Abstract
Myiasis, the invasion of live human tissue by larva of Diptera, is reported in the nasal cavity of a 5.5-year-old Iranian girl. She was referred from Golestan Province to the Shaheed Rajaei Heart Center in Tehran. In the 41th day after admission, a live parasite was found in her nasal secretions suction identified presumably as a second instar larvae of a facultative myiasis, Woholfartia nuba (Diptera: Sarcophagidae), on the basis of mtDNA-COI and morphological characteristics. Since presence of the larva was recorded after hospitalization, by definition, this infestation is considered a nosocomial myiasis.
Keywords: Nosocomial, Myiasis, Woholfartia nuba, Iran
Introduction
Among arthropod diseases affecting animals, larval infections-myiasis- of domestic and wild animals have been considered important since ancient times. Myiasis occurs worldwide, but more often in hot and humid climates. Species causing myiasis are categorized into obligatory, facultative, and accidental (Hall and Wall 1995). Common infection sites are skin wounds (Safdar et al. 2003) eyes (Razmjou et al. 2007), nose (Kim et al. 2009), and throat (Pasternak et al. 2007) and urogenital tract are less common (Salimi et al. 2010).
Nosocomial infection is defined as the infection that is caused during or after the hospitalization which was neither present nor incubating at the time of admission (Wenzel 1997). Nosocomial myiasis, although rare, is sometimes reported in debilitated, diabetics, cardiovascular, and encephalopathics patients as well as people having motor vehicle accidents. Some of the contributing factors included disturbed unconsciousness or hypoesthesia that prevents the patient’s sensation of fly contact, and paralysis or immobility that prevent from fending off the fly detected (Lettau 1991, Daniel et al. 1994, Amitay et al. 1998). Recent findings from the whole parts of the world indicate that nosocomial myiasis is probably under-reported.
Hospital-acquired myiasis usually occurs during summer months (June to September) when fly populations are in peak activity and the most identified larvae were Lucilia sericata. Although L. illustris, Cochliomyia macellaria, C. hominivorax, Sarcophaga crassipalpis, S. cruentata, Parasarcophaga argyrostoma, Musca domestica, Cuterebra buccata and Megaselia scalaris belonging to the families of Calliphoridae, Sarcophagidae, Muscidae, Cuterberidae, and Phoridae were reported as well (Table 1). There are no or a few, however, which have become obligatory parasites of warm-blooded vertebrates, like Wohlfahrtia magnifica (Schiner 1862) in the Old World, and W. vigil (Walker) and W. opaca (Coquillet) in the New World. These species are not easy to separate, and the identification of files reared from maggots must be left to the specialist (Hall and Wall 1995).
Table 1.
Summary data for reported cases of nosocomial myiasis during 1980–2010
| Family | No of reports | Country | Common reported species |
|---|---|---|---|
| Calliphoridae | 21 | USA (6) Korea (3) Canada (2) Honduras (2) Czech (2) Jamaica (1) Occupied Palestine (1) India (1) Kuwait (1) French Guiana (1) Turkey (1) |
Lucilia sericata |
| Sarco-phagidae | 4 | USA (1) England (1) Turkey (1) Italy (1) |
Sarcophaga sp |
| Muscidae | 2 | USA (1) England (1) |
Musca domestica |
| Cutere-bridae | 1 | USA (1) | Cuterebra buccata |
| Phoridae | 1 | Kuwait (1) | Megaselia scalaris |
The genus Wohlfahrtia, which comprises a fair number of species, is distributed over the Holarctic and the Ethiopian regions. This genus phylogenetically is related to Sarcophaga and some of the species also develop in decomposing organic matter. Like species of Sarcophaga, the flies of this genus are viviparous and give birth to very mobile larvae of the first stage. Each female carries approximately 120–170 larvae. They are deposited near skin lesions, even minute ones like tick bites, which are used for penetrating the tissue. The mucous membranes of nose, eyes and the female genital organs are apparently attacked without the utilization of pre-existing wounds. The larvae grow rapidly and cause widespread destruction of the healthy tissue, and after 5–7 days leave the wound for pupation. The adults are diurnal, favoring the hot hours of the day, and are not normally found on the wing in the morning, the evening or in gloomy weather. The flesh fly, Wohlfahrtia nuba (Wiedemann) is largely distributed in Africa and Asia. According to geographical distribution given by Spradbery (2002), W. nuba is distributed southerly than W. magnifica including the whole parts of Iran (except northwest), Pakistan, Saudi Arabia, countries neighborhood of west sides of the Red Sea, Egypt, Libya, Tunisia, Mauritania, Senegal and Guinea (Spradbery 2002).
Case Report
A 5.5-year-old Iranian girl was referred from Gonbad County, Golestan Province, northern Iran to the Shaheed Rajaei Heart Center in Tehran. She was operated on her heart ten days later and then was delivered to Intensive Care Unit (ICU) of the hospital. In this part of the hospital a live parasite was found in her nasal secretions suction and consignment to the department of medical entomology in the School of Public Health, Tehran University of Medical Sciences, Iran. Putative patient died 45 days after admission, unfortunately. Since this infestation manifested more than 40 days after the patient hospitalization, by definition, this may be considered nosocomial. Current morphological characters as well as sequence of a part of cytochrome oxidase subunit one of mitochondorial DNA (mtDNA-COI) was used for identification of the mentioned dipterous larva.
Special Dates
The most important dates for this myiasis report were: patient hospitalization, 25 August 2010, open heart operation, 4 September 2010, parasite separation, 5 October 2010, and expiration, 9 October 2010.
Morphological examinations
The key morphological characters including size of the larva, spinous bands, position of posterior spiracles and posterior cavity as mentioned by Zumpt (Zumpt 1965) examined under microscope (Olympus SZX12). The cephalo-pharyngeal skeleton and both anterior and posterior spiracle dissected and mounted on glass slide by Pouri solution. After desiccation the larva was keyed using valid keys (Zumpt 1965, Ferrar 1987, CDC 1994, Hall and Wall 1995, Spradbery 2002). Testing the morphological characteristics revealed that the size of the larva was as long as 5 mm, spinous bands with sclerotized spines, the posterior spiracles located in deep posterior cavity, prothoracic spiracles with 9 openings branches, peritreme present, with 2 distinct slits, spiracular slites pointing toward opening in peritreme, posterior spiracles with the incomplete peritreme, and dorsal arm of cephalo-skeleton longer than ventral arm (Fig. 1–4). On the basis of above characters the larvae presumably identical to the second instar larva of W. nuba (Diptera: Sarcophagidae).
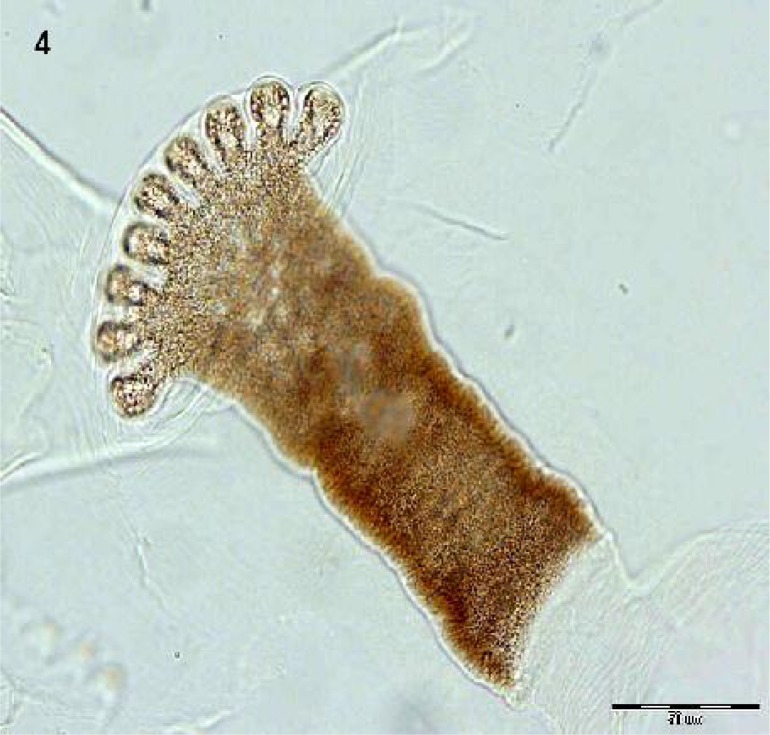
Fig. 1.
The second instar larva isolated from nasal secretions suction of a 5.5-year-old girl, 2010, Iran. Spinous band indicated just around the right down corner (Original)
Fig. 4.
Anterior spiracle of the second instar larva with 9 lobes isolated from nasal secretions suction of a 5.5-year-old girl, 2010, Iran (Original)
Molecular examinations
Except the cephalo-pharyngeal skeleton and spiracle the rest of the larva body was used for DNA extraction using the QIAGEN-DNeasy Blood and Tissue Kit (Germany). The mtDNA-COI gene extending 690 bp of 5′ fragment as applied by Lunt (Lunt et al. 1996) was amplified using primers including C1-J-2090/C1-N-2735. This region of gene was amplified in 20μl reaction mixtures containing 1μl each of the forward and reverse primers, 1μl the genomic DNA, 1U of Taq DNA polymerase, 1.5 Mm MgCl2 and 250 μm dNTPs. The PCR amplification was performed in two thermal circulations. After initial denaturation at 94 °C for 2 min, the first one performed for 5 cycles of 94 °C for 40 s, 45 °C for 40 s, and 72 °C for 1 min, and the last one repeated for 35 cycles of 94 °C for 40 s, 51 °C for 40 s, and 72 °C for 1 min. Final extension step was continued at 72 °C for 5 min. PCR products were visualized on a 1% agarose gel containing ethidium bromide and using an UV transilluminator. The PCR products were sequenced by Seqlab, a German sequencing company. Sequence of this fragment was deposited in the GenBank database with accession number JF277565.
The obtained sequence in this study was compared with the relevant sequences available in public databases using the BLAST program (http://www.ncbi.nlm.nih.gov/blast/blast.cgi) and then were aligned using ClustalW2 (http://www.ebi.ac.uk/Tools/msa/clustalw2/).
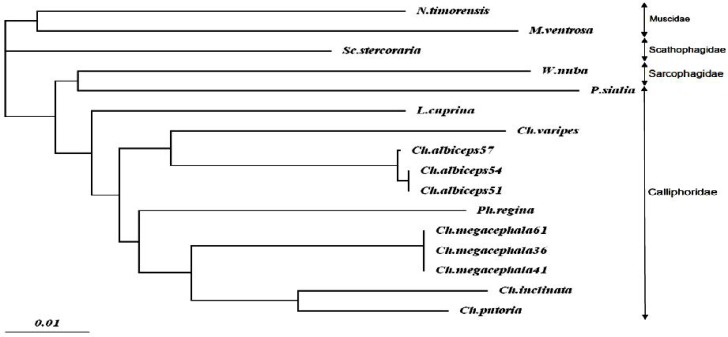
Phylogenetic relationships between the specimen of this study and some representatives of other related flies causing myiasis including EU418542, AB112857, AB112861, AJ426041, EU418536, DQ345078, AF083657, AB1128 51, AB112854, EU418545, AJ417708, AF295 559, AF104625, EU627697, and EU627705 were inferred with the algorithms implemented in ClustalW2. Sequence of COI region of two member of Muscidae (Musca ventrosa: EU 627697, Neomyia timorensis: EU627705) were used as out groups.
Sequence comparison of the specimen with the available data in Genbank using BLAST revealed maximum 93% identity with Chrysomya albiceps COI region. Other entries of the COI sequences with similarity less than 93% belonged to other dipteran flies of Calliphoridae, Muscidae, Scathophagidae and Drosophilidae families. The phylogenic trees inferred from these data showed that the specimen placed in a completely isolated branch far from other dipteran species particularly from the closely related species of Calliphoridae (Fig. 5). However, among different branches on the tree, Protocalliphora sialia (Calliphoridae) was the most associated fly to W. nuba.
Fig. 5.
The phylogenetic relationship of Wohlfahrtia nuba (JF277565) (Sarcophagidae) found in this study with other dipteran flies inferred from 645 bp of the COI gene. Sequence data of other flies are retrieved from Genbank, Calliphoridae: (Chrysomya putoria: EU418542, C. inclinata: AB112857, C. megacephala: AB112861, AJ426041, EU418536, C. albiceps: AF083657, AB112851, AB112854, C. varipes: EU418545, Phormia regina: DQ345078, Lucilia cuprina: AJ417708, Protocalliphora sialia: AF295559), Scathophagidae: (Scathophaga stercoraria: AF104625), Muscidae: (Musca ventrosa: EU627697, Neomyia timorensis: EU627705)
Discussion
This is the first report of nosocomial case caused by W. nuba in Iran. Morphological characters of the larva showed that it was approximately at the second to third instar transition (due to presence of developing pre-third slit), therefore, no more than five or six days old (60–72 hours, on average 66 h). This came from the fact that the insect rate of development is temperature dependent (Nietschke 2007, Oshaghi et al. 2009, Shiravi et al. 2011). This can be calculated using the available information on thermal requirement of W. nuba (Amudi 1993). Total developmental times of flesh flies W. nuba from first larval stage to eclosion was reported 34.7, 23.5, 18.7, 13.4 and 13.6 days when reared at 21, 25, 29, 33 and 37° C respectively. Estimated the lower developmental (tL) threshold temperatures were 12.8, 14 and 13.6 °C and the thermal constant (K) were 116.8, 152.2 and 268.2 degree days (DD) for larvae, pupae and total developmental time (egg to adult) respectively (Amudi 1993). On the other hand, the temperature of human nostril is approximately 30 °C (Greenberg 1984). Therefore based on the base threshold temperature of larvae (12.8 °C), the larvae had received 17.3 DD (30–12.8= 17.3) every day. By dividing thermal constant (K) of larval stage to this value (116.8/17.3= 6.75 day), only about 7 days was enough for the larvae to complete their development and convert to pupae. Because there are three instars at larval stage, each instar needs roughly 2.25 days (6.75/3= 2.25) to develop. Therefore, since the larva was approximately at the second to third instar transition, its age can be about 4 to 4.5 days. We also found, as expected, that this case like most of other cases (Greenberg 1984, Daniel et al. 1994, Joo and Kim 2001, Yazar et al. 2005, Sesterhenn et al. 2009) occurred during the summer when the fly population is in its greatest density.
Wohlfahrtia nuba is a facultative flesh fly and infests wounds of livestock in North Africa and the Middle East, but it probably feeds only on dead or diseased tissues rather than on living tissues (Amudi 1993). It also was found in head wounds or head cavities but not in dermal sores. Wohlfahrtia nuba appears to be an important myiasis causing agent in Saudi Arabia, because almost one third of the cases reported from different geographical localities of the country were due to this species (El-Azazy and El-Metenawy 2004). The larvae of this species are also used in maggot therapy (El-Azazy and El-Metenawy 2004). However; it was observed that W. nuba would feed on healthy tissues at the edges of a wound if all necrotic tissue was exhausted (Grantham-Hill 1933).
The parasite we identified morphologically was a second instar larva. It is worth mentioning that identification of the immature larva of parasite causing myiasis posed difficulties particularly the parasites with little available knowledge. However, the available morphological characteristics of the larva, especially the number of lobes of its anterior spiracles (Fig. 4), plus lack of similarity of its COI sequence to any other morphologically related species such as C. albiceps, C. megacephala, C. varipes, C. putoria, C. inclinata, Lucilia cuprina, L. illastris, Protocalliphora sialia, and Phormia regina, together with knowledge of the distribution of W. nuba in the region (Spradbery 2002), support correct identification of the larva as W. nuba. Generally we have severely limitations with sarcophagid flies because it is difficult or impossible to determine the species of a sarcophagid larvae and in many instances an adult specimen, based on anatomy. Up to now there are no suitable key for the identification of the immature stages of Sarcophagidae so we used combination of several valid keys (Zumpt 1965, Ferrar 1987, CDC 1994, Spradbery 2002). In addition, for most larval stages of flesh flies identification based on morphological characteristics is still impossible (Byrd and Castner 2001), so we used molecular toll for identification purposes.
Of the various types of myiasis, only the secondary (accidental, facultative) myiasis is potentially nosocomial. And in this paper we report a facultative myiasis caused by W. nuba. Flesh flies prefer sunlight rather than shaded conditions but hospital condition must be attracting for these flies (Smith 1986).
Based on Greenberg (1984) opinion, some of the factors that frequently produce myiasis include (i) helpless and debilitated individuals (ii) blood or odors of decomposition, (iii) neglect of nursing or custodial personnel, and (iv) summer season. In case of our report, facilitator conditions were included the patient’s inability to fend off flies, the initial presence of mucus in the nose and location of the hospital where was in the center of Tehran metropolitan.
Reviewing 35 reported nosocomial cases from various countries showed those myiases were caused by maggots of both facultative and obligatory parasitic species of flies. In practice, there is no indication of ‘true’ myiasis in both community-acquired cases as well as nosocomial infestations, because hospital-acquired myiasis is probably under-reported for a number of reasons and some of the cases are squelched by hospital administrators, risk managers, and public relations departments for obvious medico legal and political reasons (Smith and Clevenger 1986, Lukin 1989, Lettau 1991, Josephson and Krajden 1993, Daniel et al. 1994, Chigusa et al. 1996).
The prevention measures of nosocomial myiasis are directly related to the flies. Sanitary and efficient waste disposals supplemented by some insecticide sprays should minimize fly density. Screens or sealed windows will provide physical barriers. Wound care and dressing, as well as attention to the hygiene of the patients will also decrease the attractions of flies to the patients (Lettau 1991, Josephson and Krajden 1993).
Fig. 2.
The cephalo-pharyngeal skeleton of second instar larva isolated from nasal secretions suction of a 5.5-year-old girl, 2010, Iran (Original)
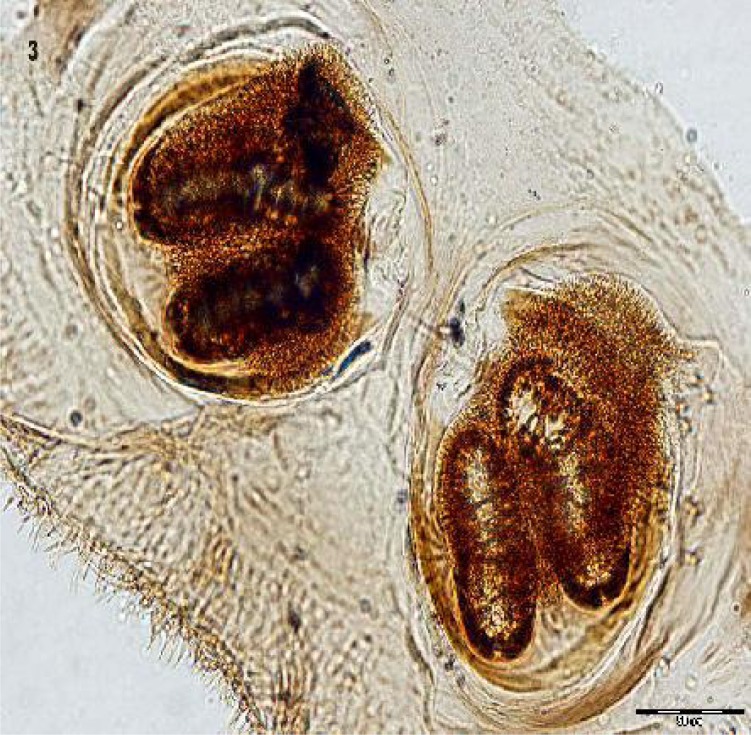
Fig. 3.
Posterior spiracles of the second instar larva isolated from nasal secretions suction of a 5.5-year-old girl, 2010, Iran (Original)
Acknowledgments
We would like to thank Dr M Hall from the Department of Entomology, Natural History Museum, London, UK, Dr T Pape from Natural History Museum of Denmark, and Dr S Tirgari from TUMS for giving some impressive comments and Eng. K Akbarzadeh for providing identification keys. This research was supported by the Tehran University of Medical Sciences. The authors declare that there is no conflict of interest.
References
- Amitay M, Efrat M, McGarry JW, Shinwell ES. Nosocomial myiasis in an extremely premature infant caused by the sheep blowfly Lucilia sericata. Pediatr Infect Dis J. 1998;17:1056–1057. doi: 10.1097/00006454-199811000-00024. [DOI] [PubMed] [Google Scholar]
- Amudi MA. Effect of temperature on the developmental stages of Wohlfahrtia nuba (Dip: Sarcophagidae) J Egypt Soc Parasitol. 1993;23:697–705. [PubMed] [Google Scholar]
- Byrd JH, Castner JL. Insects of forensic importance. In: Byrd JH, Castner JL, editors. Forensic entomology The utility of arthropods in legal investigations. CRC Press; Boca Raton: 2001. pp. 287–302. [Google Scholar]
- CDC . Pictorial Keys to Arthropods, Reptiles, Birds and Mammals of Public Health Importance. Centers for Disease Control and Prevention (CDC); Atlanta: 1994. [Google Scholar]
- Chigusa Y, Kirinoki M, Yokoi H, Matsuda H, Okada K, Yanadori A, Yamazaki S. Two cases of wound myiasis due to Lucilia sericata and L. illustris (Diptera: Calliphoridae) Med Entomol Zool. 1996;47:73–76. [Google Scholar]
- Daniel M, Sramova H, Zalabska E. Lucilia sericata (Diptera: Calliphoridae) causing hospital-acquired myiasis of a traumatic wound. J Hosp Infect. 1994;28:149–152. doi: 10.1016/0195-6701(94)90141-4. [DOI] [PubMed] [Google Scholar]
- El-Azazy OME, El-Metenawy TM. Cutaneous myiasis in Saudi Arabia. Vet Rec. 2004;154:305–306. doi: 10.1136/vr.154.10.305. [DOI] [PubMed] [Google Scholar]
- Ferrar P. A guide to the breeding habits and immature stages of Diptera Cyclorrhapha. Parts 1 and 2. Scandinavian Science Press; 1987. [Google Scholar]
- Grantham-Hill C. Preliminary note on the treatment of infected wounds with the larva of Wohlfahrtia nuba. Trans R Soc Trop Med Hyg. 1933;27:93–98. [Google Scholar]
- Greenberg B. Two cases of human myiasis caused by Phaenicia sericata (Diptera: Calliphoridae) in Chicago area hospitals. J Med Entomol. 1984;21:615. doi: 10.1093/jmedent/21.5.615. [DOI] [PubMed] [Google Scholar]
- Hall M, Wall R. Myiasis of humans and domestic animals. Adv Parasitol. 1995;35:257–334. doi: 10.1016/s0065-308x(08)60073-1. [DOI] [PubMed] [Google Scholar]
- Joo CY, Kim JB. Nosocomial submandibular infections with dipterous fly larvae. Korean J Parasitol. 2001;39:255–260. doi: 10.3347/kjp.2001.39.3.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephson RL, Krajden S. An unusual nosocomial infection: Nasotracheal myiasis. J Otolaryngol. 1993;22:46–47. [PubMed] [Google Scholar]
- Kim JS, Seo PW, Kim JW, Go JH, Jang SC, Lee HJ, Seo M. A Nasal Myiasis in a 76-Year-Old Female in Korea. Korean J Parasitol. 2009;47(4):405–407. doi: 10.3347/kjp.2009.47.4.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lettau LA. Nosocomial transmission and infection control aspects of parasitic and ectoparasitic diseases. Infect Control Hosp Epidemiol. 1991;12:179–185. doi: 10.1086/646313. [DOI] [PubMed] [Google Scholar]
- Lukin LG. Human cutaneous myiasis in Brisbane: a prospective study. Med J Aust. 1989;150:237–240. doi: 10.5694/j.1326-5377.1989.tb136454.x. [DOI] [PubMed] [Google Scholar]
- Lunt DH, Zhang DX, Szymura JM, Hewitt GM. The insect cytochrome oxidase I gene: evolutionary patterns and conserved primers for phylogenetic studies. Insect Mol Biol. 1996;5:153–165. doi: 10.1111/j.1365-2583.1996.tb00049.x. [DOI] [PubMed] [Google Scholar]
- Nietschke BS, Magarey RD, Borchert DM, Calvin DD, Jones E. A developmental database to support insect phenology models. Crop Prot. 2007;26:1444–1448. [Google Scholar]
- Oshaghi MA, Maleki-Ravasan N, Javadian E, Rassi Y, Sadraei J, Enayati AA, Vatandoost H, Zare Z, Emami SN. Application of predictive degree day model for field development of sand fly vectors of visceral leishmaniasis in northwest of Iran. J Vec Borne Dis. 2009;46:1–8. [PubMed] [Google Scholar]
- Pasternak J, Joo SH, Ganc AJ, Junior MSD, Morsh RD, Pinto TH. A case of throat Cochliomyia hominovorax infestation. Einstein. 2007;5(2):170–172. [Google Scholar]
- Razmjou H, Mowlavi GH, Nateghpour M, Solaymani-Mohamadi S, Kia EB. Ophthalmomyiasis caused by flesh fly (Diptera: Sarcophagidae) in a patient with eye malignancy in Iran. Iran J Arthropod-Borne Dis. 2007;1(2):53–56. [Google Scholar]
- Safdar N, Young DK, Andes D. Autochthonous furuncular myiasis in the United States: Case report and literature review. Clin Infect Dis. 2003;36:73–80. doi: 10.1086/368183. [DOI] [PubMed] [Google Scholar]
- Salimi M, Edalat H, Jourabchi A, Oshaghi MA. First report of human nasal myiasis caused by Eristalisin tenax in Iran (Diptera: Syrphidae) Iran J Arthropod-borne Dis. 2010;4:77–80. [PMC free article] [PubMed] [Google Scholar]
- Sesterhenn AM, Pfützner W, Braulke DM, Wiegand S, Werner JA, Taubert A. Cutaneous manifestation of myiasis in malignant wounds of the head and neck. Eur J Dermatol. 2009;19:64–68. doi: 10.1684/ejd.2008.0568. [DOI] [PubMed] [Google Scholar]
- Shiravi AH, Mostafavi R, Akbarzadeh K, Oshaghi MA. Temperature requirements of some common Iranian forensically important blow and flesh flies (Diptera) under laboratory conditions. Iran J Arthropod-borne Dis. 2011;5:54–62. [PMC free article] [PubMed] [Google Scholar]
- Smith DR, Clevenger RR. Nosocomial nasal myiasis. Arch Pathol Lab Med. 1986;110:439–440. [PubMed] [Google Scholar]
- Smith KGV. A Manual of Forensic Entomology. The Trustees of British Museum of Natural History, London and Comstock Publishing Associates; New York: 1986. [Google Scholar]
- Spradbery JP. A Manual for the Diagnosis of Screw-Worm Fly. Agriculture, Fisheries and Forestry; Australia: 2002. [Google Scholar]
- Wenzel RP. Prevention and control of nosocomial infections. 3rd ed. Williams and Wilkins Co; Blatimore: 1997. [Google Scholar]
- Yazar S, Dik B, Yalcin S, Demirtas F, Yaman O, Ozturk M, Sahin I. Nosocomial Oral Myiasis by Sarcophaga sp. In Turkey. Yonsei Med J. 2005;46:431–434. doi: 10.3349/ymj.2005.46.3.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumpt F. Myasis in Man and Animals in the old world. Butterworths; London: 1965. [Google Scholar]