Abstract
Soil nematode community response to treatments of three, four-year crop rotations (spring wheat-pea-spring wheat-flax, spring wheat-green manure-spring wheat-flax, and spring wheat-alfalfa-alfalfa-flax) under conventional and organic management, and native tall grass restoration (restored prairie) were assessed in June 2003, and July and August 2004. The research site was the Glenlea Long-term Rotation and Crop Management Study, in the Red River Valley, Manitoba, established in 1992. The nematode community varied more with sample occasion than management and rotation. The restored prairie favored high colonizer-persister (c-p) value omnivores and carnivores, and fungivores but less bacterivores. The restored prairie soil food web was highly structured, mature and low-to-moderately enriched as indicated by structure (SI), maturity (MI) and enrichment (EI) index values, respectively. Higher abundance of fungivores and channel index (CI) values suggested fungal-dominated decomposition. Nematode diversity was low even after more than a decade of restoration. A longer time may be required to attain higher diversity for this restored fragmented prairie site distant from native prairies. No consistent differences were found between organic and conventional management for nematode trophic abundance, with the exception of enrichment opportunists of the c-p 1 group which were favored by conventional management. Although EI was lower and SI was higher for organic than conventional their absolute values suggested decomposition channels to be primarily bacterial, and fewer trophic links with both management scenarios. A high abundance of fungivores in the rotation including the green manure indicates greater fungal decomposition.
Keywords: nematode diversity, organic management, prairie restoration, soil food web
Nematodes represent many trophic components of the food web (Yeates et al., 1993) and play essential roles in ecosystem functioning (Ingham et al., 1985; Ferris and Bongers, 2006). Nematode communities are sensitive to change in environmental conditions due to natural or anthropogenic causes and are therefore considered useful bioindicators for soil health assessment (Neher and Olson, 1999; Yeates and Bongers, 1999). Colonizer-persister (c-p) values of nematode taxa ordinated on a 1-5 scale based on r-k life-history characteristics are useful in interpreting the trophic status of the soil food web in different habitats (Bongers, 1990). Maturity index calculations based on these taxa rankings have been widely used to describe the effects of different crop management practices on the soil food webs (Bongers and Ferris, 1999; Porazinska et al., 1999). Further, nematode faunal analysis based on the relative weighted abundance of c-p classes provides a quantitative measure of the nematode community structure and the probable condition of the soil food web (Ferris et al., 2001). The analysis includes calculation of indices of food web enrichment (EI), structure (SI) and channel index (CI) which provides information about belowground processes. The EI indicates the response of primary decomposers (bacteria and fungi) to available resources, SI indicates prevalence of trophic linkages in the soil food web, and CI provides prevalent decomposition channels in the soil food web.
Synthetic fertilizers, pesticides, and herbicides are important inputs in conventional agricultural systems which can impact nematode community composition in different ways. For example, fertilizer application in cultivated soils decreased omnivore and fungal-feeding nematodes (Sohlenius and Wasilewska, 1984; Sohlenius and Boström, 1986) but increased both plant-parasitic and bacterial feeding nematodes (Sohlenius and Boström, 1986). Yardim and Edwards (1998) found adverse effects of insecticide applications on the abundances of bacterivores and fungivores while plant-parasites were favoured compared to an untreated control. Tenuta and Ferris (2004) demonstrated in solution studies that high c-p (3-5) but not low c-p value (1-2) nematodes were killed from low concentrations of ammonium fertilizer.
Organic farming practices do not rely on the application of synthetic inputs and soil fertility is managed through suitable crop rotation and cover crops, supplemented with animal and crop wastes or green manures (Clark et al., 1999). Organically managed soils posses greater microbial activity and bacterial feeding nematodes than those managed with conventional practices (Bulluck et al., 2002; Briar et al., 2007). Abundance of fungal- and bacterial-feeding nematodes tend to increase in organic management systems (Freckman, 1988; Griffiths et al., 1994), presumably because prey, fungi and bacterial numbers increase after application of organic amendments (Bongers and Ferris, 1999; McSorley and Frederick, 1999). In addition, other soil management practices such as tillage, irrigation and crop rotation, common to both organic and conventional management, may affect the soil ecosystem by changing the microbial and nematode trophic structure (Parmelee and Alston, 1986; McSorley and Gallaher, 1994; López-Fando and Bello, 1995; Yeates et al., 1999; Bouwman and Arts, 2000; Fu et al., 2000; Berkelmans et al., 2003; Wang et al., 2004). Berkelmans et al. (2003) observed that the increase in high c-p value nematodes from 12 years of organic management was destroyed by intensive tillage. Organic systems containing annual crops rely on frequent tillage events to reduce weeds. It is unclear if this frequent tillage negates the promotion of nematode and food web biodiversity with organic management.
Post soil disturbance (tillage, synthetic inputs), nematode communities are dominated by fast-growing, r-strategist bacterivores that, over time, transform to a more diverse community including slower-growing higher c-p (2 and 3) value bacterivores and fungivores and, ultimately, even higher c-p (4 and 5) omnivore and carnivore nematodes, once the level of disturbance is minimized or eliminated (Yeates et al., 1999; Fu et al., 2000). In previous studies, application of nematode community and faunal analysis comparing organic and conventional farming systems was done for commercial fields with different crop management histories (Neher, 1999; Neher and Olson, 1999).
Nematode community and faunal analysis has been used to assess soil food web responses to agroecosystem management in temperate, cool winter, and warm summer climate areas (Ekschmitt et al., 2001; Viketoft, 2007; Hánl, 2008). At present, nematode faunal analysis has not been applied to determining the responses to agro-ecosystem management in a temperate continental climate with short, very warm summers and long, very cold winters such as the Red River Valley of central North America at the eastern edge of the Canadian Prairies. The Red River Valley is a 11,500,000 ha region drained by the Red River (a.k.a. the Red River of the North) in North Dakota and Minnesota to Manitoba with clay soil.
Prairies are rich in nematode diversity (Todd et al., 2006) and posses the ability to provide ecosystem services such as pest regulation, prevention of nutrient losses and green house gas emissions compared to agricultural systems (Buyanovsky et al., 1987; Culman et al., 2010; Glover et al., 2010). Studies done in the past lacked an undisturbed control, such as tall grass prairie, established in close proximity as part of the same experimental setup for comparisons with crop managements systems. Moreover, there has been a lack of characterization of nematode communities following the reversion of cropland to restored prairie, especially in a northern, cold continental climate. It is uncertain if upon conversion of cultivated land to undisturbed grasslands, it can regain richness. The main objectives of this study were to determine soil nematode community responses to long-term crop management and prairie restoration using a replicated field plot design.
Materials and Methods
Study site description and treatments: This research was conducted at the Glenlea Long-Term Crop Rotation and Management Study site established in 1992, it is Canada’s oldest organic-conventional management comparison study. The site is located at the University of Manitoba, Glenlea Research Station, 20 km south of Winnipeg, MB (N 49, 39, 0/W 97, 7, 0). The soil is a Typic Hapludert of Red River or Scantenbury series (Michalyna, 1970). The texture of the soil is clay (9% sand, 26% silt, and 66% clay) with a pH of 7.4 and an organic matter concentration of 77g/kg. The climate for the site is temperate humid continental, having cold winters and warm summers. According to the climate normal database for the weather station at the Glenlea Research Station (Environment Canada 2011), January is the month averaging the lowest mean temperature (-18.5 °C) and July the warmest month (19.3 °C). Total sunshine in July averages 304 hours. Total precipitation averages 532 cm with 432 occurring as rain, and 100 as snow. The frost free period averages 122 days, beginning the last week of May and ending last week of September.
The experimental design of the study was a randomized complete block in a split plot arrangement with three replicates for crop rotation and management treatments. A treatment of restored native prairie (Restored Prairie) was also included in each replicate block. The main plot effect was the three crop rotation treatments as well as the restored prairie treatment. Crop rotations were spring wheat (Triticum aestivum L.)-pea (Pisum sativum L.)-spring wheat-flax (Linum usitatissimum L.) (WPWF), spring wheat-green manure (Fababean (Vicia faba L.))-spring wheat-flax (WGmWF) and spring wheat-alfalfa (Medicago sativa L.)-alfalfa-flax (WAAF). The split plot effect was crop management (conventional or organic). Under organic management, no fertilizers or pesticides were applied. Under conventional management, NPK fertilizers were applied to soil test recommendations (Manitoba Soil Fertility Guide) based on laboratory analysis of composite soil samples for each treatment and herbicides were applied at label rates based on economic thresholds. The size of each subplot was 4 by 25m and that for restored prairie being 25 by 25m. Flax and wheat were grown in 2003 and 2004, respectively.
The restored prairie was planted in 1992 to the native grasses Andropogon gerardii Vitman var. gerardii, Sorghastrum nutans L., Panicum virgatum L., Agropyron smithii Rydb., Elymus lanceolatus Scribn., and Smith Gould., and Elymus trachycaulus Link Gould ex Shinners, with the plots having been undisturbed and unharvested except for controlled burns in 1998 and 2002 to rejuvenate the vegetation.
Soil sampling: Soil samples from each plot were collected on three occasions, June 2003, and July and August, 2004. Fifteen soil samples were taken in a “W” pattern across each plot to a depth of 15 cm using a tube sampler (2.5 cm-diameter), with samples taken between the rows of plants and within the row of plants for the crop rotation treatments. The soil collected for a plot was mixed together to provide one sample for analysis. All samples were placed in sealed polyethylene bags, and placed in an ice chest immediately upon sampling. The soil samples were transported to the laboratory and stored at 4°C until processing. Nematodes were extracted and identified within a week of soil sample collection.
Nematode analysis: Nematode extraction, identification and enumeration were done as described by Forge and Tenuta (2008). Briefly, nematodes were extracted from 100 g soil using the Cobb sieving sugar/flotation method (Ingham, 1994) and numbers reported here per 100 g dry soil weight. The total number of nematodes in each sample was determined using a stereo microscope at 50-x magnification and the first 100 individuals were identified to genus/family level using a compound microscope at 100-400 x magnification using the taxonomic keys provided by Bongers (1994).
Soil food web analysis: All identified nematode taxa were assigned to a trophic group: bacterial- and fungal-feeder, omnivore, carnivore, root-associates and obligate plant-parasites (Yeates et al., 1993). Nematode genera were also assigned a colonizer-persister value (c-p value) (Bongers, 1990; Bongers and Bongers, 1998) and a functional guild (Ferris et al., 2001). Maturity index (MI) for free-living nematodes (all nematodes except plant-parasitic nematodes) was calculated from c-p values and abundance of the nematode taxa in each sample using the formula MI = Σνvixfi, where νvi is the c-p value of taxon i, fi is the frequency of taxon i in a sample (Bongers, 1990; 1999). Enrichment (EI) and structure (SI) indices were calculated according to Ferris et al. (2001). The two indices allow quantitative measure of the nematode community structure and the probable condition of the soil food web. Basal components (b) of the food web (bacterial- and fungal-feeders in the c-p 2 guild) calculated as b = ∑kbnb where kb is the weighted constant for the guild (Ferris et al., 2001), and n is the number of nematodes in that guild. Enrichment (e) and structure (s) components were similarly calculated, using nematode guilds indicative of enrichment (bacterial-feeders of c-p 1, and fungal-feeders of c-p 2), and guilds supporting structure (bacterial-feeders of c-p 3-5, fungal-feeders of c-p3-5, omnivores of c-p 3-5, and carnivores of c-p 3-5). The EI was calculated as 100xe/(e + b), and the SI as 100x s/(s + b). Channel Index (CI), which provides an index of nature of decomposition, was calculated as 100 x (0.8 fungivores c-p 2 / (3.2 bacterivores c-p 1 + 0.8 fungivores c-p 2) where the coefficients are the ke enrichment weightings for the respective guilds (Ferris et al., 2001). Shannon diversity (H’), was calculated for nematode diversity, using the following formulae: Shannon-Weiner Index H’ = ∑Pi (ln Pi), where Pi is the proportion of genera ni in the nematode community n (Pielou, 1977).
Statistical analysis: Repeated measures analysis of variance (PROC GLM SAS Ver. 9.00, SAS Institute, Cary, NC) was performed on nematode taxa, trophic group abundance and food web indices using a split plot experimental design with rotation as the main, management as the split effect and sample occasion as source of the repeated measures. Mean comparison tests were performed to compare rotations and sample occasions only if the ANOVA results were significant at P ≤ 0.1. Repeated measure ANOVA was also performed for comparing organic management across all rotations to the restored prairie treatment. To meet the requirements of normality (Ryan-Joiner test) and homogeneity of variance (Levene’s test) nematode taxa and trophic group abundance was transformed as ln (x+1) prior to analysis.
Results
Response to crop management and rotation: A total of 35 nematode taxa as 33 genera and 2 families were identified from the soil samples over the study (Table 1). The most abundant taxa in the cropped treatments across sample days (6% or greater of average abundance of all taxa) were Filenchus (13%), Eucephalobus (11%), Aphelenchus and Tylenchus (10%), Rhabditidae, Aphelenchoides, and Plectus sp. 1 (8%), Cephalobus and Pangrolaimus (6%). Abundance of Rhabditidae and Prismatolaimus was higher (P < 0.10) in the conventional while Tylencholaimus, Dorylaimidae and Xiphinema were lower than in the organic management treatment (Table 1). Aphelenchus was favored the most (P < 0.10) by wheat-green manure-wheat-flax, Psilenchus and Eudorylaimus by the wheat-pea-wheat-flax and Chiloplacus by the wheat-alfalfa-alfalfa-flax rotation (data not presented).
Table 1.
Mean abundancea (±1SE) of nematode individuals (per 100 g dry soil) for three sample occasions for the crop managements and restored prairie treatment. Values for conventional and organic managements are for across the three crop rotations. See text for guild descriptions.
Across cropped treatments and sample days, the relative abundance of trophic groups in decreasing order was bacterivores (40%), root associates (30%), fungivores (20%), and omnivores plus carnivores (5%). The relative abundance of obligate plant-feeders was 1% with organic management while absent in conventional management.
The abundance of trophic groups varied most with sample day than management and rotation (Table 2). Abundance of four of the six trophic groups varied significantly with sample day (P < 0.05). However, there was no apparent trend in the abundance on sample days for the four trophic groups. Bacterivores in c-p 1 class were marginally favored (P = 0.06) with conventional management (Table 2). Likewise, there was a weak (P = 0.07) difference in abundance of fungivores with the wheat-green manure-wheat-flax being higher than the wheat-pea-wheat-flax rotation (Table 2).
Table 2.
Nematode trophic group abundance (± SE) for conventional and organic management and three crop rotations, and the restored prairie treatment. See text for rotation description.
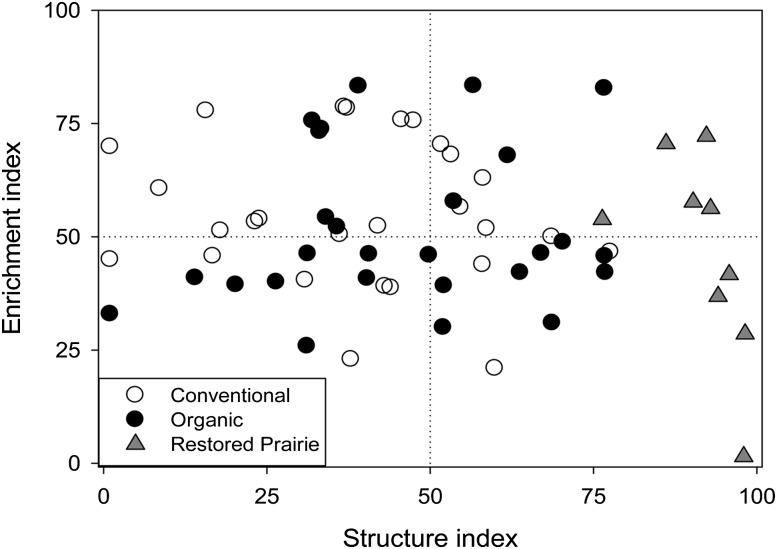
The soil food web indices including EI and SI varied most with sample day (P = 0.001) but also with management (P = 0.01) (Table 3). CI varied only with sample days but not with management (P > 0.1). No differences were observed for nematode diversity among the management scenarios. The EI was highest on June 2003 which corresponded to the lowest MI of the sample days. The SI was highest on July 2004 and lowest on Aug 2004. CI was highest in August 2004 and lowest in June 2003. The MI and SI were significantly higher in the organic while EI was lower than the conventional management. Plotting EI versus SI revealed conventional management exhibiting majority of data points in the range of enriched-highly enriched but low structured, whereas majority of the data points for the organic management scored low enriched-moderately enriched and low structured-structured soil food web.
Table 3.
Nematode community indices (MI: maturity; EI: enrichment; SI: structure; CI: channel and H’: Shannon diversity index) for conventional and organic management and, three crop rotations and a restored prairie. See text for rotation description.
Response to prairie restoration: Fewer taxa were obtained in the soil samples collected from restored prairie with a total of 26, of which 23 were genera and 2 were families over the study (Table 1). Bacterivore genera including Panagrobelus, Acrobeloides, Cervidellus and Chiloplacus and Alaimus were not observed in the restored prairie treatment. Mononchus, Paratylenchus and Xiphinema of low abundance with organic management were also not present in the restored prairie. Further, the restored prairie was dominated by fewer taxa. The highest relative abundance of taxa across sample days was Tylencholaimus (37%), Filenchus (25%), and Tylenchus (12%). The other taxa had relative abundance no greater than 3%. Rhabditidae, Panagrolaimus, Cephalobus, Eucephalobus, Plectus sp. 1, Aphelenchoides and Aphelenchus were lower (P < 0.10) in the restored prairie while Tylencholaimus, Mesodorylaimus and Filenchus were higher in restored prairie than with organic management. Hemicycliophora was observed in the restored prairie and not in organic management (Table 1).
Across sample occasions, the relative abundance of trophic groups in decreasing order was root-associates (44%), fungivores (34%), bacterivores (11%), omnivores plus carnivores (8%), and obligate plant-feeders (4%) of the total nematode abundance.
Sample occasion effect was significant (P < 0.05) for bacterivores and fungivores but not for other trophic groups (Table 2). Abundance of bacterivores in c-p 1 class (P = 0.007) and other bacterivores c-p2-4 was lower (P < 0.006) in the restored prairie while fungivores (P = 0.01), carnivore plus omnivore (P = 0.03) and root associates (P = 0.04) were higher than in the organic management treatment (Table 2). No differences (P > 0.1) were observed for the obligate plant-feeders.
The restored prairie had higher MI (P = 0.001) and SI (P= 0.001), while EI was marginally lower (P = 0.09) and H’ was significantly lower (P = 0.001) than the organic management (Table 3). Sample occasion x management interaction was weakly significant (P = 0.07) for MI but not EI, SI, CI and H’. Plotting of EI versus SI revealed low-moderately enriched but highly structured soil food web (Figure 1).
Fig.1.
Comparison of nematode food web enrichment and structure conditions in conventional and organic managements, and restored prairie treatment. Data points represent enrichment and structure index scores for all three sampling times.
Discussion
The nematode community in the restored prairie treatment had fewer genera than the adjacent cropping system. This contrasts with other studies where high nematode diversity has been reported from prairie landscapes (Todd, 1996; DuPont et al., 2010; Cullman et al., 2010). General trend of nematode community in terms of diversity and trophic group abundance in the cropping systems at the study site were similar to those of other agricultural systems and regions (Freckman and Ettema, 1993; Neher and Campbell, 1994; Neher, 1999) ). Nematode abundance varied over the sampling time across the management and rotation treatments. However, no apparent trend of nematode community over the sample occasions precludes establishing any cause and effect relationship for sample time.
Prairie restoration: The nematode assemblages of native prairie lands are characterized by low abundance of bacterivores and correlate with low nutrient enrichment (Todd, 1996; Todd et al., 1999; Todd et al., 2006). Our study results showed an agreement with this pattern. Both total abundance and the relative abundance of bacterivores in the restored prairie treatment were lower compared to cropping management treatment. The relative abundance of bacterivores was only 11% as opposed to 40% in the cropping management. In particular c-p1 enrichment opportunists contributed little to the total abundance of bacterivores in the restored prairie treatment. Correspondingly nitrogen concentration assessed in the restored prairie soil samples was also lower than the cropping management treatments (Welsh et al., 2009). This may be an indication of the system recovery after restoration of an agricultural land to native prairie tall grasses.
Restored prairie treatment favored root associates (Tylenchidae) the most and these constituted a greater proportion of the total than all other trophic groups. Although root associated nematodes are common with prairie grasses (Todd, 1996; Todd et al., 1999), their feeding habits probably vary; as an aggregate they are considered either root hair or fungal feeders (Yeates et al., 1993; Okada et al., 2002). Perennial prairie grasses have deeper roots with more extensive root hairs than the crop plants (Glover et al., 2010), which may have supported higher numbers of root associates by providing more feeding sites. Likewise fungivore/s in this group may have responded to the availability of greater food resources as evident from the higher percent of arbuscular mycorrhizae fungi observed on the prairie grass root system at the same study site (Welsh, 2007). We speculate that the combination of factors favored these nematodes in the restored prairie. Root hair feeder/s belonging to this group are not expected to cause measurable damage to the plants, rather have been reported to increase C and N mineralization, microbial activity and plant growth (Bardgett et al., 1999). As a fungal feeder/s it would result in lending more weight to the inference of slow fungal-dominated decomposition channels. Either way, high occurrence of root associates suggests them as an ecologically significant nematode group in regulating the restored prairie soil food web in this cold continental climatic condition.
Greater c-p value nematodes are generally associated with low stress and undisturbed environments (Bongers, 1999; Ferris et al., 2001). Higher buildup of greater c-p (3-5) value nematodes in the restored prairie treatment resulted in higher SI and MI values suggesting more structured food webs than those supported by the cropping system. Both lack of synthetic applications and physical disturbances in the restored prairie treatment likely attributed to the greater buildup of higher c-p value nematodes.
The EI is a measure of the abundance of enrichment opportunists relative to the abundance of basal taxa. It quantifies the level of resource enrichment and serves as an indicator of soil productivity (Ferris et al., 2001). A typical undisturbed natural system like prairie grasslands would have a soil food web exhibiting low EI while agricultural systems possess high EI values (Ferris et al., 2001). Range of EI values indicated low-moderately enriched soil food web in the Restored Prairie treatment (Figure 1). Higher EI values than expected in some samples only resulted due to correspondingly low abundance of basal taxa, though the c-p 1 bacterivores (enrichment indicators) were low in numbers. In addition, higher abundance of fungivores observed in the restored prairie soil sample implies fungal decomposition and less bacterial decomposition pathways. Considered together, the restored prairie treatment exhibited a soil food web with low enriched conditions and greater contribution of fungal-decomposition pathways. Slow decomposition posses potential benefits of nutrient conservation by preventing excessive N leaching and losses to denitrification (Glover et. al., 2010). Nematode community assessment however, provides only putative evidence of the benefits of converting crop land to tall grasses, further work will be required for quantification of nutrient losses from the site.
Prairie landscapes are considered not only rich in nematode diversity but also comprised of unique nematode genera rarely observed in agricultural systems (Todd et al., 2006; Culman et al., 2010). No distinct genera (except for Hemicycliophora) were observed, but rather a rapid decline in the diversity was noted with a major influence on several bacterivores and obligate plant-feeders. Secondly, there was a rapid shift in the relative abundance of several taxa. For example Rhabditidae, Panagrolaimus, Cephalobus, Eucephalobus, Plectus sp. 1, Aphelenchoides and Aphelenchus were in low abundance, while Tylencholaimus, Mesodorylaimus and Filenchus were very high in abundance. Even though there was no external application of mineral fertilizers, shifts in major soil nutrients were observed in the restored prairie (Welsh et al., 2009). Soil phosphorus levels were unusually higher while N was lower than the adjacent cropping system. It appears that a switch in above ground vegetation from crop plants to tall grasses and/or changes in nutrient dynamics influenced the below ground nematode community. Responses of below ground communities to shifts in land use related to plant cover and soil properties has been discussed in other studies (Yeates 1991; Yeates and Bongers, 1999; Cadet et al., 2003). Further, it was likely that in our climatic condition the time lapse since restoration was not enough to attain higher diversity, possibly due to low migration of taxa from other native prairie locations to this restored fragmented prairie site, surrounded mainly by croplands (Yeates, 1991). Low nematode diversity, even after more than a decade of converting agricultural land to native prairie grassland, highlights the importance of native prairies especially when such landscapes have significantly shrunk in North America due to anthropogenic activities (Wolters et al., 2000; Todd et al., 2006).
Crop management effects: Abundance of c-p 1 bacterivores was marginally higher in the conventional than the organic management while no differences were seen for the abundance of other trophic groups. C-p 1 bacterivore nematodes are more opportunistic in response than other bacterivores to resource enrichment (Ferris et al., 2004). Higher number of c-p 1 bacterivores also translated to marginally higher EI values suggesting a soil food web to be slightly more bacterial dominated than the organic management. Application of external organic matter inputs such as manures and crop residues increases energy availability and leads to increase of c-p 1 bacterivores abundance in particular (Alon and Steinberger, 1999; Bulluck and Ristaino, 2002; Ferris et al., 2004). In the present study, crop managements did not differ in terms of application of external inputs of organic matter. Therefore, increase in opportunistic nematode taxa in the organic management was not expected. In contrast, synthetic fertilizer and herbicide applications in conventional management plots appeared to have enhanced the decomposition process and resulted in marginally higher abundance of c-p 1 bacterivores under this cold continental climate system.
Total abundance of omnivores plus carnivores did not differ among the managements, probably due to soil disturbances in both systems (Freckman and Ettema, 1993; Fiscus and Neher, 2002). However, higher numbers of c-p 4 fungivore (Tylencholaimus) and correspondingly lower abundance of basal taxa (c-p 2 value) translated to significantly higher SI and MI values in the organic than the conventional management. Structured systems as inferred from higher MI and SI values are expected to demonstrate top-down regulation and reduction in plant-parasitic nematode pressure (Sánchez-Moreno and Ferris, 2007). Lack of differences for omnivores plus carnivores and very low build up of obligate plant-feeders in both managements revealed no synchrony among predators and prey in our study results.
Although nematode classifications into trophic groups masked the differences, individual nematode genus/family comparisons revealed some differences among the managements. Conventional plots underwent both chemical (synthetic inputs) and mechanical disturbance (mainly tillage) while organic management had only mechanical disturbance. Some nematode genera are sensitive to chemical inputs but tolerant to mechanical disturbances while others could be sensitive or tolerant to both (Fiscus and Neher, 2002). Differences in disturbances among the managements would explain why selective nematode genera were favored by one management over the other in the present study.
Crop rotations did not affect nematode community except for fungivores and CI values being marginally higher in the rotation wheat-green manure-wheat-flax, than the other two rotations. Although contribution of bacterivores to nutrient mineralization is estimated to be higher than the fungivores (Ferris et al., 1996; Chen and Ferris, 1999; Okada and Ferris, 2001), simultaneously higher abundance of fungivores indicate slow decomposition with protection from nutrient leaching and availability for the next crop in rotation (Ferris et al., 2004). Similar benefit can be expected from having faba beans as a green manure crop in the rotation.
Of the factors examined, sample occasion resulted in the greatest variation in nematode community composition. Determination of the drivers of the variation caused by sample occasion was not possible with the undertaken three samplings. Future studies of management effects on nematode community composition should contain more sample occasions. Despite just three sample occasions, several responses in nematode community composition were observed with prairie restoration, crop management and rotation. The restored prairie soil food web was highly structured, mature and low-to-moderately enriched as indicated by structure (SI), maturity (MI) and enrichment (EI) index values, respectively. Both, high population of fungivores and low numbers of enrichment opportunist bacterivores suggested slow fungal-dominated decomposition channels at the restored prairie site with potential benefits of preventing nutrients losses. Despite being restored with tall grasses for about ten years, the restored prairie treatment lacks undisturbed prairie characteristics of even greater nematode diversity and species richness. A longer time span may be required to attain higher levels of nematode diversity for this restored fragmented prairie site surrounded by croplands. No consistent differences were observed between organic and conventional for the abundance of nematode trophic groups except for enrichment opportunist c-p 1 bacterivores marginally favored by the conventional management. Although EI was lower and SI was higher for organic than conventional management, their absolute values suggested decomposition channels to be primarily bacterial and relatively fewer trophic links with both management regimes. A high abundance of fungivores in the rotation including the green manure crop indicates greater fungal decomposition than the other two rotations.
Acknowledgments
We wish to acknowledge the technical assistance of Keith Bamford, responsible for management of the Glenlea Long Term Crop Rotation study. The funding for this project was provided by the Natural Sciences and Engineering Research Council of Canada and the Covering New Ground and Agricultural Sustainability Initiative programs of Manitoba Agriculture, Food and Rural Initiatives.
Literature Cited
- Alon A, Steinberger Y. Effect of nitrogen amendments on microbial biomass, above-ground biomass and nematode population in the Negev Desert soil. Journal of Arid Environments. 1999;41:429–441. [Google Scholar]
- Bardgett RD, Cook R, Yeates GW, Denton CS. The influence of nematodes on below-ground processes in grassland ecosystems. Plant and Soil. 1999;212:23–33. [Google Scholar]
- Berkelmans R, Ferris H, Tenuta M, Bruggen AHC van. Effects of long-term crop management on nematode trophic levels other than plant feeders disappear after 1 year of disruptive soil management. Applied Soil Ecology. 2003;23:223–235. [Google Scholar]
- Bongers T. The maturity index: an ecological measure of environmental disturbance based on nematode species composition. Oecologica. 1990;83:14–19. doi: 10.1007/BF00324627. [DOI] [PubMed] [Google Scholar]
- Bongers T. 1994. De Nematoden van Nederland. Utrecht, Netherlands: Koninklijke Nederlandse Natuurhistorische Vereniging. [Google Scholar]
- Bongers T. The maturity index, the evolution of nematode life-history traits, adaptive radiation and cp-scaling. Plant and Soil. 1999;212:13–22. [Google Scholar]
- Bongers T, Bongers M. Functional diversity of nematodes. Applied Soil Ecology. 1998;10:239–251. [Google Scholar]
- Bongers T, Ferris H. Nematode community structure as a bioindicator in environmental monitoring. Trends Ecology and Evolution. 1999;14:224–228. doi: 10.1016/s0169-5347(98)01583-3. [DOI] [PubMed] [Google Scholar]
- Bouwman LA, Arts WBM. Effects of soil compaction on the relationships between nematodes, grass production and soil physical properties. Applied Soil Ecology. 2000;14:213–222. [Google Scholar]
- Briar SS, Grewal PS, Somasekhar N, Stinner D, Miller SA. Soil nematode community, organic matter, microbial biomass and nitrogen dynamics in field plots transitioning from conventional to organic management. Applied Soil Ecology. 2007;37:256–266. [Google Scholar]
- Bulluck LR, III, Barker KR, Ristaino JB. Influences of organic and synthetic soil fertility amendments on nematode trophic groups and community dynamics under tomatoes. Applied Soil Ecology. 2002;21:233–250. [Google Scholar]
- Bulluck LR, III, Ristaino JB. Effect of synthetic and organic soil fertility amendments on southern blight, soil microbial communities and yield of processing tomatoes. Phytopathology. 2002;92:181–189. doi: 10.1094/PHYTO.2002.92.2.181. [DOI] [PubMed] [Google Scholar]
- Buyanovsky GA, Kucera CL, Wagner GH. Comparative analyses of carbon dynamics in native and cultivated ecosystems. Ecology. 1987;68:2023–2031. doi: 10.2307/1939893. [DOI] [PubMed] [Google Scholar]
- Cadet P, Pate E, N′Diaye-Faye N. Nematode community changes and survival rates under natural fallow in the sudano-sahelian area of Senegal. Pedobiologia. 2003;47:149–160. [Google Scholar]
- Chen J, Ferris H. The effects of nematode grazing on nitrogen mineralization during fungal decomposition of organic matter. Soil Biology and Biochemistry. 1999;31:1265–1279. [Google Scholar]
- Clark MS, Horwarth WR, Shennan C, Scow KM, Lanini WT, Ferris H. Nitrogen, weeds and water as yield-limiting factors in conventional, low-input, and organic tomato systems. Agriculture Ecosystems and Environment. 1999;73:257–270. [Google Scholar]
- Culman SW, DuPont ST, Glover JD, Buckley DH, Fick GW, Ferris H, Crews TE. Long-term impacts of high-input annual cropping and unfertilized perennial grass production on soil properties and belowground food webs in Kansas, USA. Agriculture Ecosystems and Environment. 2010;137:13–24. [Google Scholar]
- DuPont ST, Culman SW, Ferris H, Buckley DH, Glover JD. No-tillage conversion of harvested perennial grassland to annual cropland reduces root biomass, decreases active carbon stocks, and impacts soil biota. Agriculture Ecosystems and Environment. 2010;137:25–32. [Google Scholar]
- Ekschmitt K, Bakonyi G, Bongers M, Bongers T, Boström S, Dogan H, Harrison A, Nagy P, O'Donnell AG, Papatheodorou EM, Sohlenius B, Stamou GP, Wolters V. Nematodes community structure as indicator of soil functioning in European grassland soils. European Journal of Soil Biology. 2001;212:163–268. [Google Scholar]
- Environment Canada. 2011. Canadian Climate Normals 1971-2000. Glenlea Manitoba Weather Station. http://climate.weatheroffice.gc.ca/climate_normals/index_e.html (last accessed June 19, 2011).
- Ferris H, Bongers T. Nematode indicators of organic enrichment. Journal of Nematology. 2006;38:3–12. [PMC free article] [PubMed] [Google Scholar]
- Ferris H, Bongers T, De Goede RGM. Nematode faunal indicators of soil food web condition. Journal of Nematology. 1999;31:534–535. [Google Scholar]
- Ferris H, Bongers T, De Geode RGM. A framework for soil food web diagnostics: extension of the nematode faunal analysis concept. Applied Soil Ecology. 2001;18:13–29. [Google Scholar]
- Ferris H, Venette RC, Lau SS. Dynamics of nematode communities in tomatoes grown in conventional and organic farming systems and their impact on soil fertility. Applied Soil Ecology. 1996;3:161–175. [Google Scholar]
- Ferris H, Venette RC, Scow KM. Soil management to enhance bacterivore and fungivore nematode populations and their nitrogen mineralization function. Applied Soil Ecology. 2004;24:19–35. [Google Scholar]
- Fiscus DA, Neher DA. Distinguishing sensitivity of free-leaving soil nematode genera to physical and chemical disturbances. Ecological Applications. 2002;12:565–575. [Google Scholar]
- Forge T, Tenuta M. 2008. Indicators of soil food web properties. Pp. 577–588 in M. R. Carter and E. D. Gregorich, ed. Soil Sampling and Methods of Analysis (2nd ed.), Boca Raton: Canadian Society of Soil Science, CRC Press. [Google Scholar]
- Freckman DW. Bacterivorous nematodes and organic matter decomposition. Agriculture Ecosystems and Environment. 1988;24:195–217. [Google Scholar]
- Freckman DW, Ettema CH. Assessing nematode communities in agroecosystems of varying human intervention. Agriculture Ecosystems and Environment. 1993;45:239–261. [Google Scholar]
- Fu S, Coleman DC, Hendrix PF, Crossley DA., Jr Responses of trophic groups of soil nematodes to residue application under conventional tillage and no tillage regimes. Soil Biology and Biochemistry. 2000;32:1731–1741. [Google Scholar]
- Glover JD, Culman SW, DuPont ST, Broussard W, Young L, Mangan ME, Mai JG, Crews TE, DeHaan LR, Buckley DH, Ferris H, Turner RE, Reynold HL, Wyse DL. Harvested perennial grasslands provide ecological benchmarks for agricultural sustainability. Agriculture Ecosystems and Environment. 2010;137:3–12. [Google Scholar]
- Griffiths BS, Ritz K, Wheatley RE. Nematodes as indicators of enhanced microbiological activity in a Scottish organic farming system. Soil Use Management. 1994;10:20–24. [Google Scholar]
- Hánl L. Nematode assemblages indicate soil restoration on colliery spoils afforested by planting different tree species and by natural succession. Applied Soil Ecology. 2008;40:86–99. [Google Scholar]
- Ingham RE. 1994. Nematodes. Pp. 459–490 in R.W. Weaver, S. Angle, P. Bottomley, B. Bezdicek, S. Smith, A. Tabatabai and A. Wollum, ed. Methods of Soil Analysis. Part 2. Microbiological and Biochemical Properties no. 5. Madison: Soil Science Society of America Press. [Google Scholar]
- Ingham RE, Trofymow JA, Ingham ER, Coleman DC. Interactions of bacteria, fungi, and their nematode grazers: Effects on nutrient cycling and plant growth. Ecological Monographs. 1985;55:119–140. [Google Scholar]
- López-Fando C, Bello A. Variability in soil nematode populations due to tillage and crop rotation in semi-arid mediterranean agrosystems. Soil and Tillage Research. 1995;36:59–72. [Google Scholar]
- McSorley R, Frederick JJ. Nematode population fluctuations during decomposition of specific organic amendments. Journal of Nematology. 1999;31:37–44. [PMC free article] [PubMed] [Google Scholar]
- McSorley R, Gallaher RN. Effect of tillage and crop residue management on nematode densities on corn. Suppl. Journal of Nematology. 1994;26:669–674. [PMC free article] [PubMed] [Google Scholar]
- Michalyna W. Report of the detailed soil survey of Glenlea Research Station. Canada-Manitoba Soil Survey. Township 8, Range 3E. 1970 [Google Scholar]
- Neher DA. Nematode communities in organically and conventionally managed agricultural soils. Journal of Nematology. 1999;31:142–154. [PMC free article] [PubMed] [Google Scholar]
- Neher DA, Campbell CL. Nematode communities and microbial biomass in soils with annual and perennial crops. Applied Soil Ecology. 1994;1:17–28. [Google Scholar]
- Neher DA, Olson RK. Nematode communities in soils of four farm cropping management systems. Pedobiologia. 1999;43:430–438. [Google Scholar]
- Okada H, Ferris H. Temperature effects on growth and nitrogen mineralization of fungi and fungal feeding nematodes. Plant and Soil. 2001;234:253–262. [Google Scholar]
- Okada H, Tsukiboshi T, Kadota I. Mycetophagy in Filenchus misellus (Andrassy, 1958) Lownsbery & Lownsbery, 1985 (Nematoda: Tylenchidae), with notes on its morphology. Nematology. 2002;4:795–801. [Google Scholar]
- Parmelee RW, Alston DG. Nematode trophic structure in conventional and no tillage agroecosystems. Journal of Nematology. 1986;18:403–407. [PMC free article] [PubMed] [Google Scholar]
- Pielou EC. 1977. Mathematical Ecology. New York: Wiley. [Google Scholar]
- Porazinska DL, Duncan LW, McSorley R, Graham JH. Nematode communities as indicators of status and processes of a soil ecosystem influenced by agricultural management practices. Applied Soil Ecology. 1999;13:69–86. [Google Scholar]
- Sánchez-Moreno S, Ferris H. Suppressive service of the soil food web: effects of environmental management. Agriculture Ecosystems and Environment. 2007;119:75–87. [Google Scholar]
- Sohlenius B, Wasilewska L. Influence of irrigation and fertilization on the nematode community in a Swedish pine forest soil. Journal of Applied Ecology. 1984;21:327–342. [Google Scholar]
- Sohlenius B, Boström S. Short-term dynamics of nematode communities in arable soil - Influence of nitrogen fertilization in barley crops. Pedobiologia. 1986;29:183–191. [Google Scholar]
- Tenuta M, Ferris H. Sensitivity of nematode life-history groups to ions and osmotic tensions of nitrogenous solutions. Journal of Nematology. 2004;36:85–94. [PMC free article] [PubMed] [Google Scholar]
- Todd TC. Effects of management practices on nematode community structure in tallgrass prairie. Applied Soil Ecology. 1996;3:235–246. [Google Scholar]
- Todd TC, Blair JM, Milliken GA. Effects of altered soil-water availability on a tallgrass prairie nematode community. Applied Soil Ecology. 1999;13:45–55. [Google Scholar]
- Todd TC, Powers TO, Mullin PG. Sentinel Nematodes of land-use change and restoration in tallgrass prairie. Journal of Nematology. 2006;38:20–27. [PMC free article] [PubMed] [Google Scholar]
- Viketoft M. Plant induced spatial distribution of nematodes in a semi-natural grassland. Nematology. 2007;9:131–142. [Google Scholar]
- Wang KH, McSorley R, Marshall AJ, Gallaher RN. Nematode community changes associated with decomposition of Crotalaria juncea amendment in litterbags. Applied Soil Ecology. 2004;27:31–45. [Google Scholar]
- Welsh C. 2007. Organic crop management can decrease labile soil P and promote mycorrhizal association of crops. M.S. dissertation. University of Manitoba, Winnipeg, Manitoba, Canada. [Google Scholar]
- Welsh CM, Tenuta M, Flaten DN, Thiessen-Martens JR, Entz MH. High yielding organic crop management decreases plant available but not recalcitrant soil phosphorus. Agronomy Journal. 2009;101:1027–1035. [Google Scholar]
- Wolters V, Silver WL, Bignell DE, Coleman DC, Lavelle P, Van Der Putten WH, De Ruiter P, Rusek J, Wall DH, Wardle DA, Brussaard L, Dangerfield JM, Brown VK, Giller KE, Hooper DU, Sala O, Tiedje J, Van Veen JA. Effects of global changes on above- and belowground biodiversity in terrestrial ecosystems: Implications for ecosystem functioning. Bioscience. 2000;50:1089–1098. [Google Scholar]
- Yardim EN, Edwards CA. The effects of chemical pest, disease and weed management practices on the trophic structure of nematode populations in tomato agroecosystems. Applied Soil Ecology. 1998;7:137–147. [Google Scholar]
- Yeates GW. Impact of historical changes in land use on the soil fauna. New Zealand Journal of Ecology. 1991;15:99–106. [Google Scholar]
- Yeates GW, Bongers T. Nematode diversity in agroecosystems. Agriculture Ecosystems and Environment. 1999;74:113–135. [Google Scholar]
- Yeates GW, Bongers T, De Goede RGM, Freckman DW, Georgieva SS. Feeding habits in soil nematode families and genera- an outline for soil ecologists. Journal of Nematology. 1993;25:315–331. [PMC free article] [PubMed] [Google Scholar]
- Yeates GW, Wardle DA, Watson RN. Response of soil nematode populations, community structure, diversity and temporal variability to agricultural intensification over a seven-year period. Soil Biology and Biochemistry. 1999;31:1721–1733. [Google Scholar]