Abstract
Background
Neoadjuvant chemoradiotherapy before total mesorectal excision for rectal cancer is associated with improved local tumor control, primary tumor regression and pathologic downstaging. Therefore, tumor response in the bowel wall has been proposed to be used to identify patients for organ-preserving strategies.
Objective
To determine rate of residual lymph node involvement following neoadjuvant chemoradiotherapy among patients with ypT0-2 residual bowel wall tumor and to comparatively assess their oncologic outcomes following TME.
Design
Retrospective consecutive cohort study, 1993 to 2008.
Setting and patients
Patients with stage cII-III rectal carcinoma treated with preoperative chemoradiotherapy and total mesorectal excision.
Main Outcome Measures
Rate of lymph node metastasis by ypT stage. Recurrence- free survival and frequencies of distant metastasis and local recurrence.
Results
Among all 406 ypT0-2 patients, 66 (16.3%) had lymph node metastasis: 20.8% among ypT2, 17.1% among ypT1, and 9.1 % among ypT0 patients. Local recurrences (2.0% vs. 5.5%; P=0.038) but not distant metastases (9.3% vs. 13.5%; P=0.38) occurred more frequently in ypN+ vs ypN0 patients. Recurrence-free survival was 87.5% among ypT0-2N0 and 83.6% for ypT0-2N+ (P=0.28). The lack of difference in recurrence-free survival persisted after covariate adjustment (HR, 1.29; 95% CI, 0.77–2.16; P=0.37). However, among ypT3-4patients, 5-year recurrence-free survival was significantly lower with lymph node metastasis (HR, 1.51; 95% CI, 1.07–2.12; P=0.019).
Limitations
Low local recurrence event rate limited further comparison by ypT0-2 subgroups.
Conclusions
Residual mesorectal lymph node metastasis risk remains high even with good neoadjuvant chemoradiotherapy response within the bowel wall. Complete removal of the mesorectal burden results in excellent disease control. Given the uniquely good outcomes with standard therapy among patients with ypT0-2 disease, use of ypT stage to stratify patients for local excision risks undertreatment of an unacceptably high proportion of patients.
Keywords: Rectal cancer, Neoadjuvant therapy, Chemoradiotherapy, Treatment response, Lymph node
INTRODUCTION
The current treatment standard for patients with locally advanced rectal cancer is neoadjuvant chemoradiation therapy (CXRT) followed by radical, total mesorectal excision (TME). Multidisciplinary treatment with CXRT has been associated with improved local tumor control with the added effect of tumor regression and potential for downstaging.1 In fact, the response to neoadjuvant treatment has been shown to be a surrogate for long-term outcomes.2,3 Based on this, and in an effort to avoid the potential morbidity and impaired long-term functional outcomes associated with radical resection, there has been an increasing interest for organ-preserving strategies with local excision or watchful waiting in the management of patients with rectal cancer and good response to neoadjuvant CXRT.4,5 Furthermore, these patients with a good response (ypT0-2, N0) to neoadjuvant CXRT have a low risk for both local recurrence and distant failure following TME when compared to patients with poor response.2,3,6 Since at pathologic evaluation approximately 50% of patients treated with neoadjuvant CXRT will have ypT0-2 disease and approximately 15–20% of all treated patients will have a pathologic complete response (ypT0N0), such a strategy has the potential to impact a large number of patients with rectal cancer.
However, one important determinant of local and distant recurrence risk may be the status of the mesorectal lymph nodes. A major effect of the TME technique is the complete excision of the tumor and associated mesorectum, both with wide clearance around the tumor avoiding a positive resection margin and also with complete excision of potentially tumor-bearing mesorectal lymph nodes. Although response at the primary tumor site within the bowel may provide insight into the status of residual disease within the mesorectum, before the residual tumor within the bowel wall (ypT status) can be used to stratify subsequent treatment, the potential for mesorectal nodal involvement must be assessed. However, the reported rate of metastatic lymph nodes in ypT0 tumors ranges from 2 to 17% leading some groups to consider organ preserving and “wait and see” strategies within this group.3,4,7–10 Still others have explored organ-preserving strategies even for patients with residual ypT1-2 tumors after neoadjuvant CXRT, although less is known about the incidence and impact of ypN+ disease among these patients.11,12
Therefore, the purpose of this study was to determine the incidence of residual lymph node involvement following neoadjuvant CXRT among patients with ypT0-2 residual tumor within the bowel wall and to comparatively assess their oncologic outcomes following TME.
METHODS
Patients
We performed a retrospective consecutive cohort study of patients with biopsy-proven, locally advanced (stage cII-III)rectal cancers treated with preoperative CXRT followed by radical resection at the University of Texas MD Anderson Cancer Center between 1993 and 2008 who were identified from our colorectal cancer database and tumor registry. Patients with ypT0-2 posttreatment status were examined in detail for the primary analysis and compared to those with ypT3-4 tumors. Patients with concurrent distant metastasis at diagnosis, or those with concurrent inflammatory bowel disease, hereditary colorectal cancer syndromes, concurrent malignancy, emergent surgery, prior history of immunotherapy or radiotherapy to the pelvis, or prior history of malignancy were excluded. This study was approved by the MD Anderson Institutional Review Board.
Clinical Staging, Treatment, and Pathologic Evaluation
All patients underwent physical examination including full colonoscopic evaluation to exclude synchronous tumors, as well as digital rectal examination and proctoscopic evaluation to identify the tumor distance from the anal verge. Pretreatment clinical stage was assessed based on endorectal ultrasound, high resolution rectal cancer magnetic resonance imaging, or computed tomography. Patients were treated with neoadjuvant CXRTwith a median radiotherapy dose of 50.4 Gy and concurrent chemotherapy (mainly single-agent infusional 5-fluorouracil or capecitabine). Operations using total mesorectal excision principles generally were performed 6 to 8 weeks following completion of chemoradiotherapy.
Standard pathologic tumor staging of the resected specimen was performed in accordance with the guidelines of the College of American Pathologists, with histopathologic diagnosis performed by dedicated gastrointestinal cancer pathologists.13 Patients were categorized according to ypT stage and by the presence of lymph node metastasis. All tumors were wholly submitted for evaluation (in the event of clinical complete response, the entire scar was submitted) and serially sectioned every 2–3 mm. All lymph nodes were examined with one to three separate sections per node. Postoperative follow-up consisted of routine clinical examination with carcinoembryonic antigen measurement every 3 to 6 months and cross-sectional imaging every 6 to 12 months for 5 to 7 years.
Statistical Analysis
Nonparametric data were compared using the Wilcoxon rank sum test and categorical data were summarized by frequency within each cohort, and comparisons were performed using the chi square test for proportions. For recurrence-free survival (RFS) analysis, cases were identified as failures at the time of disease recurrence or death from any cause (i.e., noncancer deaths were not censored). Five-year LR and RFS rates were determined by the Kaplan-Meier method, and univariate comparisons were performed using the log-rank test. Cox proportional hazards regression analysis was performed for multivariate comparisons with the final model developed using backwards selection starting from the fully saturated model and with inclusion of additional clinically relevant terms. P values < .05 were considered significant.
Results
Patient Population and Tumor Characteristics
Among a total of 725 patients who had been treated for rectal cancer with neoadjuvant CXRTduring the study period, 406 had pathologically proven ypT0-2 cancers and were included in our primary analysis. Median follow-up time for the ypT0-2 cohort was 79.5 months (interquartile range [IQR], 47 to 126 months) and was not different between the ypN0 and ypN+ groups (P=0.84). The median age was 57 years (IQR, 48 to 66 years). Most tumors were located in the mid and distal rectum with a median distance from the anal verge of 5 cm (IQR, 3 to 8 cm). The majority of the patients on preoperative evaluation were clinically node positive. The median dose of delivered radiation was 50.4 Gy (IQR, 45 to 52.5 Gy) and surgery was performed at a median of 7 (IQR,6 to 8) weeks after completion of chemoradiotherapy. All patients underwent total or tumor-specific mesorectal excision depending on the extent of the tumor. Three hundred patients (73.9%) underwent sphincter-preserving operation (Table 1). The cohort with ypT3-4 residual disease was similar with respect to age and tumor location but due to the poorer survival, median follow-up was shorter at 57 months (IQR 36–90 months).
Table 1.
Clinical and tumor characteristics according to the lymph node metastasis in patients with ypT0-2
| Characteristics | yT0-2N0 | yT0-2N+ | P | |||
|---|---|---|---|---|---|---|
|
| ||||||
| n | % | n | % | |||
|
| ||||||
| Overall | 340 | 66 | ||||
| Age, years a | 59 (49–67) | 55 (46–66) | 0.072 | |||
| < 50 | 89 | 26% | 23 | 35% | 0.347 | |
| 50–65 | 150 | 44% | 25 | 38% | ||
| > 65 | 101 | 30% | 18 | 27% | ||
|
| ||||||
| Sex | 0.951 | |||||
| Female | 125 | 37% | 24 | 36% | ||
| Male | 215 | 63% | 42 | 64% | ||
|
| ||||||
| Location | 0.460 | |||||
| >10 cm | 17 | 5% | 2 | 3% | ||
| 6–10 cm | 138 | 41% | 25 | 38% | ||
| ≤ 5cm | 176 | 52% | 35 | 53% | ||
| Unknown | 9 | 3% | 4 | 6% | ||
|
| ||||||
| Clinical stage | 0.189 | |||||
| II | 144 | 42% | 23 | 35% | ||
| III | 173 | 51% | 41 | 62% | ||
| Unknown | 23 | 7% | 2 | 3% | ||
|
| ||||||
| Tumor grade | 0.301 | |||||
| G1, G2 | 295 | 87% | 53 | 80% | ||
| G1, G2 | 35 | 10% | 9 | 14% | ||
| Unknown | 10 | 3% | 4 | 6% | ||
|
| ||||||
| Examined lymph nodesa | 11 (6–15) | 11 (7–16) | 0.698 | |||
|
| ||||||
| Lymphovascular invasionb | <.001 | |||||
| No | 256 | 75% | 40 | 61% | ||
| Yes | 10 | 3% | 10 | 15% | ||
| Unknown | 74 | 22% | 16 | 24% | ||
|
| ||||||
| Radiation dose, Gyc | 50.4 (45–52.5) | 40.4 (45–52.5) | 0.373 | |||
|
| ||||||
| Sphincterpreservation | 0.814 | |||||
| No | 88 | 26% | 18 | 27% | ||
| Yes | 252 | 74% | 48 | 73% | ||
|
| ||||||
| Adjuvant chemotherapyd | 282 | 83% | 60 | 91% | 0.104 | |
| Fluoropyrimidine onlye | 238 | 84% | 44 | 73% | 0.041 | |
| Oxaliplatin-basede | 44 | 16% | 16 | 27% | ||
Median value with interquartile range
Based on final resection specimen
Tumor location was analyzed based on distance (cm) from anal verge
Percentage among patients received adjuvant chemotherapy
Comparison of fluoropyrimidine only versus oxaliplatin-based
Pathologic Results and Tumor Characteristics
Within the primary study cohort of ypT0-2 patients, the median total number of lymph nodes examined was 11(IQR, 6–15) and did not differ between the ypN0 and ypN+ patients. Most patients were cT3-4 prior to neoadjuvant treatment (91%, 86%, and 86% for ypT0, ypT1, and ypT2, respectively) indicating significant tumor downstaging. Overall, 66(16.3%) patients had lymph node metastasis. Patients with higher ypT categories following chemoradiotherapy were more likely to also have positive ypN status (P<0.001). By ypT stage, the numbers of ypN+ tumors were 13 (9.1%) for ypT0 12 (17.1%) for ypT1, and 41(20.8%) for ypT2. As expected, when positive lymph nodes were present, the median number of positive nodes was 1 (IQR 1–2) for ypT0-2 and 2 (IQR 1–3) for ypT3-4.
Patients were categorized into two groups based on the identification of lymph node metastasis: ypN0and ypN+. Median age and sex were not significantly different between groups. Presence of lymphovascular invasion was more frequently observed in the ypN+ group (P<0.001). The patients who were ypN+ were also more likely to have been cN+ (63% vs 51%). Treatment factors that did not differ between the two groups included the total dose of radiation, the time interval between treatment and resection, and whether or not adjuvant chemotherapy was given. Although a single-agent fluoropyrimidine (5-fluorouracil or capecitabine) was the most common regimen, there was an association between receipt of combination chemotherapy that included oxaliplatin and ypN+status, but this difference was small (Table 1).
Recurrence and Survival
Recurrences were observed in 44 patients overall, 6 had local recurrence only, 34 had systemic recurrence only, and 4 had both local and systemic recurrences. Overall, the 5-year risk for local recurrence was 2.0% among ypT0-2N0 patients and 5.5% among ypT0-2N+ patients (P=0.038). Recurrences were local only in 3 of 340 ypN0 patients and 3 of 66 ypN+ patients. Distant recurrences occurred in 27 ypN0 and 7 ypN+ patients (Table 2).
Table 2.
Recurrences stratifiedby ypT stageand ypN status
| ypT0 | ypT1 | ypT2 | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| ypN0 | ypN+ | ypN0 | ypN+ | ypN0 | ypN+ | |
|
| ||||||
| 129 (90.9) | 13 (9.1) | 58 (82.9) | 12 (17.1) | 151 (79.2) | 41 (20.8) | |
| Local recurrence only, n (%a) | 0 | 1 (7.6) | 0 | 0 | 3 (1.9) | 2(4.9) |
| Systemic recurrence only, n (%a) | 8 (6.2) | 1 (7.6) | 4 (6.9) | 1 (8.3) | 15 (9.7) | 5 (12.2) |
| Both local and systemic recurrence, n (%a) | 1 (0.8) | 0 | 2 (3.4) | 0 | 0 | 1 (2.4) |
|
| ||||||
| 5-year RFS | 91.9% | 83.3% | 81.0% | 78.7% | ||
| P b | 0.43 | 0.67 | ||||
frequency
univariate, log rank
For the cohort of ypT0-2 patients, overall survival and RFS at 5 years were 87.5% and 83.6%, respectively. Stratified by ypN status, the 5-year RFS rates were 85.2%, and 79.6% for the ypT0-2N0 and ypT0-2N+ groups, respectively (P=0.28). In contrast, the 5-year RFS rates were 66.7% and 50.4% for the ypT3-4N0and ypT3-4N+ patients, respectively, (P=0.010) (Figure 1).
Figure 1.
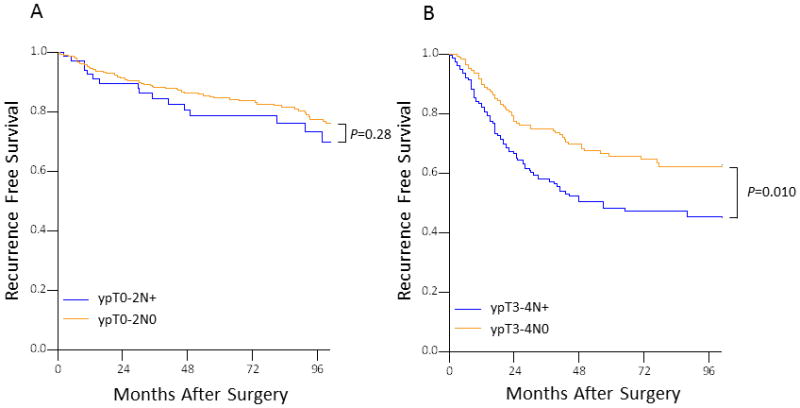
Five-year recurrence-free survival according to lymph node status by ypT stage.
The results of the multivariate Cox regression analysis for RFS are shown in Table 3. In adjusted analysis RFS was not associated with lymph node metastasis in patients with ypT0-2disease (hazard ratio, 1.29; 95% CI, 0.77 to 2.16; P=0.37). Although among patients with ypT3-4 disease, adjusted 5-year RFS was significantly lower in patients with lymph node metastasis (HR, 1.51; 95% CI, 1.07–2.13; P=0.019). Five-year RFS for patients with ypT0-2N+ was higher than that of those with ypT3-4N0, but this did not reach statistical significance (HR, 1.41; 95% CI, 0.84–2.38, P=0.19).
Table 3.
Multivariable Cox regression model for recurrence-free survivalamong ypT0-2 patients
| Variable | ypT0-2
|
|
|---|---|---|
| Hazard ratio | 95% CI | |
| LN metastasis | ||
| ypN0 | 1 | |
|
| ||
| ypN+ | 1.29 | 0.77–2.16 |
|
| ||
| Age, years | ||
| ≤ 60 | 1 | |
|
| ||
| 61–75 | 1.32 | 0.74–2.34 |
|
| ||
| > 75 | 2.06 | 1.18–3.59 |
|
| ||
| ypT Stage | ||
| 0 | 1 | |
|
| ||
| 1 | 1.44 | 0.76–2.74 |
|
| ||
| 2 | 1.62 | 1.99–2.68 |
|
| ||
| Tumor grade | ||
| G1, G2 | 1 | |
|
| ||
| G3, G4 | 0.73 | 0.35–1.52 |
|
| ||
| Lymphovascular invasion | ||
| None | 1 | |
|
| ||
| Present | 2.25 | 1.00–5.08 |
|
| ||
| Adjuvant chemotherapy | ||
| No | 1 | |
|
| ||
| Yes | 0.45 | 0.28–0.70 |
Discussion
The results of the present study show that among patients with locally advanced rectal cancer with a good response to neoadjuvant CXRT who have ypT0-2 residual disease following neoadjuvant CXRT, one in six patients will have histologically confirmed residual lymph node metastases. Even among patients who have complete tumor regression within the bowel wall following neoadjuvant CXRT, at least one in eleven will have histologically confirmed lymph node metastases. Despite this risk, multidisciplinary treatment that includes surgical resection with TME is associated with excellent long-term outcomes with no significant differences in RFS for patients with ypT0-2N+ disease when compared to those with ypT0-2N0 disease. This demonstrates the potential curative role of complete resection of the primary tumor and the associated lymph node basin among even patients with demonstrated residual lymph node disease.
It has been increasingly recognized that the response to neoadjuvant CXRT is an important surrogate indicator of long-term outcomes.2,3 It is, in part, based on this observation that there has been a growing interest in organ-preserving strategies without mesorectal excision for patients with good clinical response to neoadjuvant CXRT and confirmed to have ypT0 to ypT2 residual disease. However, before considering strategies that exclude mesorectal excision, the risk for lymph node disease must be characterized. The presence of lymph node metastases is generally accepted to be one of the most important prognostic factors for patients with rectal cancer and the decrease in local recurrence risk with TME is largely attributable to en-bloc removal of the mesorectum and its lymph nodes.14,15
Unfortunately, no reliable technique currently exists to predict the status of the bowel wall or mesorectum following neoadjuvant CXRT without definitive resection. Complete clinical responses may be identified in 4–27% of patients, however, despite a clinical complete response, residual tumor within the bowel wall may still be a significant concern.16–19 Furthermore, despite advances in technology, there still remains no reliable preoperative imaging modality that can predict pCR and mesorectal sterilization.20–24 We have previously observed an apparent nodal sterilization rate of 56% among cN+ patients.3 As a result, there has been great interest in assessing the status of the tumor in the bowel wall by biopsy or local excision as a marker of the status of the mesorectum and some teams have advocated local excision alone for tumors that are ypT0-2. Unfortunately, the results of this study show that despite significant response within the bowel wall, residual lymph node metastasis is not an ignorable concern.
The rate of lymph node metastasis among patients with ypT0 disease in prior reports has ranged from 2–17%.25,26 Because of the relative rarity of this event, and generally small sample sizes, there has been significant variation in this estimate, however, our identification rate of 9.1% is in line with one of the largest pooled datasets that reported a rate of 8.7% among 333 ypT0 patients as well as our own prior report of 122 patients with ypT0-2 cancers.16,26 As with previous reports of lymph node involvement among ypT1-2 patients, we have demonstrated a positive association with advancing ypT stage; however, our observed rates of 17.1% for ypT1 and 20.8% for ypT2 patients are higher than prior estimates of approximately <10% among ypT1 and approximately 20% among ypT2 patients.8,25 This may, in part, be due to issues related to smaller sample size in prior studies, pretreatment tumor burden, or the completeness of the lymph node evaluation, although the median number of 11 lymph nodes examined is in line with most prior reports. Furthermore, the median time to resection following completion of CXRT of 7 weeks is also in line with prior reports. Our institutional protocol has been to entirely embed the primary tumor and lymph nodes for serial sectioning and the resultant standardized approach may have improved the detection rate, however, the rate of bowel wall sterilization (ypT0) was comparable to prior reports. A standardized surgical approach with TME or TSME may also have contributed. Finally, nearly 20 patients with ypT0 disease were treated with local excision for reasons of comorbidity or refusal of radical resection during the study period.27 Even in the unlikely circumstance that all of these patients were ypN0, the rate of ypN+ would have still been 8%. Regardless, the results indicate that in patients treated with preoperative chemoradiotherapy, despite a good response within the bowel wall, there remains a significant risk for residual mesorectal lymph nodal disease.
But when the multidisciplinary treatment approach for these patients includes definitive resection, there is excellent disease control with low rates of local or distant recurrence. In fact, a difference in RFS could not be observed amongypT0-2N+ patients when compared to the ypT0-2N0 group (HR 1.35, 95% CI: 0.80–2.27). Consistent with a previously report from population-based data, for patients with poor response to CXRT with ypT3-4 tumors, residual lymph node involvement was correlated with decreased recurrence-free survival despite radical resection.28 Although patients who are pT1-2N1 without preoperative CXRT may also have favorable local disease control with TME, their risk for distant failure is significantly higher than for those patients who are pT1-2N0.29 Furthermore, data from the Dutch TME trial demonstrates that despite preoperative XRT, lymph node metastasis remains a major determinant of local control.30 Thus, it may be postulated that the local and systemic disease risk among patients with moderate response to neoadjuvant CXRT and resultant ypT1-2N+ disease may be effectively controlled by complete surgical resection, supporting the need to include the residual mesorectal disease in the treatment strategy. Furthermore, the poor subsequent surgical salvage potential for patients who recur following treatment without definitive mesorectal excision cannot be ignored.31
However, despite the high risk for mesorectal lymph node involvement, the majority of patients with ypT0-2 disease will still have negative lymph nodes at resection. As a result, several small series of patients treated with organ-preserving approaches have shown promising results. But the challenge is to identify patients with ypN+ disease and appropriately select the patients eligible for this approach as the use of residual tumor within the bowel wall up to ypT2 as criteria would leave an unacceptable number of patients at risk for undertreatment. In fact, the number needed to harm with this approach ranges from only 6 patients with stratification at ypT2 to 11 patients with stratification at ypT0. It is increasingly clear that other both imaging and molecular markers identified both before and after treatment will need to be combined with the clinical evaluation to identify eligible patients.24,32,33 Furthermore, because of the underlying excellent prognosis of these patients with standard multidisciplinary treatment, any new treatment strategy must be compared against the oncologic outcomes observed with radical resection.
This is one of the largest evaluations of lymph node metastasis among patients with good bowel wall response to neoadjuvant chemoradiotherapy within a single institution. Our analysis identified a high risk for residual mesorectal lymph node metastasis among patients even with complete pathologic response. However, complete removal of the mesorectal burden in this population results in excellent disease control and comparable outcomes with patients without lymph node metastasis. Given the uniquely good outcomes with standard therapy among patients with ypT0-2 disease, the use of ypT stage to stratify patients for local excision or a wait and see approach risks undertreatment of an unacceptably high proportion of patients. Thus, organ-preserving strategies for these patients will need to consider both imaging and molecular biomarkers in addition to clinical response to identify eligible patients.
Acknowledgments
Supported in part by Grant No. K07-CA1331987 from the National Institutes of Health (NIH, GJC), and Grant No. CA016672 from NIH through MD Anderson’s Cancer Center Support.
Footnotes
Podium presentation (S6) at the 2012 American Society of Colon and Rectal Surgeons Annual Scientific Meeting, San Antonio, TX. June 2-6, 2012.
Author Contributions: Conception & Design (IJP, GJC); Acquisition of Data (YNY, JNS, MAR, BF, SN, GJC); Analysis & Interpretation (IJP, CH, GJC); Drafting of Manuscript (IJP, GJC); Critical Revision (IJP, YNY, CH, GJC); Funding (GJC); Final Approval (all authors).
References
- 1.Hartley A, Ho KF, McConkey C, Geh JI. Pathological complete response following pre-operative chemoradiotherapy in rectal cancer: analysis of phase II/III trials. Br J Radiol. 2005;78:934–938. doi: 10.1259/bjr/86650067. [DOI] [PubMed] [Google Scholar]
- 2.Maas M, Nelemans PJ, Valentini V, et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. 2010;11:835–844. doi: 10.1016/S1470-2045(10)70172-8. [DOI] [PubMed] [Google Scholar]
- 3.Park IJ, You YN, Agarwal A, et al. Neoadjuvant treatment response as an early response indicator for patients with rectal cancer. J Clin Oncol. 2012;30:1770–1776. doi: 10.1200/JCO.2011.39.7901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Habr-Gama A, Perez RO, Nadalin W, et al. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results. Ann Surg. 2004;240:711–718. doi: 10.1097/01.sla.0000141194.27992.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maas M, Beets-Tan RG, Lambregts DM, et al. Wait-and-see policy for clinical complete responders after chemoradiation for rectal cancer. J Clin Oncol. 2011;29:4633–4640. doi: 10.1200/JCO.2011.37.7176. [DOI] [PubMed] [Google Scholar]
- 6.Collette L, Bosset JF, den Dulk M, et al. Patients with curative resection of cT3–4 rectal cancer after preoperative radiotherapy or radiochemotherapy: does anybody benefit from adjuvant fluorouracil-based chemotherapy? A trial of the European Organisation for Research and Treatment of Cancer Radiation Oncology Group. J Clin Oncol. 2007;25:4379–4386. doi: 10.1200/JCO.2007.11.9685. [DOI] [PubMed] [Google Scholar]
- 7.Read TE, Andujar JE, Caushaj PF, et al. Neoadjuvant therapy for rectal cancer: histologic response of the primary tumor predicts nodal status. Dis Colon Rectum. 2004;47:825–831. doi: 10.1007/s10350-004-0535-x. [DOI] [PubMed] [Google Scholar]
- 8.Bujko K, Nowacki MP, Nasierowska-Guttmejer A, et al. Prediction of mesorectal nodal metastases after chemoradiation for rectal cancer: results of a randomised trial: implication for subsequent local excision. Radiother Oncol. 2005;76:234–240. doi: 10.1016/j.radonc.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 9.Hughes R, Glynne-Jones R, Grainger J, et al. Can pathological complete response in the primary tumour following pre-operative pelvic chemoradiotherapy for T3-T4 rectal cancer predict for sterilisation of pelvic lymph nodes, a low risk of local recurrence and the appropriateness of local excision? Int J Colorectal Dis. 2006;21:11–17. doi: 10.1007/s00384-005-0749-y. [DOI] [PubMed] [Google Scholar]
- 10.Pucciarelli S, Capirci C, Emanuele U, et al. Relationship between pathologic T-stage and nodal metastasis after preoperative chemoradiotherapy for locally advanced rectal cancer. Ann Surg Oncol. 2005;12:111–116. doi: 10.1245/ASO.2005.03.044. [DOI] [PubMed] [Google Scholar]
- 11.Perez RO, Habr-Gama A, Proscurshim I, et al. Local excision for ypT2 rectal cancer--much ado about something. J Gastrointest Surg. 2007;11:1431–1440. doi: 10.1007/s11605-007-0271-3. [DOI] [PubMed] [Google Scholar]
- 12.Bujko K, Sopylo R, Kepka L. Local excision after radio(chemo)therapy for rectal cancer: is it safe? Clin Oncol (R Coll Radiol) 2007;19:693–700. doi: 10.1016/j.clon.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 13.Washington MK, Berlin J, Branton P, et al. Protocol for the examination of specimens from patients with primary carcinoma of the colon and rectum. Arch Pathol Lab Med. 2009;133:1539–1551. doi: 10.5858/133.10.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wibe A, Eriksen MT, Syse A, Myrvold HE, Soreide O. Total mesorectal excision for rectal cancer--what can be achieved by a national audit? Colorectal Dis. 2003;5:471–477. doi: 10.1046/j.1463-1318.2003.00506.x. [DOI] [PubMed] [Google Scholar]
- 15.Heald RJ, Ryall RD. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet. 1986;1:1479–1482. doi: 10.1016/s0140-6736(86)91510-2. [DOI] [PubMed] [Google Scholar]
- 16.Bedrosian I, Rodriguez-Bigas MA, et al. Predicting the node-negative mesorectum after preoperative chemoradiation for locally advanced rectal carcinoma. J Gastrointest Surg. 2004;8:56–63. doi: 10.1016/j.gassur.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 17.Hiotis SP, Weber SM, Cohen AM, et al. Assessing the predictive value of clinical complete response to neoadjuvant therapy for rectal cancer: an analysis of 488 patients. J Am Coll Surg. 2002;194:131–136. doi: 10.1016/s1072-7515(01)01159-0. [DOI] [PubMed] [Google Scholar]
- 18.Garcia-Aguilar J, Shi Q, Thomas CR, Jr, et al. A phase II trial of neoadjuvant chemoradiation and local excision for T2N0 rectal cancer: preliminary results of the ACOSOG Z6041 trial. Ann Surg Oncol. 2012;19:384–391. doi: 10.1245/s10434-011-1933-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith FM, Chang KH, Sheahan K, Hyland J, O’Connell PR, Winter DC. The surgical significance of residual mucosal abnormalities in rectal cancer following neoadjuvant chemoradiotherapy. Br J Surg. 2012;99:993–1001. doi: 10.1002/bjs.8700. [DOI] [PubMed] [Google Scholar]
- 20.Gollub MJ, Gultekin DH, Akin O, et al. Dynamic contrast enhanced-MRI for the detection of pathological complete response to neoadjuvant chemotherapy for locally advanced rectal cancer. Eur Radiol. 2012;22:821–831. doi: 10.1007/s00330-011-2321-1. [DOI] [PubMed] [Google Scholar]
- 21.Curvo-Semedo L, Lambregts DM, Maas M, et al. Rectal cancer: assessment of complete response to preoperative combined radiation therapy with chemotherapy--conventional MR volumetry versus diffusion-weighted MR imaging. Radiology. 2011;260:734–743. doi: 10.1148/radiol.11102467. [DOI] [PubMed] [Google Scholar]
- 22.Vliegen RF, Beets-Tan RG, Vanhauten B, et al. Can an FDG-PET/CT predict tumor clearance of the mesorectal fascia after preoperative chemoradiation of locally advanced rectal cancer? Strahlenther Onkol. 2008;184:457–464. doi: 10.1007/s00066-008-1858-7. [DOI] [PubMed] [Google Scholar]
- 23.Leibold T, Akhurst TJ, Chessin DB, et al. Evaluation of (1)F-FDG-PET for early detection of suboptimal response of rectal cancer to preoperative chemoradiotherapy: a prospective analysis. Ann Surg Oncol. 2011;18:2783–2789. doi: 10.1245/s10434-011-1634-2. [DOI] [PubMed] [Google Scholar]
- 24.Chang GJ, You YN, Park IJ, et al. Pretreatment high-resolution rectal MRI and treatment response to neoadjuvant chemoradiation. Dis Colon Rectum. 2012;55:371–377. doi: 10.1097/DCR.0b013e31824678e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guillem JG, Diaz-Gonzalez JA, Minsky BD, et al. cT3N0 rectal cancer: potential overtreatment with preoperative chemoradiotherapy is warranted. J Clin Oncol. 2008;26:368–373. doi: 10.1200/JCO.2007.13.5434. [DOI] [PubMed] [Google Scholar]
- 26.Yeo SG, Kim DY, Kim TH, et al. Pathologic complete response of primary tumor following preoperative chemoradiotherapy for locally advanced rectal cancer: long-term outcomes and prognostic significance of pathologic nodal status (KROG 09–01) Ann Surg. 2010;252:998–1004. doi: 10.1097/SLA.0b013e3181f3f1b1. [DOI] [PubMed] [Google Scholar]
- 27.Callender GG, Das P, Rodriguez-Bigas MA, et al. Local excision after preoperative chemoradiation results in an equivalent outcome to total mesorectal excision in selected patients with T3 rectal cancer. Ann Surg Oncol. 2010;17:441–447. doi: 10.1245/s10434-009-0735-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang GJ, Rodriguez-Bigas MA, Eng C, Skibber JM. Lymph node status after neoadjuvant radiotherapy for rectal cancer is a biologic predictor of outcome. Cancer. 2009;115:5432–5440. doi: 10.1002/cncr.24622. [DOI] [PubMed] [Google Scholar]
- 29.Gunderson LL, Sargent DJ, Tepper JE, et al. Impact of T and N stage and treatment on survival and relapse in adjuvant rectal cancer: a pooled analysis. J Clin Oncol. 2004;22:1785–1796. doi: 10.1200/JCO.2004.08.173. [DOI] [PubMed] [Google Scholar]
- 30.Peeters KC, Marijnen CA, Nagtegaal ID, et al. The TME trial after a median follow-up of 6 years: increased local control but no survival benefit in irradiated patients with resectable rectal carcinoma. Ann Surg. 2007;246:693–701. doi: 10.1097/01.sla.0000257358.56863.ce. [DOI] [PubMed] [Google Scholar]
- 31.Friel CM, Cromwell JW, Marra C, Madoff RD, Rothenberger DA, Garcia-Aguilar J. Salvage radical surgery after failed local excision for early rectal cancer. Dis Colon Rectum. 2002;45:875–879. doi: 10.1007/s10350-004-6320-z. [DOI] [PubMed] [Google Scholar]
- 32.Lambregts DM, Maas M, Bakers FC, et al. Long-term follow-up features on rectal MRI during a wait-and-see approach after a clinical complete response in patients with rectal cancer treated with chemoradiotherapy. Dis Colon Rectum. 2011;54:1521–1528. doi: 10.1097/DCR.0b013e318232da89. [DOI] [PubMed] [Google Scholar]
- 33.Garcia-Aguilar J, Chen Z, Smith DD, et al. Identification of a biomarker profile associated with resistance to neoadjuvant chemoradiation therapy in rectal cancer. Ann Surg. 2011;254:486–493. doi: 10.1097/SLA.0b013e31822b8cfa. [DOI] [PMC free article] [PubMed] [Google Scholar]


