Abstract
We demonstrated that β-(1-Azulenyl)-L-Alanine, a fluorescent pseudoisosteric analog of tryptophan, exhibits weak environmental dependence and thus allows for using weak intrinsic quenchers, such as methionines, to monitor protein-protein interactions while not perturbing them.
Fluorescence spectroscopy is an excellent and versatile tool for studying protein folding, dynamics, aggregation and local environment.1 Intrinsic probes for such studies are of great value, as they are potentially less disruptive to the structure of the biological molecule of interest. Tryptophan’s (Trp) ability to be excited separately from all other amino acids, high sensitivity to the local environment and low abundance in proteins is particularly well suited for such studies.2 Tryptophan emission is highly dependent on solvent exposure and susceptible to quenching due to electron transfer to the backbone and the neighboring side chains3, 4 resulting in the apparent Trp quantum yield (Φ) typically ranging from 0.35 to 0.01, or even less,5 and emission maximum (λmax) shifting from ca. 360 nm to ca. 310 nm.6 While extremely useful in protein folding studies,7 the concomitant change in Φ and λmax can complicate analysis when several events (such as binding, quenching and drastic change of local environment) occur simultaneously. Additionally, labeling of a distinct site in a macromolecule is often necessary which may require significant sequence changes through mutagenesis for proteins that naturally contain multiple intrinsic fluorophores.
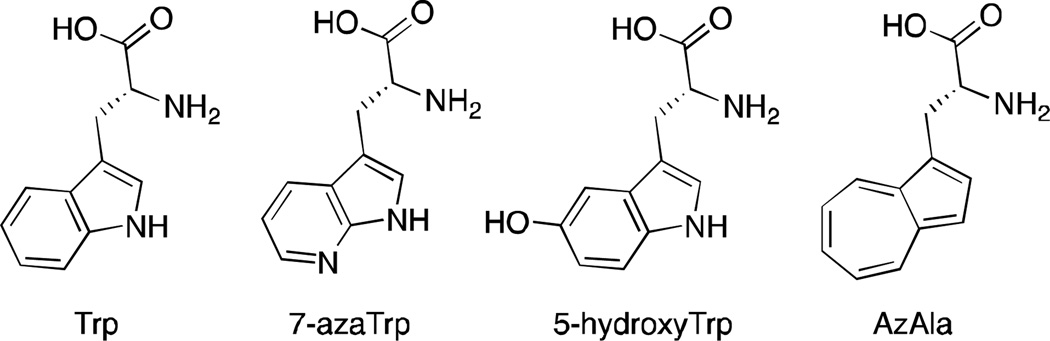
One approach to address this problem is to substitute the residue of interest with an unnatural analog, which can be selectively excited above ~310 nm, where Trp absorption is negligible. This could be achieved by developing iso- or pseudoisosteric mimics with distinct fluorescent signatures that only minimally perturb the native protein’s properties.2, 7–9 Some of such analogs, such as 7-azatryptophan and 5-hydroxytryptophan (Scheme 1), can be incorporated into proteins using the cellular machinery.10 However these, along with other probes,2 do not allow for easy decoupling of the contribution of the environment from the quenching due to local interactions.
Scheme 1.
Structures of Trp and some of its analogs.
Large shifts of quantum yields and emission maxima positions could potentially change FRET and quenching efficiencies in fluorescence studies. As the rate of energy transfer is dependent on Φ and overall efficiency of transfer is dependent on the overlap integral, any in situ changes in fluorophore environment can impact FRET efficiency without any change in distance between the donor and acceptor.1 Thus, a need exists for a tryptophan mimic that is relatively insensitive to its environment. Here, we show that azulene, a pseudoisosteric hydrocarbon analog of indole fulfills that role. Azulene has a number of advantageous properties: it can be excited independently of Trp, the fluorescence emission photophyscis are simple, and it does not possess functional groups that are sensitive to the local environment.
The use of β-(1-Azulenyl)-L-Alanine (AzAla), an amino acid containing azulene in its side-chain, as a tryptophan mimic was first proposed by Hudson et al.11 Its practical use was hindered by the lack of efficient synthetic routes to AzAla until the syntheses of the racemic12 and the enantiomerically pure derivatives13 was reported. Mutation of Trp to AzAla has been shown to have a minimal effect on efficacy of short peptides targeting GPCR receptors in cell assays,13 but the biophysical characterization of this probe was limited to studies of its interaction with synthetically introduced free radicals.14–16 Here we demonstrate that AzAla’s insensitivity to the environment allows for investigation of biophysical phenomena by via a structurally conservative, single fluorescent probe, which does not require additional functionalization. Together with AzAla’s similarity to Trp, it provides a powerful chemical biology tool for looking at protein-protein interactions.
Azulene derivatives have rich absorption spectra with bands distinctly different from those of tryptophan. In particular, the S2-S0 transition is completely separate from the 280 nm region with the extinction coefficients (~4200 cm−1M−1 for AzAla) and quantum yield values comparable to those of tryptophan (ESI†, S11-S13). Moreover, the narrower profile of the azulene emission results in nearly the same apparent emission intensity for AzAla (ESI†, S11). Fluorescence lifetime measurements show simple monoexponential fluorescence decay (ESI†, S11) of AzAla fluorescence in both the free and peptide incorporated forms. This offers another advantage over Trp, which exhibits very complex fluorescence decay behavior and simplifies analysis.17
Initial studies were performed after AzAla was converted to the corresponding diamide, N-Acetyl-(AzulylAlanine)-propylamide (NAAzAP) to better represent protein environment and to minimize any pH effects. Fluorescence spectra of NAAzAP measured in different solvents show only a 6 nm red shift (unlike blue shifts of Trp and indole-based Trp mimics) with concomitant relatively small increase in intensity upon lowering solvent polarity (12%–78%, depending on the solvent, with no clear trend that links fluorescence intensity and solvent polarity, ESI†, S11). Fluorescence spectra were obtained by exciting at 342 nm, which approximately corresponds to an isosbestic point of AzAla absorption in different solvents.
But would this environmental insensitivity still hold in a biochemically relevant situation? Lipid bilayers offer a medium where the polarity of the local environment significantly impacts the behavior of fluorophores. We chose a well-studied polyleucine Leu19 model that spans the hydrophobic portion of the bilayer flanked by two lysine residues on both sides to ensure proper transmembrane (TM) orientation of the peptide.18 A series of peptides were prepared where AzAla was introduced in the middle of the peptide (Z13), near the terminus (Z3), and in the middle of the peptide with a neighboring aspartate (D9Z13) (Table 1). Using this system we explored the properties of AzAla in the middle of the hydrophobic bilayer, in the headgroup region and the influence of neighboring polar groups.
Table 1.
AzAla-labeled transmembrane peptides used in this study.
| Peptide | Sequencea |
|---|---|
| Z13 | |
| Z3 | |
| D9Z13 |
Z signifies AzAla.
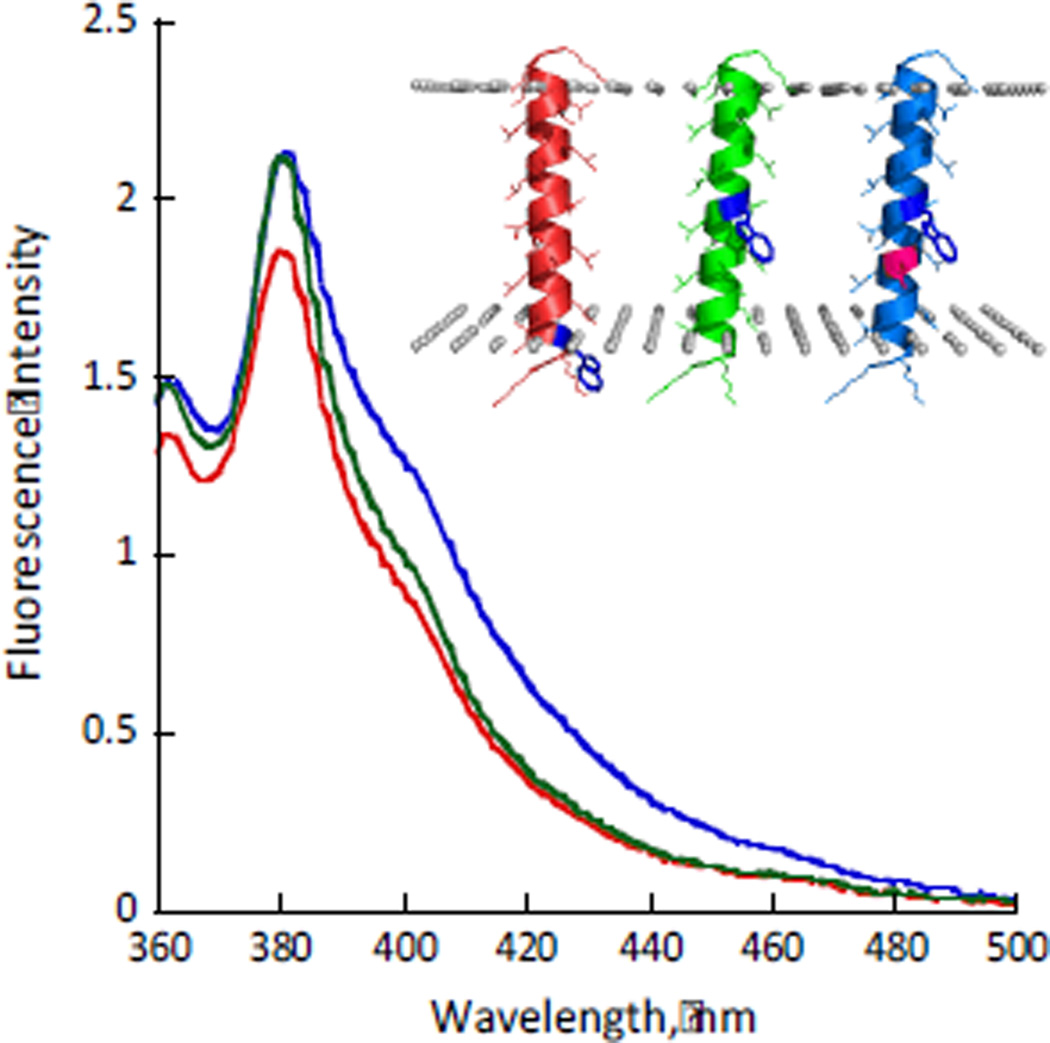
The peptides were reconstituted in dioleoyl phosphatidyl choline (DOPC) lipid vesicles at 1:125 peptide:lipid ratio. The fluorescence spectra of all of the peptides showed λmax of ~381 nm and only minimal changes (~15%) in quantum yield, confirming that AzAla fluorescence does not depend on insertion depth. Additionally, an aspartic acid residue one helical turn away from AzAla, known to significantly red shift Trp fluorescence even in the TM region,18, 19 did not influence the AzAla fluorescence (Fig. 1). The proper insertion of the peptides in the bilayer was confirmed by quenching experiments using aqueous and membrane-embedded quenchers.20 The fluorophores at the center of the TM sequence exhibit high susceptibility to quenching by 10-doxyl nonadecane (10-DN, confined to the hydrophobic portion of the bilayer) and significantly lower susceptibility to water-soluble acrylamide. Conversely, the shallowly located AzAla in Z3 is much more susceptible to the aqueous acrylamide and less susceptible to the 10-DN (ESI†, S16).
Fig. 1.
Fluorescence emission spectra of Z3 (red), Z13 (green) and D9Z13 (blue) in DOPC lipid bilayers (λex = 342 nm). The inset shows location of the azulene moiety in the bilayer predicted by E(z)-potential (the borders of the hydrophobic portion are shown by grey spheres).25
Fluorescence quenching is an excellent tool for studying protein dynamics and folding.21, 22 Since AzAla is quite insensitive to the local environment, we expected that its properties would be much more affected by weaker quenchers commonly present in proteins (e.g. histidine, methionine, etc.), providing a valuable tool for studying protein-protein interactions. Collisional quenching experiments showed that NAAzAP’s fluorescence is indeed quenched in the presence of free methionine and imidazole (ESI†, S18) but not by tyrosine or tryptophan under the same conditions.
To exploit the sensitivity to weak quenchers, and thereby test the utility of the probe in a biological context, we monitored the intractions of AzAla substituted peptides with eukaryotic protein calmodulin (CaM), involved in multiple signal transduction pathways.26 We chose this system as recognition of Trp in the CaM-binding domains is part of the natural interaction and CaM has a large number of methionines. Additionally, binding of CaM to its partners has previously been studied spectroscopically.22, 23 Quenching of Trp fluorescence by methionines in CaM is known,24 but these effects are masked by the increase of Trp’s quantum yield upon partitioning in the hydrophobic cleft of CaM.
As the CaM-binding partner, we chose a 20-residue domain of smooth muscle myosin light chain kinase (smMLCK). The smMLCK binds CaM in an anti-parallel orientation (the peptide N-terminus is associated with the protein C-terminus) with a low nM binding affinity in the presence of Ca2+.26 Incorporation of AzAla into the peptide did not change its quantum yield (ESI†, S12-S13) and the fluorescence lifetime properties (monoexponential decay model, ESI†, S11), compared to the diamide version. A surface plasmon resonance study showed that CaM exhibits similar binding affinity to both native and the AzAla-mutated peptides (ESI†, S19-S20).
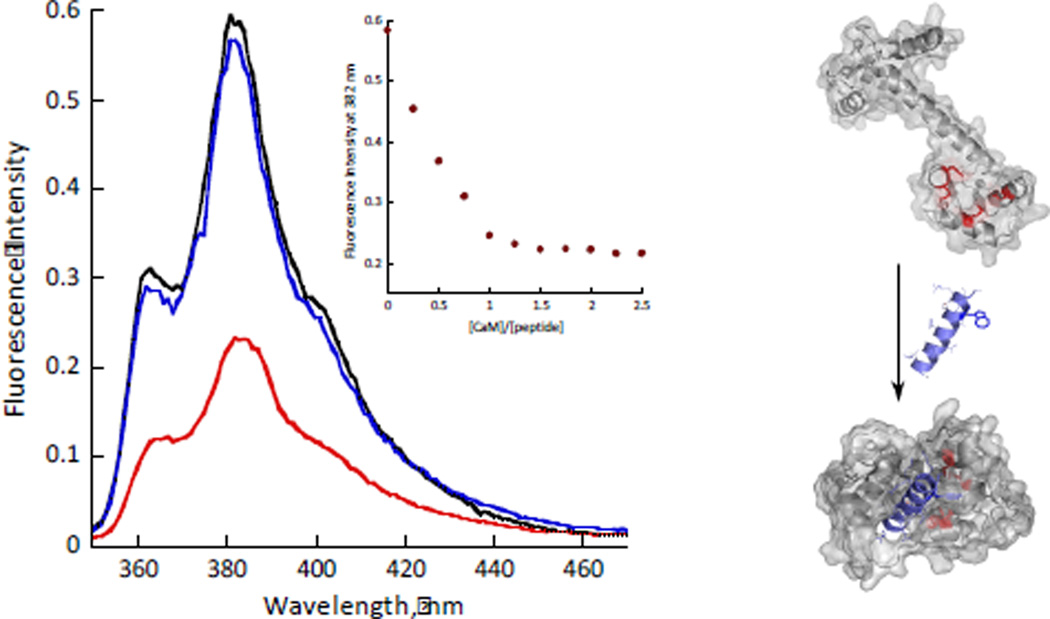
Having established that AzAla does not significantly perturb the CaM-smMLCK interaction, we measured fluorescence emission spectra of AzAla-labeled smMLCK in the presence of CaM. An overall decrease in fluorescence is observed and the 1:1 binding stoichiometry was easily determined. AzAla fluorescence could be fully restored by sequestering Ca2+ required for CaM-smMLCK association with EDTA (Fig. 2). Binding of the smMLCK peptide to CaM was also followed by fluorescence polarization and yielded consistent results (ESI†, S21). Further, the quenching observed during CaM-smMLCK association is not specific to a particular peptide sequence, but rather represents a common phenomenon based on weak quencher interaction with AzAla. This was shown by the CaM-melittin association. Melittin has strong affinity for CaM, a similar position of the Trp in the binding pocket upon association with the protein, but distinctly different sequence and a parallel binding topology.27 Despite these changes, the fluorescence profiles of AzAla-labeled melittin upon association with CaM was essentially identical to that of the AzAla smMLCK (ESI†, S22).
Fig. 2.
Left - Fluorescence emission spectra (λex = 342 nm) of smMLCK peptide (10 µM peptide, 50 mM HEPES, pH 7, 100 mM NaCl) before (black), after (red) addition of 12 µM of CaM and after addition of 2 mM EDTA (blue). The inset shows the corresponding titration curve. Right – A schematic representation of CaM-smMLCK interaction (respective PDB entries: 1cll and 2o5g). Met residues near AzAla are shown in red.
We have demonstrated the unique ability of AzAla to serve as an environment-insensitive and non-disruptive biochemical probe that can be selectively excited at 342 nm. The minimal changes in quantum yield and emission maxima in different microenvironments allow for observations of subtle effects caused by weak quenchers inherently present in proteins. While currently no published method exists to introduce AzAla into larger proteins using cellular machinery,28 in vitro methods based on chemical modification of suppressor tRNAs, known to incorporate a large variety of chemically diverse unnatural amino acids into proteins,29 are likely to succeed for AzAla. Additionally, semi-synthetic routes provide ample options for its incorporation into larger biomolecules.30 Alternatively, azulene could be introduced through reactive probes that utilize the reactivity of Lys and Cys side chains, similar to traditional fluorescent labeling methods. This approach will allow for expansion of this methodology to a broader range of protein-protein interfaces at a lesser synthetic cost. Future applications will also include reporting more accurate measurements of FRET, not skewed by local microenvironment changes. While considerable attention has been devoted to fluorescent probes that are highly sensitive to their local environment, our results highlight the opportunities provided by environmental insensitivity that have not been similarly exploited in the past
Supplementary Material
Acknowledgments
We thank Bruce Hudson, James Hougland and William DeGrado for valuable discussions, Joel Bennett for use of Biacore 300 instrument, Alex Siemiarczuk and Clark Allen for the help with acquisition of the fluorescence lifetime data. This work was funded by Syracuse University (start-up funds to I.V.K.) and the NIH (grant 1R15GM094330 to G.A.C.).
Footnotes
Electronic Supplementary Information (ESI) available: details of AzAla preparation, spectroscopic characterization, fluorescence quenching. See DOI: 10.1039/b000000x/
References
- 1.Lakowicz JR. Principles of Fluorescence Spectroscopy. New York: Springer; 2006. [Google Scholar]
- 2.Sinkeldam RW, Greco NJ, Tor Y. Chem. Rev. 2010;110:2579–2619. doi: 10.1021/cr900301e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen Y, Barkley MD. Biochemistry. 1998;37:9976–9982. doi: 10.1021/bi980274n. [DOI] [PubMed] [Google Scholar]
- 4.Callis PR, Vivian JT. Chem. Phys. Lett. 2003;369:409–414. [Google Scholar]
- 5.Eftink MR. In: Methods of Biochemical Analysis. Suelter CH, editor. New York: John Wiley and Sons; 1991. pp. 127–125. [Google Scholar]
- 6.Gilardi G, Mei G, Rosato N, Canters GW, Finazzi-Agro A. Biochemistry. 1994;33:1425–1432. doi: 10.1021/bi00172a020. [DOI] [PubMed] [Google Scholar]
- 7.Royer CA. Chem. Rev. 2006;106:1769–1784. doi: 10.1021/cr0404390. [DOI] [PubMed] [Google Scholar]
- 8.Eftink M. In: Methods Enzymol. Brand L, Johnson ML, editors. 1997. p. 221. [DOI] [PubMed] [Google Scholar]
- 9.Eftink M, Shastry MCR. In: Methods Enzymol. Brand L, Johnson ML, editors. 1997. p. 258. [DOI] [PubMed] [Google Scholar]
- 10.Budisa N, Pal PP. Biol. Chem. 2004;385:893–904. doi: 10.1515/BC.2004.117. [DOI] [PubMed] [Google Scholar]
- 11.Hudson BS, Harris DL, Ludescher RD, Ruggiero A, Cooney-Freed A, Cavalier, in S. In: Application of Fluorescence in the Biomedical Sciences. Taylor RD, et al., editors. New York: A.R. Liss; 1986. pp. 159–202. [Google Scholar]
- 12.Klemm LH, Hudson BS, Lu JJ. Org. Prep. Proced. 1989;21:633–641. [Google Scholar]
- 13.Loidl, H.-S. Musiol G, Budisa N, Huber R, Poirot S, Fourmy D, Moroder L. J. Peptide Sci. 2000;6:139–144. doi: 10.1002/(SICI)1099-1387(200003)6:3<139::AID-PSC240>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 14.Sartori E, Toffoletti A, Corvaja C, Moroder L, Formaggio F, Toniolo C. Chem. Phys. Lett. 2004;385:362–367. [Google Scholar]
- 15.Mazzuca C, Stella L, Venanzi M, Formaggio F, Toniolo C. Biophys. J. 2005;88:3411–3421. doi: 10.1529/biophysj.104.056077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Venanzi M, Valeri A, Palleschi A, Stella L, Moroder L, Formaggio F, Toniolo C, Pispisa B. Biopolymers. 2004;75:128–139. doi: 10.1002/bip.20110. [DOI] [PubMed] [Google Scholar]
- 17.Szabo AG, Rayner DM. J. Am. Chem. Soc. 1980;102:554–563. [Google Scholar]
- 18.Caputo GA, London E. Biochemistry. 2004;43:8794–8806. doi: 10.1021/bi049696p. [DOI] [PubMed] [Google Scholar]
- 19.Jones JD, Gierasch LM. Biophys. J. 1994;67:1534–1545. doi: 10.1016/S0006-3495(94)80627-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caputo GA, London E. Biochemistry. 2003;42:3265–3274. doi: 10.1021/bi026696l. [DOI] [PubMed] [Google Scholar]
- 21.Culik RM, Jo H, DeGrado WF, Gai F. J. Am. Chem. Soc. 2012;134:8026–8029. doi: 10.1021/ja301681v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldberg JM, Batjardal S, Petersson EJ. J. Am. Chem. Soc. 2010;132:14718–14720. doi: 10.1021/ja1044924. [DOI] [PubMed] [Google Scholar]
- 23.Jo H, Culik RM, Korendovych IV, DeGrado WF, Gai F. Biochemistry. 2010;49:10354–10356. doi: 10.1021/bi101711a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weljie AM, Vogel HJ. Protein Engineeing. 2000;13:59–66. doi: 10.1093/protein/13.1.59. [DOI] [PubMed] [Google Scholar]
- 25.Senes A, Chadi DC, Law PB, Walters RF, Nanda V, DeGrado WF. J. Mol. Biol. 2007;366:436–438. doi: 10.1016/j.jmb.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 26.O'Neil KT, DeGrado WF. Trends in Biochem. Sci. 1990;15:59–64. doi: 10.1016/0968-0004(90)90177-d. [DOI] [PubMed] [Google Scholar]
- 27.Scaloni A, Miraglia N, Orru S, Amodeo P, Motta A, Marino G, Pucci P. J. Mol. Biol. 1998;277:945–958. doi: 10.1006/jmbi.1998.1629. [DOI] [PubMed] [Google Scholar]
- 28.Budisa N. Angew. Chem. Int. Ed. 2004;43:6426–6463. doi: 10.1002/anie.200300646. [DOI] [PubMed] [Google Scholar]
- 29.Hohsaka T, Sisido M. Curr. Opin. Chem. Biol. 2002;6:809–815. doi: 10.1016/s1367-5931(02)00376-9. [DOI] [PubMed] [Google Scholar]
- 30.Kent SBH. Chem. Soc. Rev. 2009;38:338–351. doi: 10.1039/b700141j. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.