activity. The larval stage of I. ricinus has been activated during a period from November until February, this activity stopped in spring and summer, which seems to be due to the biological properties of I. ricinus whose activity decreases and even stopped in the hot and dry months of the year. Gray (1985) determined the activity of the larval stage of I. ricinus in Ireland showed bimodal activity. The comparison of our result and those achieved in Ireland showed a difference in the monthly activity period.
It can be concluded that the activity of I. ricinus in Mazandaran is limited to the cold seasons of the year, this condition caused enough coldness, and humidity (over 80%) for tick host finding in fall and winter, therefore, the wide and extensive occurrence of I. ricinus could be observed on animals.
Acknowledgments
The authors would like to appreciate to the Network Amol Veterinary Experts and Specialized Veterinary Faculty Research-Shahriar Unit. The authors declare that there is no conflict of interests.
References
- Davudi J, Hoghooghi Rad N, Golzar Abdi Sh. Ixodid tick species infesting cow and their seasonality in west Azerbaijan Res. J Parasitol. 2008;3:98–103. [Google Scholar]
- Estrada- Pena A, Bouattour A, Camicas JL, Walker AR. Tick of domestic animals in Mediterranean region. A guide to identification of species. Bioscience reports; London, UK: 2004. [Google Scholar]
- Foldvari G, Morialigeti1 M, Solymosi N, Lukas Z, Majoros G, Kósa P, Farkas1 R. Hard Ticks Infesting Dogs in Hungary and their Infection with Babesia and Borrelia Species. Parasitol Res. 2007;101:S25–S34. [Google Scholar]
- Gray JS. Studies on the life cycle and seasonal activity of I. ricinus in Ireland. Acarology VI. 1980;2:1200–1203. [Google Scholar]
- Gray JS. Studies on the larval activity of the tick I. ricinus L, in Co. Wicklow, Ireland. Exp Appl Acarol. 1985;1:307–316. doi: 10.1007/BF01201570. [DOI] [PubMed] [Google Scholar]
- Grey J, Kahl O, Janetzeki C, Stein J. Studies of ecology of I ricinus in a deer forest in County Galway, Ireland. J Med Entomol. 1992;29:915–920. doi: 10.1093/jmedent/29.6.915. [DOI] [PubMed] [Google Scholar]
- Hrklova G, Novdkovi MC, Hytra M, Kosfov C, Pefko B. Monitoring The Distribution and Abundance of I. ricinus Ticks In Relevance of Climate Change and Prevalence of Boweliu bursdorfert Sensu lato in northern Slovakia (Liptovskf valley) Folia Veterinaria. 2008;52(2):62–63. [Google Scholar]
- Hubalek Z, Holouzka J, Juricova Z. Prevalence rates of Borrelia burgdorferi sensulato in host-seeking I. ricinus ticks in Europe. Folia Parasitol. 1998;45:67–72. doi: 10.1007/s004360050378. [DOI] [PubMed] [Google Scholar]
- Kalsbeek V, Frandsen F. The seasonal activity of I. ricinus ticks in Denmark. Anz, Schadlingskde. Pflanzenschutz, Umweltschutz. 1996;69:160–161. [Google Scholar]
- Kettle DC. Medical and veterinary entomology. Second edition. CAB International; Cambridge: 1995. [Google Scholar]
- Kott I, Valter J, Daniel M, Kiil B. Activity of tick, Ixodesr icinus in dependence from the weather (in Czech) 2004. Stdtnl zdrayotni ustav Praha, Praha.
- Mackay A, Foil L. Seasonal and geographical of adult I. scapularis say (Acari: Ixodidae) in Louisiana. J Vector Ecol. 2005;30(2):325–330. [PubMed] [Google Scholar]
- Miroslawa H. Ecological aspects of distribution of the population of I. ricinus and theirs infection with borrellia burgdorferi in western Pomerania (NW Poland) Zoolog Pol. 2004;49(1–4):77–84. [Google Scholar]
- Nabian S, Rahbari S, Shayan P, Haddadzadeh HR. Current status of tick funa in north of Iran. Iran J Parasitol. 2007;2(1):12–17. [Google Scholar]
- Nillson A. Seasonal occurrence of I. ricinus (Acari) in vegetation and on small mammals in southern Sweden. Hol Ecol. 1998;11:161–165. [Google Scholar]
- O’connel S, Granstrom M, Gray JS, Stonek G. Epidemiology of European Lyme boreliosis. Zent Bl Bacteriol. 1998;287:229–240. doi: 10.1016/s0934-8840(98)80124-2. [DOI] [PubMed] [Google Scholar]
- Rahbari S. some on ecological aspects of tick fauna of west Azarbidjan, Iran. J Appl Anim Res. 1995;7:189–194. [Google Scholar]
- Rahbari S, Nabian S, Shayan P, Hadadzadeh HR. Status of Haemaphysalis tick infestation in domestic ruminants in Iran. Korean J Parasitol. 2007;45(2):129–132. doi: 10.3347/kjp.2007.45.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razavi SM, Seifi S. Identification of hard ticks’ species (Ixodidae) in cattle of amol area. J Fac Vet Med. 2006;61(3):217–220. [Google Scholar]
- Razmi GR, Glinsharifodini M, Sarvi S. Prevalence of ixodid ticks on cattle in Mazandaran province, Iran. Korean J Parasitol. 2007;45(4):307–310. doi: 10.3347/kjp.2007.45.4.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salari Sh, Vatandoost H, Telmadarraiy Z, Entezar Mahdi R, Kia Eb. Seasonal activity of ticks and their importance in tick- borne infectious disease in west Azerbaijan. Iran J Arthropod-Borne Dis. 2008;2(2):28–34. [Google Scholar]
- Salim abadi Y, Tlmadarraiy Z, Vatandoost H, Chinikar S, Oshaghi MA, Moradi M, Mirabzadeh ardakan E, Hekmat S, Nasiri A. Hard tick on domestic ruminants and their seasonal population dynamics in Yazd Province, Iran. Iran J Arthropod-Borne Dis. 2010;4(1):66–71. [PMC free article] [PubMed] [Google Scholar]
- Talleklint L, Jaenson TGT, Mather T. Seasonal variation in the capacity of the bank vole to infected larvae ticks with the Lyme disease spirochete, Borrelia burgdorferi. J Med Entomol. 1993;30:812–815. doi: 10.1093/jmedent/30.4.812. [DOI] [PubMed] [Google Scholar]
- Talleklint L, Jaenson TGT. Relationships between I. ricinus density and the prevalence of infection with Borrelia like spirochetes and density of infected ticks. J Med Entomol. 1966;33:805–811. doi: 10.1093/jmedent/33.5.805. [DOI] [PubMed] [Google Scholar]
- Tuncer D, Mutlu G, Karaer Z, Sayin F. Seasonal Occurrence of ticks on and borrelia burgdorferi influence in I. ricinus in Antalya region. Acta parazittologica Turcica. 2004;28(3):158–160. [Google Scholar]
- Yossefi MR, Keighobadi M, Asnaashari MY. Ixodid tick species infestating sheep and cattle in kelardasht part (Chaloos) Iran. J Entomol. 2008;5(1):56–58. [Google Scholar]