Abstract
Linalool is either a toxic compound to a few species of plant parasitic nematodes or attractive to entomopathogenic nematodes. This compound is produced and emitted by several host plants of Globodera rostochiensis and G. pallida, the potato cyst nematodes (PCN). With the aim to reveal the effect of linalool on PCN, laboratory assays were carried out. Survival of PCN second-stage juveniles (J2s) in water + linalool control did not differ; thus, proving linalool to be nontoxic to PCN. Behavioral assays carried out in Petri dishes revealed attractiveness in the form of positive response of J2s of both PCN species towards linalool. Based on these behavioral assays, sensitivity to linalool of G. rostochiensis J2s was higher compared with that of G. pallida J2s. Linalool is the first compound of plant origin to elicit positive response in both PCN species.
Keywords: Chemoattraction; behavior; Globodera rostochiensis; Globodera pallida, linalool; potato cyst nematodes; second-stage juvenile; Solanaceae
Eggs of potato cyst nematodes (PCN), Globodera rostochiensis (Wollenweber) Behrens and G. pallida (Stone) Behrens, can remain unhatched for many years in the soil (Turner, 1996). In the presence of a suitable Solanaceae host plant after overwintering, PCN second-stage juveniles (J2s) emerge from the cysts and search for host plant roots using chemoreception (e.g. Steiner, 1925; Devine and Jones, 2003). Weischer (1959) and Clarke and Hennessy (1984) have demonstrated that potato root diffusates stimulated locomotory activity, movement and migration, of G. rostochiensis J2s. Behavioral assays carried out by Devine and Jones (2003) revealed the presence of chemoattractants, chemostats and chemorepellents for G. rostochiensis and G. pallida juveniles in several potato root diffusate fractions. Identification of bioactive compounds that elicit behavioral responses to PCN J2s could provide us with better understanding of their behavior and might lead to novel control strategies. Solanaceae release a great variety of chemicals (Eich, 2008) into the soil, many of which are common amongst the major host plants of PCN. One of these chemicals is linalool (3,7-dimethyl octa-1,6-dien-3-ol) and it is known to be toxic to some plant parasitic nematodes such as Meloidogyne incognita (Ibrahim et al., 2006), M. arenaria (Walker and Melin, 1996), M. javanica, Anguina tritici, Heterodera cajani, Tylenchulus semipenetrans (Malik et al., 1987; Sangwan et al., 1990) and Bursaphelenchus xylophilus (Kong et al., 2007). On the other hand, linalool is known as attractant to the following entomopathogenic nematode species: Diplogasteroides magnus (Hong et al., 2008), Heterorhabditis megidis (Köllner et al., 2008), and Pristionchus maupasi (Hong and Sommer, 2006). Thus, the role of this compound in host plant-PCN interaction remains unknown. Our aim was to reveal the effect of linalool on J2s of both PCN species.
Materials and Methods
Potato cyst nematodes: Globodera rostochiensis Ro1 (Ecosse population, Germany) and G. pallida Pa2 (Kalle population, Germany) were supplied by Julius Kühn Institute (Quedlinburg, Germany). Cysts were placed on a filter paper moistened with distilled water (dW) and kept at 21°C for seven days and then the cysts were gently crushed to expose hatched J2/unhatched eggs that were washed by dW to a Petri dish with its bottom covered by a layer of 1.5 % agar (Carl Roth, Karlsruhe, Germany). In a few hours the water was absorbed and actively moving J2s were used for testing.
Potato root diffusate: Potato root diffusate (PRD) was prepared according to Pridannikov et al. (2007), except filtering of PRD. Three Désirée variety potato tubers were planted in 400 ml pots filled with autoclaved sand and were maintained under 60% relative humidity, 14:10 hr light:dark period and 22°C in a greenhouse. After three weeks the potato tubers were pulled out and the intact roots were rinsed with dW to remove sand particles. Intact roots were then immersed into 50 ml dW for 24 hr at 22°C and the obtained PRD was kept at 4°C.
Linalool: Linalool (Sigma-Aldrich, Seelze, Germany) was racemic with purity 97%. Due to its limited solubility in water, an aqueous linalool emulsion of 0.56 × 10-1 M concentration was made. A dilution series to 0.56 × 10-6 M was prepared; the pH differed from 6.57 to 6.82, depending on the concentration. To calculate the content of linalool in PRD, headspace solid-phase microextraction (polydimethylsiloxane fiber - Supelco, Bellefonte, USA) (Pawliszyn, 1997) and gas chromatography with flame ionization detector (Clarus 500, Perkin Elmer, Shelton, USA), Stabilwax column (Restek, Bellefonte, USA), and external standard method (Rouessac and Rouessac, 2007) were used.
Toxicity test: Fifty G. rostochiensis and G. pallida J2s were placed in separate watch glasses containing 2 μl of dW. Two milliliters of 0.56 × 10-3 M linalool solution or dW (control) was added. The watch glasses were placed inside Petri dishes and kept at room temperature in the dark. To minimize evaporation, both linalool solution and control were replaced every three days. Mortality of J2s was recorded daily for 12 days. The nematodes were defined as dead if their body was straight or did not move after mechanical prodding while observed under a binocular microscope (Kong et al., 2006). Mortality percentages were counted from four replicates.
Behavioral tests: Behavioral tests for both G. rostochiensis and G. pallida were carried out following Rühm et al. (2003). Petri dishes, 32-mm-diam., with their bottom coated by 1-2-mm thick film of 1.5 % agar (Carl Roth, Karlsruhe, Germany) were used. A filter paper disk (Schleicher & Schuell MicroScience, Niedersachsen, Germany) 5-mm-diam. was placed in the center of each dish. One microliter either of linalool, dW, or 7 μl of PRD were spread on the filter paper. In order to increase the number of attracted J2s, another 7 μl of PRD were spread on a filter after 1 hr. After 2 hr, the paper was removed and four J2s were placed at equal distances from each other on a circular line (marked on the outer surface), which divided dish area into two equal parts; and the nematode position was recorded after 1 hr. For nematodes located in the inner area of the behavioral arena, attractiveness to the stimulus was considered and counted as a positive response, and for those found in the outer area as a negative response. Potato root diffusate and dW were used as controls. Four nematodes in 5 replicates were tested, the test was repeated 6 times (120 nematodes in total) to evaluate the effect of each linalool concentration, PRD and dW. Data was analyzed by χ2-test using software Statistica 6.0 (StatSoft, Tulsa, USA). Behavioral response of PCN J2s to dW was considered and counted as ‘no attraction‘.
Results and Discussion
Toxicity: In water, G. rostochiensis and G. pallida J2s survived for 12 days, i.e. during the period of observation with survival up to 99%. In water + linalool (linalool solution equal to 0.56 ×10-3 M) during the same period, survival of G. rostochiensis J2s was 95 ± 2.1%, and that of G. pallida J2s was 97 ± 1.7%. No statistically significant difference in J2 survival in water + linalool compared with the water control was recorded. The high concentration of linalool solution had no toxic effect to J2s, so lower concentrations were not tested. Therefore, we hypothesize that in case host plants would emit this compound in high concentrations, the compound would not be hazardous. Based on this hypothesis, we assume linalool is unlikely to be a Solanaceae plant defensive (toxic) compound against PCN.
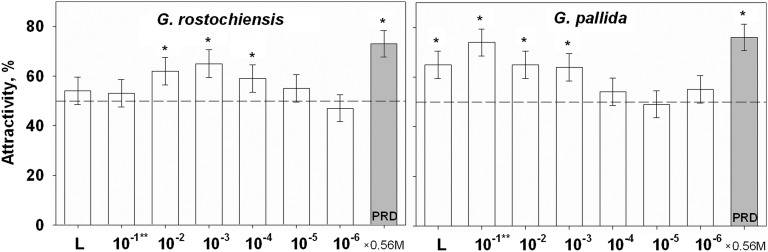
Behavior: In our study, linalool was not repellent to J2s; in fact, this compound was attractive to both PCN species (Fig. 1). Our findings contradict earlier reports on the toxic properties of linalool to some plant parasitic nematodes (Malik et al., 1987; Sangwan et al., 1990; Walker and Melin, 1996; Ibrahim et al., 2006; Kong et al., 2007). However, some interspecific behavioral differences were noted in response to linalool. The lowest concentration of linalool attractive to G. rostochiensis J2s was 0.56 × 10-4 M; J2s exhibited similar response to PRD (P > 0.05). However, a higher concentration of linalool, 0.56 × 10-3 M, was needed to evoke a positive response to G. pallida J2s. Thus, sensitivity to linalool by G. rostochiensis J2s was higher compared with that of G. pallida J2s. The range of linalool concentrations eliciting a positive response was differed as well. For example, G. rostochiensis J2s responded to linalool concentrations ranging from 10-2 to 10-4 M, while G. pallida J2s responded from undiluted linalool to 0.56 × 10-3 M concentration. In addition, high concentrations of linalool (undiluted and emulsion 0.56 × 10-1 M) did not elicit a positive response to G. rostochiensis J2s, as no statistically significant difference was found compared with the water control (P > 0.05). The reduced response at very high concentrations of an allelochemical due to possible saturation of chemoreceptors has been reported previously ( Diez and Dusenbery, 1989; Stamps and Linit, 1998). In G. pallida the same effect, loss of attractiveness at higher concentrations, was absent, due to less sensitivity to the compound compared with sensitivity of G. rostochiensis. The behavioral threshold to linalool was 0.56 × 10-3 M in G pallida and 0.56 × 10-4 M in G. rostochiensis.
Fig. 1.
Chemoattraction in the form of positive response of Globodera rostochiensis and Globodera pallida J2s to different linalool (L, undiluted) concentrations (**emulsion and solutions) (M) and potato root diffusate (PRD). Open column indicates response to linalool; grey column to PRD. Response to water was counted as zero and was detracted from that to linalool and PRD solutions (in water). Columns marked with asterisk and containing no mark differs significantly (χ2-test P < 0.05).
Devine and Jones (2003) demonstrated that potato root diffusate-hatched juveniles of both PCN species were attracted to different fractions of the diffusate, whereas water-hatched J2s were attracted to the same potato root diffusate fractions. Therefore, water-hatched juveniles were more homogenous in their response to potato root diffusate chemicals compared with potato root diffusate-hatched juveniles. Water-hatched juveniles used in our test differed in their sensitivity, i.e. threshold,) to a single compound, thus, interspecific differences even within water-hatched G. rostochiensis J2s and G. pallida J2s were revealed. If behavioral response to linalool differs in potato root diffusate-hatched and water-hatched juveniles remains to be investigated.
The linalool concentration in PRD used in the assay was 9.7 × 10-6 M, i.e. much lower than that attractive in the assay (i.e. 10-3-10-4 M). However, we assume under natural conditions roots could emit higher linalool concentrations compared with those obtained in our PRD, because the PRD-making procedure included removal of sand by washing, thus leading to some loses of chemicals on a root surface including linalool.
Several compounds have been reported to stimulate egg hatching in PCN (e.g. Devine et al., 1996; Byrne et al., 2001; Maher, 2001), as well as few compounds attractive to adult males (Riga et al., 1997). However, no plant origin compounds that elicit a chemoresponse to PCN juveniles have been identified, yet. Our tests revealed that linalool released by Solanaceae roots does not function as a defensive compound to PCN J2s but it may function as a kairomone enabling the nematodes to detect plants and may serve as a nonspecific attractant. Several fractions of potato root diffusate contain compounds that elicit chemoattraction to PCN J2s (Devine and Jones, 2003). Thus, many other compounds in addition to linalool could be involved in nematode attraction to Solanaceae roots, and increasing response to host specifity. Linalool is the first compound present in host plants that elicits chemoattraction to juveniles of both PCN species.
Literature Cited
- Byrne JT, Maher NJ, Jones PW. Comparative responses of Globodera rostochiensis and G. pallida to hatching chemicals. Journal of Nematology. 2001;33:195–202. [PMC free article] [PubMed] [Google Scholar]
- Clarke AJ, Hennessy J. Movement of Globodera rostochiensis (Woll.) juveniles stimulated by potato root exudate. Nematologica. 1984;30:206–212. [Google Scholar]
- Devine KJ, Jones PW. Investigations into the chemoattraction of the potato cyst nematodes Globodera rostochiensis and G. pallida towards fractionated potato root leachate. Nematology. 2003;5:65–75. [Google Scholar]
- Devine KJ, Byrne JT, Maher NJ, Jones PW. Resolution of natural hatching factors for the golden potato cyst nematode, Globodera rostochiensis. Annals of Applied Biology. 1996;129:323–334. [Google Scholar]
- Diez JA, Dusenbery DB. Repellent of root-knot nematodes from exudate of host roots. Journal of Chemical Ecology. 1989;15:2445–2455. doi: 10.1007/BF01020375. [DOI] [PubMed] [Google Scholar]
- Eich E. 2008. Solanaceae and Convolvulaceae: Secondary metabolites: Biosynthesis, chemotaxonomy, biological and economic significance (a handbook). Berlin: Springer-Verlag:638. [Google Scholar]
- Hong RL, Sommer RJ. Chemoattraction in Pristionchus nematodes and implications for insect recognition. Current Biology. 2006;16:2359–2365. doi: 10.1016/j.cub.2006.10.031. [DOI] [PubMed] [Google Scholar]
- Hong RL, Svatoš A, Herrmann M, Sommer RJ. Species–specific recognition of beetle cues by nematode Pristionchus maupasi. Evolution & Development. 2008;10:273–279. doi: 10.1111/j.1525-142X.2008.00236.x. [DOI] [PubMed] [Google Scholar]
- Ibrahim SK, Traboulsi AF, El-Haj S. Effect of essential oils and plant extracts on hatching, migration and mortality of Meloidogyne incognita. Phytopathologia Mediterranea. 2006;45:238–246. [Google Scholar]
- Köllner TG, Held M, Lenk C, Hiltpold I, Turlings TCJ, Gershenzon J, Degenhardt J. A Maize (E) – β-Caryophyllene synthase implicated in indirect defense responses against herbivores is not expressed in most American maize varieties. The Plant Cell. 2008;20:482–492. doi: 10.1105/tpc.107.051672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J-Ok, O, Lee SM, Moon YS, Lee SG, Ahn YJ. Nematicidal activity of plant essential oils against Bursaphelenchus xylophilus (Nematoda: Aphelenchoididae) Journal of Asia-Pacific Entomology. 2006;9:173–178. [Google Scholar]
- Kong J-Ok, Park Il–K, Choi K-S, Shin S-Ch., Ahn Y-J. Nematicidal and propagation activities of thyme red and white oil compounds toward Bursaphelenchus xylophilus (Nematoda: Parasitaphelenchidae) Journal of Nematology. 2007;39:237–242. [PMC free article] [PubMed] [Google Scholar]
- Maher NJ. 2001. The hatching mechanisms and hatching behavior of Globodera rostochiensis and G. pallida. PhD dissertation, The National University of Ireland-Cork, Republic of Ireland. [Google Scholar]
- Malik MS, Sangwan NK, Dhindsa KS, Verma KK, Bhatti DS. Nematicidal efficacy of some monoterpenes and related derivatives. Pesticides. 1987;21:30–32. [Google Scholar]
- Pawliszyn J. 1997. Solid phase microextraction: Theory and practice. New York: Wiley-VCH:247. [Google Scholar]
- Pridannikov MV, Petelina GG, Palchuk MV, Masler EP, Dzhavakhiya VG. Influence of components of Globodera rostochiensis cysts on the in vitro hatch of second-stage juveniles. Nematology. 2007;9:837–844. [Google Scholar]
- Riga E, Perry RN, Barrett J, Johnston MRL. Electrophysiological responses of males of the potato cyst nematodes, Globodera rostochiensis and G. pallida to some chemicals. Journal of Chemical Ecology. 1997;23:417–428. Rouessac, F., and Rouessac, A. 2007. Chemical analysis: Modern instrumentation methods and techniques, ed. 2. England: John Wiley and Sons:470. [Google Scholar]
- Rühm R, Dietsche E, Harloff H-J, Lieb M, Franke S, Aumann J. Characterization and partial purification of a white mustard kairomone that attracts the beet cyst nematode. Heterodera schachtii. Nematology. 2003;5:17–22. [Google Scholar]
- Sangwan NK, Verma BS, Verma KK, Dhindsa KS. Nematicidal activity of some essential plant oils. Pesticide Science. 1990;28:331–335. [Google Scholar]
- Stamps WT, Linit MJ. Chemotactic response of propogative and dispersal forms of the pine wood nematode Busrsaphelenchus xylophilus to beetle and pine derived compounds. Fundamental and Applied Nematology. 1998;21:243–250. [Google Scholar]
- Steiner G. The problem of host selection and host specialization of certain plant infesting nematodes and its application in the study of nemic pests. Phytopathology. 1925;15:499–534. [Google Scholar]
- Turner SJ. Population decline of potato cyst nematodes (Globodera rostochiensis, G. pallida) in field soils in Northern Ireland. Annals of Applied Biology. 1996;129:315–322. [Google Scholar]
- Walker JT, Melin JB. Mentha x piperita, Mentha spicata and effects of their essential oils on Meloidogyne in soil. Journal of Nematology. 1996;24:629–635. [PMC free article] [PubMed] [Google Scholar]
- Weischer B. Experimentelle Untersuchungen über die Wanderung von Nematoden. Nematologica. 1959;4:172–186. [Google Scholar]