Abstract
The plum curculio, Conotrachelus nenuphar, is a major pest of stone and pome fruit (e.g., apples, pears, peaches, cherries, etc.). Entomopathogenic nematodes (Steinernema spp. and Heterorhabditis spp.) may be used to control the larval stage of C. nenuphar following fruit drop. Indeed, certain entomopathogenic nematodes species have previously been shown to be highly effective in killing C. nenuphar larvae in laboratory and field trials. In field trials conducted in the Southeastern, USA, Steinernema riobrave has thus far been shown to be the most effective species. However, due to lower soil temperatures, other entomopathogenic nematode strains or species may be more appropriate for use against C. nenuphar in the insect’s northern range. Thus, the objective of this study was to conduct a broad screening of entomopathogenic nematodes. Under laboratory conditions, we determined the virulence of 13 nematode strains (comprising nine species) in two different soils (a loam and clay-loam) and three different temperatures (12°C, 18°C, and 25°C). Superior virulence was observed in S. feltiae (SN strain), S. rarum (17 C&E strain), and S. riobrave (355 strain). Promising levels of virulence were also observed in others including H. indica (HOM1 strain), H. bacteriophora (Oswego strain), S. kraussei, and S. carpocapsae (Sal strain). All nematode treatments were affected by temperature with the highest virulence observed at the highest temperature (25°C). In future research, field tests will be used to further narrow down the most suitable nematode species for C. nenuphar control.
Keywords: biological control, Conotrachelus nenuphar, entomopathogenic nematode, Heterorhabditis, plum curculio, Steinernema
The plum curculio, Conotrachelus nenuphar (Herbst), is a major pest of pome and stone fruit in North America (Racette et al., 1992; Horton and Johnson, 2005). Adult weevils enter orchards from overwintering sites in the spring to feed and oviposit in fruit. Attacked fruit aborts or is deformed rendering it non-saleable. Larvae continue to develop in fallen fruit, exit as fourth instars, and burrow into the soil (1 - 8 cm) to pupate (Racette et al., 1992). After emergence, adults feed on fruit and migrate to litter surrounding the orchard to overwinter (Racette et al., 1992; Olthof and Hagley, 1993). In the southern United States, an additional generation may occur on many peach cultivars prior to overwintering (Horton and Johnson, 2005).
Current control recommendations for C. nenuphar consist solely of above-ground applications of chemical insecticides to suppress adults (Horton et al., 2011). Due to environmental and regulatory concerns, research on developing alternative control strategies is warranted. Entomopathogenic nematodes are one of the potential control options (Shapiro-Ilan et al., 2004, 2008).
Entomopathogenic nematodes in the genera Steinernema and Heterorhabditis are obligate parasites of insects (Poinar, 1990). These nematodes have a mutualistic relationship with a bacterium (Xenorhabdus spp. and Photorhabdus spp. for steinernematids and heterorhabditids, respectively) (Poinar, 1990). Infective juveniles (IJs), the only free-living stage, enter hosts through natural openings (mouth, anus, and spiracles), or in some cases, through the cuticle. After entering the host’s hemocoel, nematodes release their symbiotic bacteria, which are primarily responsible for killing the host, defending against secondary invaders, and providing the nematodes with nutrition (Dowds and Peters, 2002). The nematodes molt and complete up to three generations within the host after which IJs exit the cadaver to search out new hosts (Kaya and Gaugler, 1993). Entomopathogenic nematodes are effective at controlling a variety of economically important pests including the larvae of several weevil species (Coleoptera: Curculionidae) (Shapiro-Ilan et al., 2005). Due to the nematode’s sensitivity to desiccation and ultraviolet (UV) radiation (Kaya, 1990) below-ground stages of C. nenuphar are the preferred targets for nematode applications (Shapiro-Ilan et al., 2004, 2008).
Application of certain entomopathogenic nematode species has shown efficacy in laboratory and field trials when targeting the larval stage of C. nenuphar in soil (Tedders et al., 1983; Olthof and Hagley, 1993; Shapiro-Ilan et al., 2002, 2004, 2008). In the laboratory, when six nematode species were compared for virulence to C. nenuphar larvae, Steinernema feltiae (Filipjev) and S. riobrave Cabanillas, Poinar and Raulston were pathogenic whereas Heterorhabditis bacteriophora Poinar, H. marelata Liu and Berry, H. megidis Poinar, Jackson and Klein, and S. carpocapsae (Weiser) were not (Shapiro-Ilan et al., 2002). In subsequent field trials conducted in a peach orchard in Byron, Georgia, S. riobrave exhibited high levels of suppression (control averaged 94% in four trials), whereas S. feltiae was not effective (Shapiro-Ilan et al., 2004). In contrast, Alston et al. (2005) observed suppression of C. nenuphar larvae using S. feltiae in field tests conducted in Utah; albeit the levels of control were low to moderate (< 40% corrected mortality). The ability of S. riobrave to cause high levels of suppression, e.g., > 90%, when targeting C. nenuphar larvae was also confirmed in later studies (Shapiro-Ilan et al., 2008; Pereault et al., 2009).
Our overall goal is to develop an integrated multi-stage management program for C. nenuphar suppression; application of entomopathogenic nematodes for control of soil-dwelling stages is intended to be part of that strategy. The tactic will be initially tested in the eastern range of C. nenuphar within the US. As indicated above S. riobrave has exhibited high levels of efficacy in the Southeastern US (i.e., Georgia), yet it is possible other entomopathogenic nematode species or strains will be more appropriate for use against C. nenuphar in the insect’s northern range, e.g., in the mid-Atlantic and New England states. Two environmental factors that can vary across latitudinal regions and influence nematode efficacy are soil type and temperature (Kaya, 1990; Grewal et al., 1994; Shapiro et al., 1999; Shapiro-Ilan et al., 2006). Thus our objective in this study was to conduct a broad screening of entomopathogenic nematode species and strains at three different temperatures and in two soil types (one soil typical of the fruit growing region in the mid-Atlantic and one typical of New England).
Materials and Methods
Insects, nematodes, and soils: C. nenuphar were reared at the USDA-ARS laboratory in Kearneysville, WV at 25°C and 14L:10D on a diet of green thinning apples based on the methods of Amis and Snow (1985). Fourth-instar C. nenuphar were collected in drop trays upon exit from fruit and separated into groups of 100. To limit self-inflicted injury during holding and transport, groups of 100 larvae were placed in 200 ml plastic cups (Dixie, Georgia-Pacific Consumer Products, Atlanta, GA) filled with superfine grade vermiculite (W.R. Grace and Company, Cambridge, MA). Larvae were shipped overnight to Byron, GA for experimentation in an insulated Styrofoam cooler with 2 -1oC cold packs (U-Tek, Thermosafe Brands, Arlington Heights, IL). Upon receipt, weevil larvae were separated from vermiculite by screening and larvae were used in experiments within 2 days. Dead larvae or larvae that appeared damaged were discarded.
Nematodes were reared on commercially obtained last instar Galleria mellonella (L.) at 25°C according to procedures described in Kaya and Stock (1997). Following harvest, nematodes were stored at 13°C for less than 2 wk before experimentation. Two soil types were included in this study, with both collected from apple orchard plots; one from the mid-Atlantic (Kearneysville, WV) and the other from New England (Lebanon, New Hampshire). Hereafter they will be referred to in the text as the NH and WV soils. The NH soil is classified as a loam with 38:50:12 percentage sand:silt:clay and a pH of 6.2. The WV soil is classified as a clay loam with 28:44:28 percentage sand:silt:clay and a pH of 7.18. The soil was autoclaved prior to shipment and kept at least two wks at room temp prior to use (Kaya and Stock, 1997).
Virulence bioassays: Virulence screening in the two different soils was conducted separately using identical methods. In each soil a series of three rounds of screening were conducted (hereafter referred to as Rounds 1, 2, and 3); a total of 13 nematode strains comprising nine species were compared. In Round 1, seven nematode strains were compared. Round 2 compared the remaining six strains that were not tested in the first round, as well as one nematode that was common to both Round 1 and Round 2 (i.e., S. riobrave 355 strain), which was used to facilitate qualitative comparisons between the two rounds (see Table 1 and 2 for a list of nematodes in each round). Subsequently, in Round 3, a “best candidate” screening was conducted consisting of the five or six most promising strains from the first two rounds (see Figs. 1 and 2 for the strains tested). All Rounds (within soil type) were run simultaneously at 12°C, 18°C and 25°C. The choice of temperatures was based on a range that might occur during the first C. nenuphar-induced fruit drop in mid-Atlantic and New England states.
Table 1.
Average percentage survival (± SEM) of Conotrachelus nenuphar larvae following exposure to entomopathogenic nematodesa in a loam soil (from New Hampshireb).
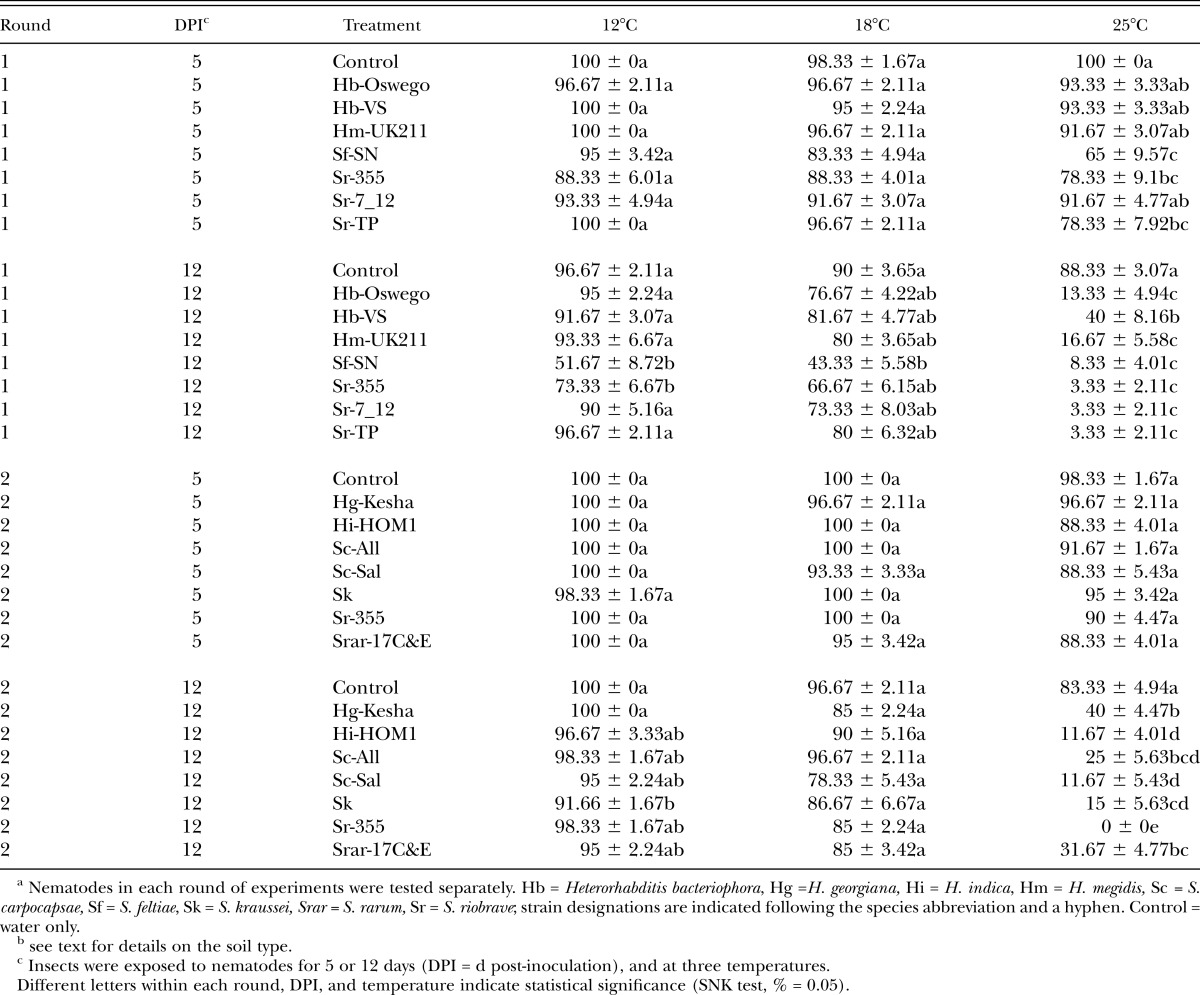
Table 2.
Average percentage survival (± SEM) of Conotrachelus nenuphar larvae following exposure to entomopathogenic nematodesa in clay loam soil (from West Virginiab).
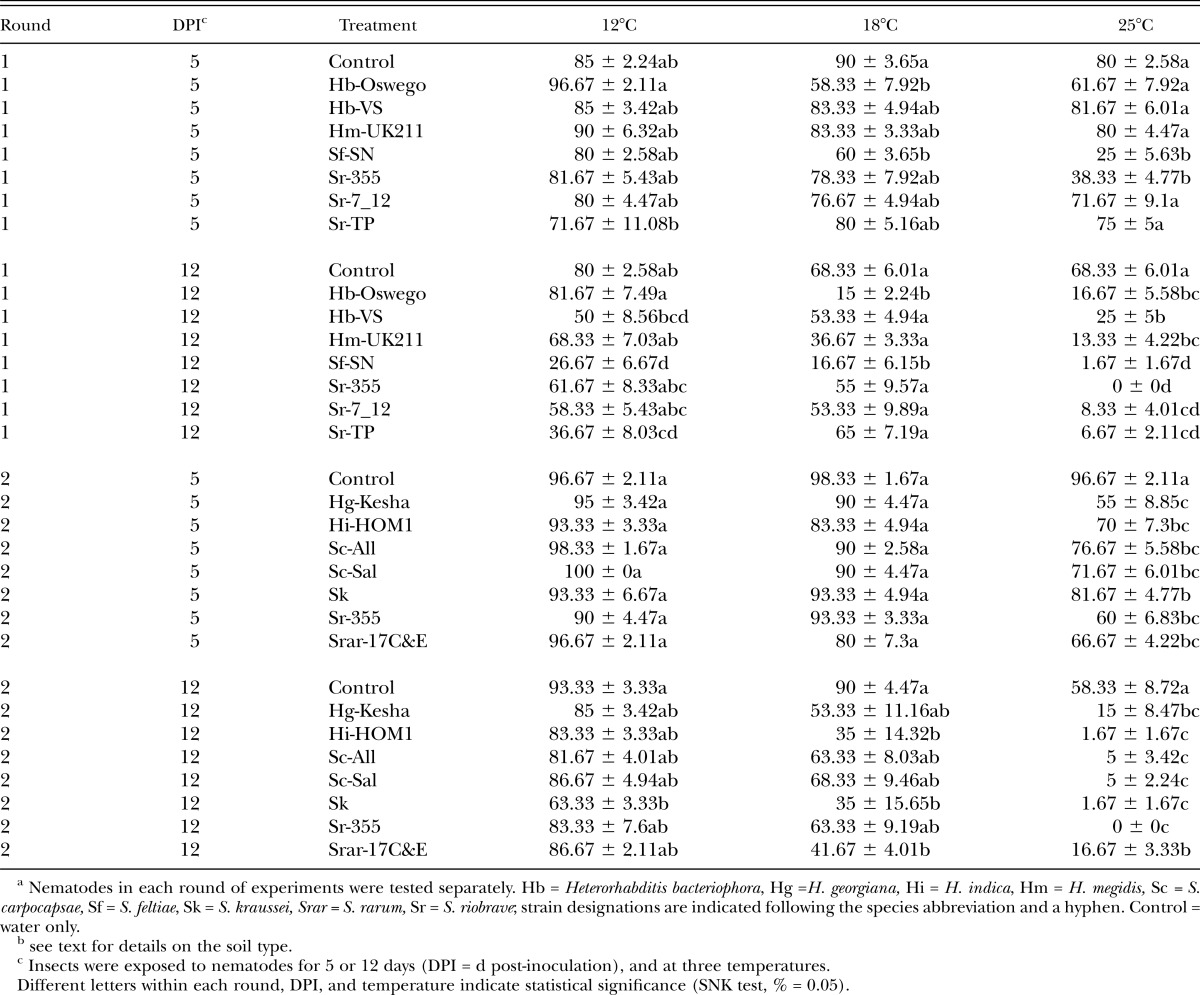
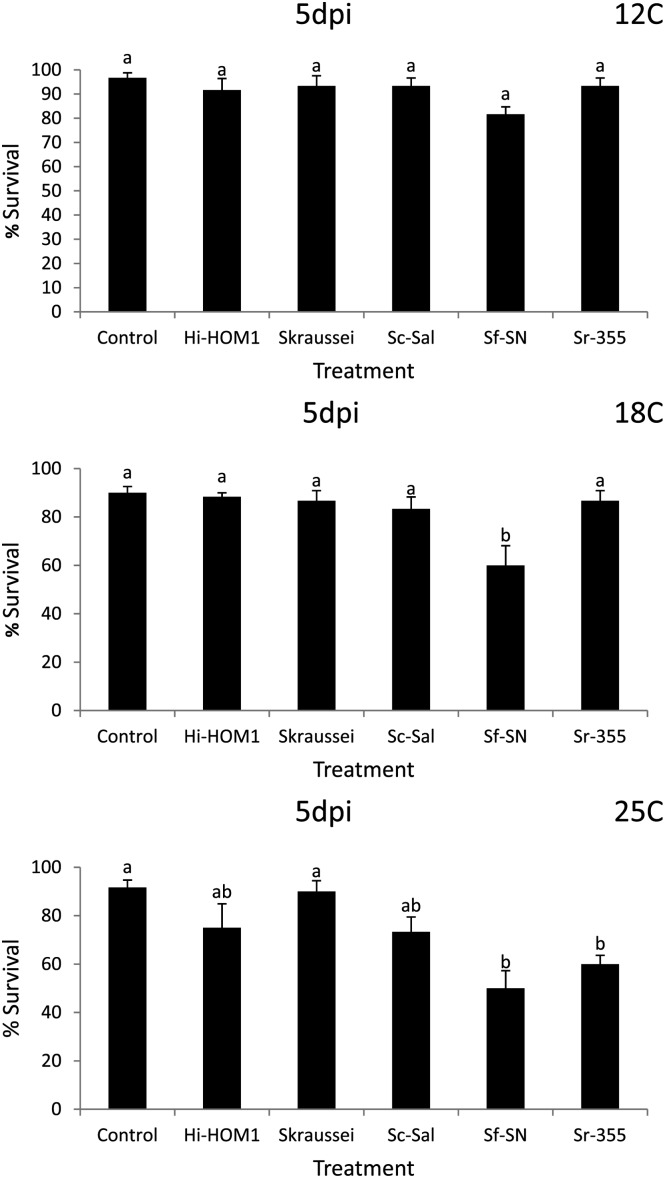
Fig. 1.
Average percentage survival (± SEM) of Conotrachelus nenuphar larvae following a 5 d exposure (5 dpi) to entomopathogenic nematodes in a loam soil (from New Hampshire). Hi = Heterorhabditis indica, Sc = Steinernema carpocapsae, Sf = S. feltiae, Sk = S. kraussei, Sr = S. riobrave; strain designations are indicated after the species abbreviation and hyphen. Control = water only. Different letters above bars indicate statistically significant differences (SNK test, % = 0.05).
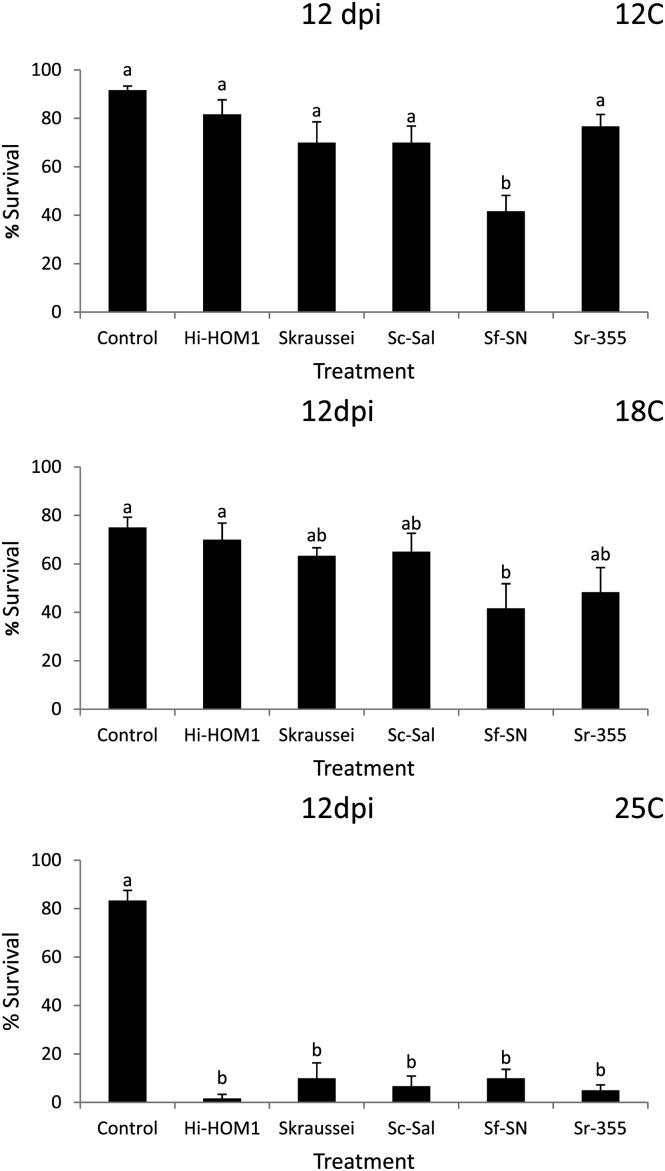
Fig. 2.
Average percentage survival (± SEM) of Conotrachelus nenuphar larvae following a 12 d exposure (12 dpi) to entomopathogenic nematodes in a loam soil (from New Hampshire). Hi = Heterorhabditis indica, Sc = Steinernema carpocapsae, Sf = S. feltiae, Sk = S. kraussei, Sr = S. riobrave; strain designations are indicated after the species abbreviation and hyphen. Control = water only. Different letters above bars indicate statistically significant differences (SNK test, % = 0.05).
Nematode virulence to C. nenuphar larvae was assessed based on procedures described by Shapiro-Ilan et al. (2002, 2003). Experiments were conducted in plastic cups (Bioserv Inc., Frenchtown, NJ). The cups (3-4 cm i.d., 3.5 cm deep) were filled with 20 g of oven-dried WV soil or 15 g of NH soil (which was equivalent to approximately 17.01cm3 volume for both soils). Approximately 500 IJs were applied to each cup in 0.5 ml tap water; prior to addition of nematodes, tap water was added to the soil so that final moisture level in each cup was at field capacity (30% for NH soil and 32% for WV soil). Controls, which were included in each assay, received water only. After application of nematodes and water, one weevil larva was added to each cup. Subsequently, cups were stored in the three temperatures, and survival of C. nenuphar was determined 5 d and 12 d post-treatment. The experiments were arranged in a completely randomized factorial design with temperature and nematode treatment constituting the main effects. All experiments included three replicates of 10 cups per treatment. Using identical experimental parameters, all experiments were repeated once in time with a fresh batch of nematodes (i.e., there were two trials per experiment).
Statistical analysis: Within each experiment, treatment effects were analyzed using ANOVA. If a significant F-test was detected, the Student-Newman-Keuls’ (SNK) test was used to further specify treatment differences (SAS Version 9.1, SAS Institute, Inc., Cary, NC). Initially, main effects in the factorial design (nematode and temperature) were analyzed for interactions. As significant main effect interactions were detected, a complete analysis of simple effects was conducted (Cochran and Cox, 1957). Specifically, within each temperature treatments were compared to the control and to each other. Additionally, although our primary goal was to compare virulence among nematode strains and species, we also evaluated the impact of temperature on virulence within each nematode treatment. For analysis of temperature effects, control mortality was corrected using Abbott’s formula (Abbott, 1925) so that potential differences in natural mortality among temperatures would not be a factor. Prior to analysis, percentage survival was transformed by arcsine of the square root (Southwood 1978, Steel and Torrie 1980). Non-transformed means are presented in the Results section. The alpha level for all statistical tests was 0.05.
Results
Assessment of main effects and justification to focus on simple effects: Interactions between main effects were detected in both soils. Significant interactions between temperature and nematode treatment were detected in all analyses at 12 d post-treatment (both soils and all 3 rounds) (P = 0.0001 in all analyses except Round 2 in the WV soil P =0.0109). Main effect interactions were also detected 5 d post-treatment in the WV soils (P = 0.0001 except Round 2 in the WV soil P = 0.0121), but the interactions were not significant 5 d post-treatment for the NH soil (P = 0.1256, 0.0871, and 0.2138 for Rounds 1, 2 and 3, respectively). In these cases, where the main effects were independent, temperature had a significant impact on C. nenuphar survival across nematode treatments (P = 0.0001 for all 3 rounds). Specifically in the NH soil at 5 d post-treatment, in Rounds 1 and 3 C. nenuphar survival was highest at 12°C followed by 18°C and 25°C had lowest survival; in Round 2 survival was higher at 25°C than 18°C and 12°C (which were not different from each other) (data not shown). Rounds 1 and 3 (at 5 d post-treatment) also exhibited significant treatment effects across temperatures in the NH soil (P = 0.0001 for both rounds); in both experiments only the S. feltiae (SN) and S. riobrave (355) treatments caused lower survival relative to the control (data not shown). Treatment effects in Round 2 across temperatures (5 d post-treatment) were not detected (P = 0.3994). Despite these instances in which main effects were independent, and given that significant interactions among main effects were detected in the majority of analyses (in 8 out of the 12 analyses), a complete analysis of simple nematode treatment effects (within each temperature) and temperature effects (within each treatment) was deemed to be in order. The results of these analyses follow.
Assessment of nematode virulence in the NH (loam) soil: Differences in nematode virulence to C. nenuphar were detected in the NH soil at all 3 temperatures (Tables 1 & 3: Figs. 1 & 2). In Round 1 at 5 d post-treatment, treatment differences were not detected at 12°C or 18°C analyses (although ANOVA indicted a significant treatment effect at 12°C, the SNK test did not differentiate among treatments) (Tables 1 & 3). At 25°C survival of C. nenuphar was lower in the S. feltiae (SN) treatment than all others except S. riobrave (355) and S. riobrave (TP) (these three treatments were also the only ones different from the control) (Tables 1 & 3). At 12 d post-treatment, S. riobrave (355) and S. feltiae (SN) exhibited higher virulence than all other treatments at 12°C, and S. feltiae (SN) was superior at 18°C; at 25°C all treatments suppress C. nenuphar survival relative to the control, and the nematode treatments were not different from each other except higher C. nenuhphar survival was observed in the H. bacteriophora (VS) treatment (Tables 1 & 3).
Table 3.
ANOVA statistics from laboratory experiments testing virulence of entomopathogenic nematodes to Conotrachelus nenuphar larvae in a loam soil (from New Hampshire).

In Round 2, treatment differences were not detected at 5 d post-treatment (although ANOVA indicted a significant treatment effect at 18°C, the SNK test did not differentiate among treatments) (Tables 1 & 3). At 12 d post-treatment and 12°C (in Round 2) S. kraussei (Steiner) was the only treatment that caused lower C. nenuphar survival relative to the control, yet at 18°C treatment differences were not elucidated (despite a significant ANOVA), and at 25°C the lowest survival was detected in S. riobrave (355) followed by S. carpocapsae (Sal) and H. indica Poinar Karunakar and David (HOM1 strain) (Tables 1 & 3). Based on the virulence levels observed in Round 1 and Round 2, we chose H. indica (HOM1), S. carpocapsae (Sal), S. feltiae (SN), S. kraussei and S. riobrave (355) for further study in Round 3.
In Round 3, at 5 d post-treatment, S. feltiae (SN) was the only treatment that caused a reduction in C. nenuphar survival relative to the control at 18°C, and S. feltiae (SN) and S. riobrave (355) were the only treatments that caused lower C. nenuphar survival than the control at 25°C (no differences were detected at 12°C) (Table 3; Fig. 1). At 12 d post-treatment (in Round 3) S. feltiae (SN) was the only treatment that reduced C. nenuphar survival compared with the control at 12°C and 18°C (Table 3; Fig. 2). At 25°C all treatments caused lower C. nenuphar survival than the control and no differences were detected among the nematode strains and species (Table 3; Fig. 2).
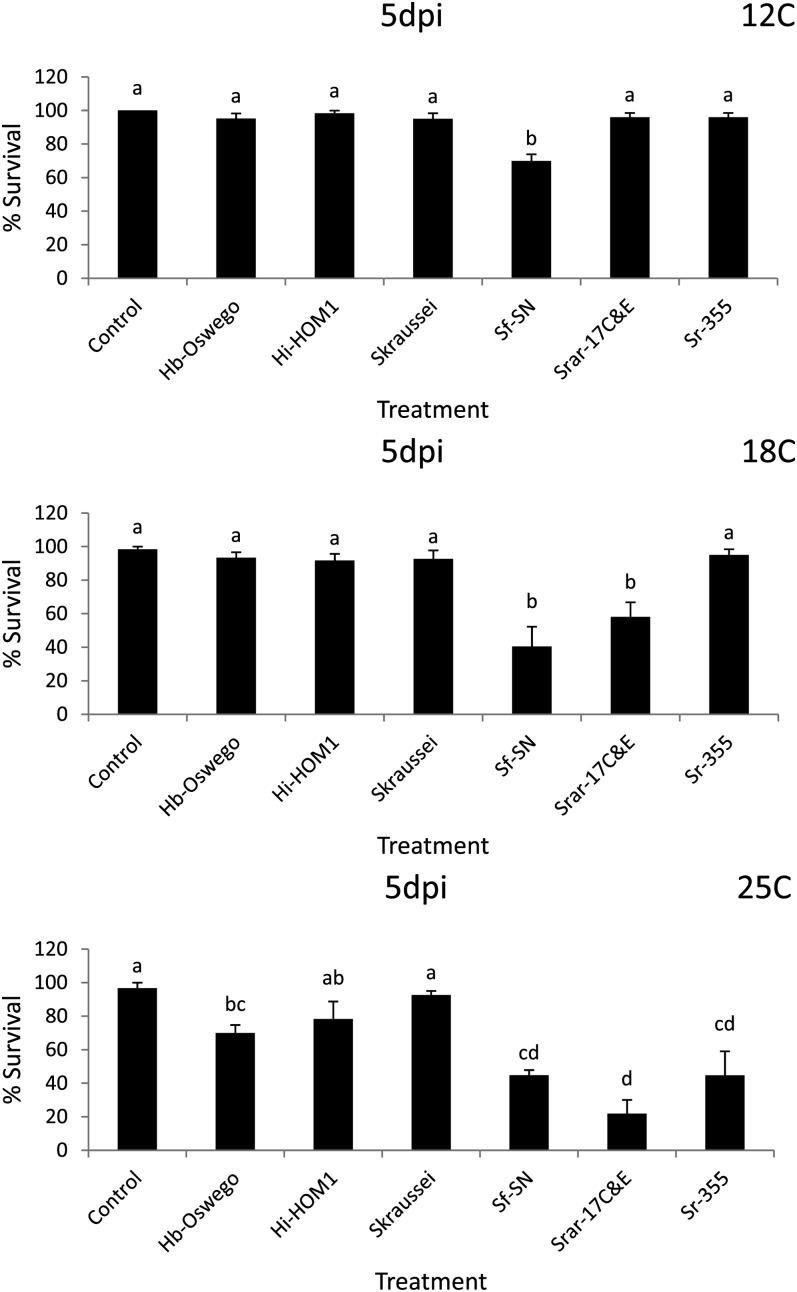
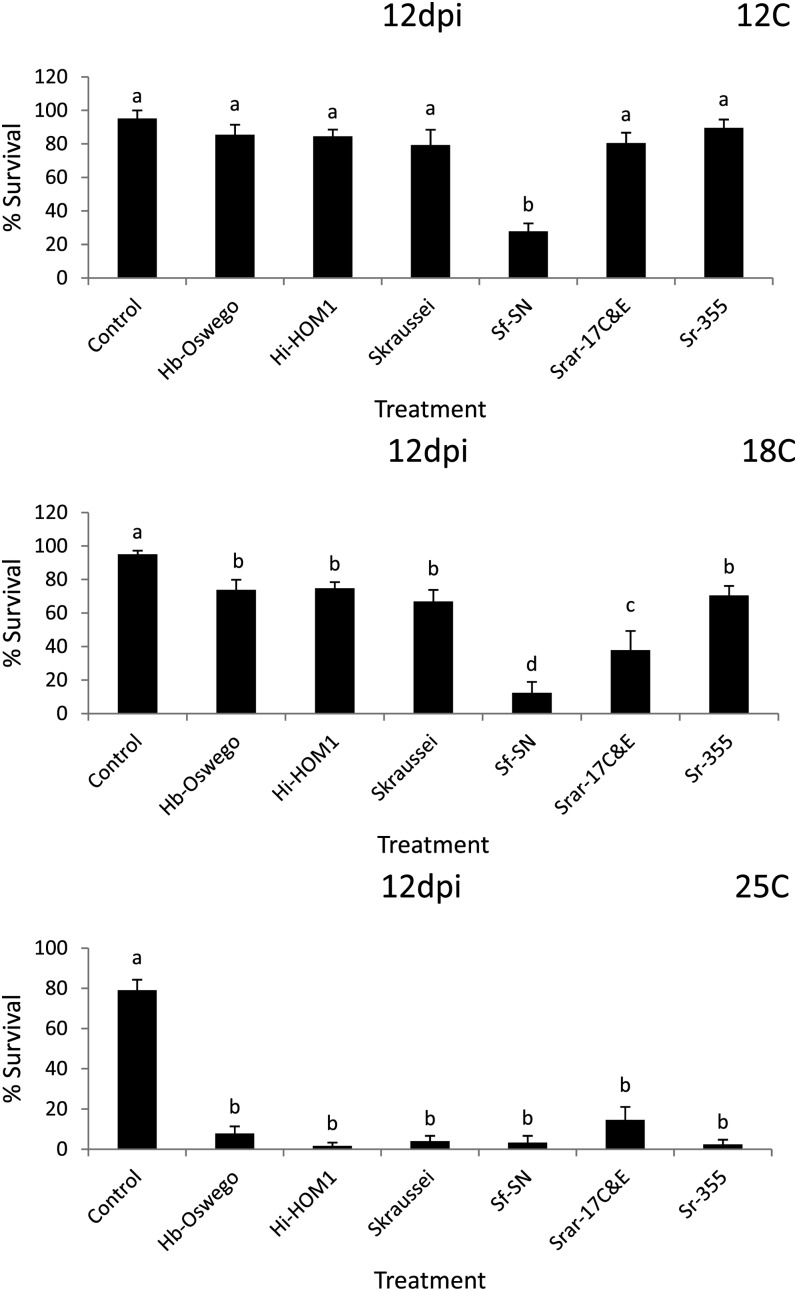
Assessment of nematode virulence in the WV (clay loam) soil: Differences in virulence were detected in the WV soil at all three temperatures (Tables 2 & 4; Figs. 3 & 4). In Round 1 at 5 d post-treatment no differences were detected among treatments relative to the control at 12°C, whereas at 18°C S. feltiae (SN) and H. bacteriophora (Oswego) caused lower C. nenuphar survival than the control, and at 25°C S. feltiae (SN) and S. riobrave (355) caused lower survival than the control (Tables 2 & 4). After 12 d post-treatment (in Round 1), C. nenuphar survival was lower in the S. feltiae (SN), S. riobrave (TP), and H. bacteriophora (VS) treatments than the control at 12°C, and at 18°C S. feltiae (SN) and H. bacteriophora (Oswego) caused lower C. nenuphar survival than the control (all other treatments were not different from the control) (Tables 2 & 4). At 25°C (Round 1 and 12 d post-treatment), all treatments caused lower survival than the control and survival was lower in the S. feltiae (SN) and S. riobrave (355) compared with other treatments except for S. riobrave (7-12 and TP strains).
Table 4.
ANOVA statistics from laboratory experiments testing virulence of entomopathogenic nematodes to Conotrachelus nenuphar larvae in a clay loam soil (from West Virginia).
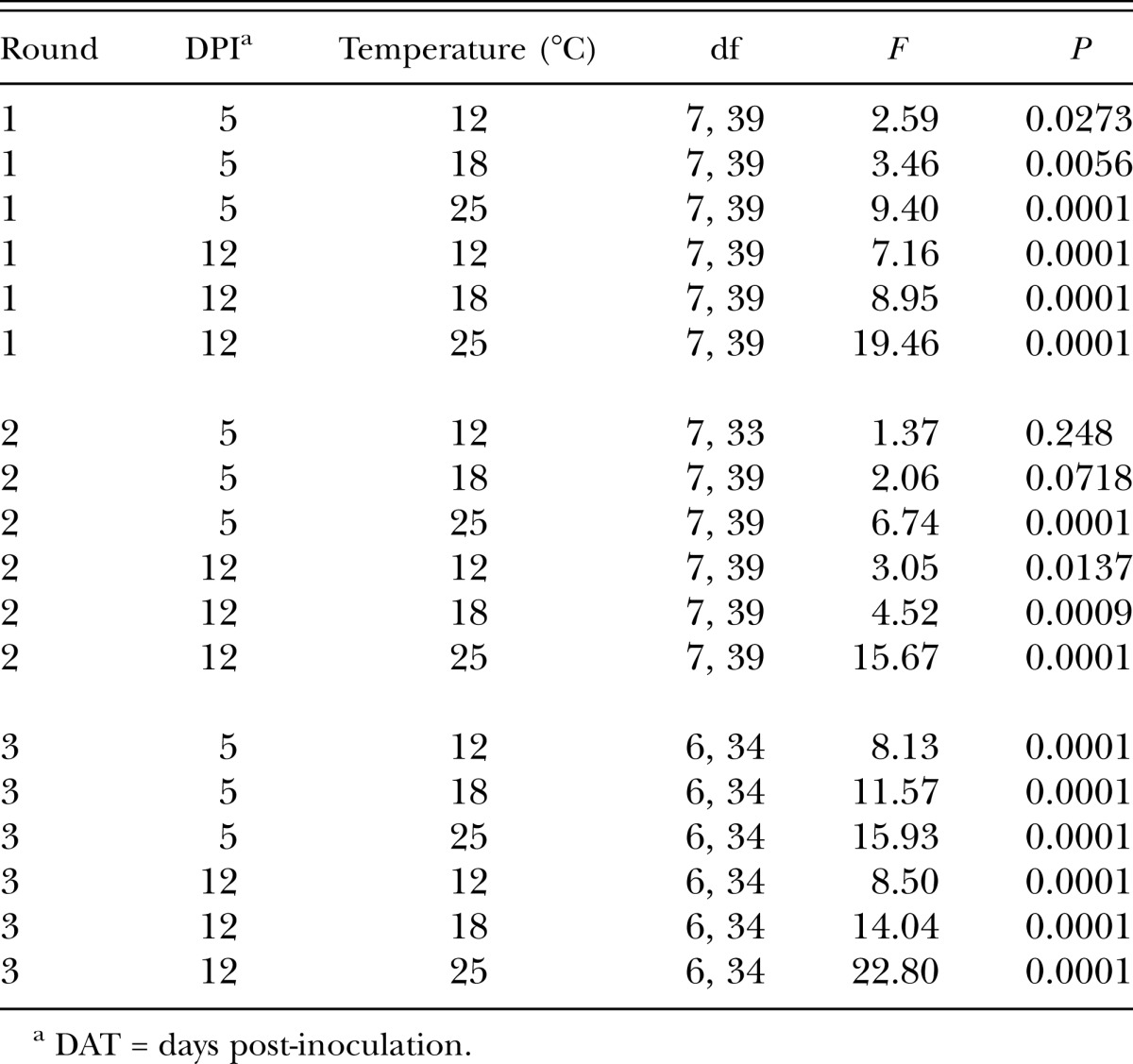
Fig. 3.
Average percentage survival (± SEM) of Conotrachelus nenuphar larvae following exposure to 5 d exposure (5 dpi) entomopathogenic nematodes in clay loam soil (from West Virginia). Hb = Heterorhabditis bacteriophora, Hi = H. indica, Sf = Steinernema feltiae, Sk = S. kraussei, Srar = S. rarum, Sr = S. riobrave; strain designations are indicated after the species abbreviation and hyphen. Control = water only. Different letters above bars indicate statistically significant differences (SNK test, % = 0.05).
Fig. 4.
Average percentage survival (± SEM) of Conotrachelus nenuphar larvae following exposure to 12 d exposure (12 dpi) entomopathogenic nematodes in clay loam soil (from West Virginia). Hb = Heterorhabditis bacteriophora, Hi = H. indica, Sf = Steinernema feltiae, Sk = S. kraussei, Srar = S. rarum, Sr = S. riobrave; strain designations are indicated after the species abbreviation and hyphen. Control = water only. Different letters above bars indicate statistically significant differences (SNK test, % = 0.05).
In Round 2 at 5 d post-treatment, no treatment effects were detected at 12°C and 18°C (Tables 2 & 4); at 25°C all treatments caused lower survival than the control (Tables 2 & 4). At 12 d post-treatment (in Round 2) S. kraussei was the only treatment that caused lower C. nenuphar survival than the control at 12°C, and S. kraussei, H. indica (HOM1), and S. rarum (de Doucet) (17C&E strain) caused a reduction in C. nenuphar survival relative to the control at 18°C (Tables 2 & 4). At 25°C, all treatments reduced C. nenuphar survival and no differences were detected among them except survival was higher in the S. rarum (17C&E) treatment than all others except H. georgiana Nguyen, Shapiro-Ilan and Mbata (Kesha strain) (Tables 2 & 4). Based on the results of Rounds 1 and 2, we chose H. bacteriophora (Oswego), H. indica (HOM1), S. feltiae (SN), S. kraussei, S. rarum (17C&E), and S. riobrave (355) for further study in Round 3.
In Round 3, at 5 d post-treatment S. feltiae (SN) was the only treatment that caused a significant reduction in C. nenuphar survival relative to the control at 12°C, and S. feltiae (SN) and S. rarum (17C&E) were the only treatments with lower C. nenuphar survival than the control at 18°C (Table 4; Fig. 3). At 25 °C (in Round 3, 5 d post-treatment) all nematode treatments suppressed C. nenuphar survival except H. indica (HOM1) and S. kraussei; S. rarum (17C&E) exhibited higher virulence than all other treatments except S. feltiae (SN) and S. riobrave (355) (Table 4; Fig. 3). After 12 d post-treatment at 12°C S. feltiae (SN) was the only treatment causing lower survival than the control, at 18°C S. feltiae (SN) caused the lowest survival followed by S. rarum (17C&E) (the other treatments caused lower survival than the control and were different from each other), and at 25°C all nematode treatments suppressed C. nenuphar with no differences detected among them (Table 4; Fig. 4).
Assessment of temperature effects within treatments: Temperature effects on corrected C. nenuphar survival were detected within nematode treatments in all 6 assay rounds (three rounds x 2 soils). At 5 d post-treatment significant temperature effects were observed in some treatments but not others (data not shown), yet by 12 d post-treatment temperature effects were observed in all treatments (P < 0.01 in all analyses except P = 0.0334 for the H. bacteriophora [VS] treatment in WV soil Round 1, and P = 0.0160 in the S. feltiae [SN] treatment NH soil Round 3). In all treatments (all rounds and all soils at 12 d post-treatment) C. nenuphar survival was lower at 25°C than 12°C except in the H. bacteriophora (VS) treatment for Round 1 WV soil where survival was lower at 25°C than 18°C and 12°C was intermediate. C. nenuphar survival was also lower (12 d post-treatment) at 25°C than 18°C in all treatments except in the H. bacteriophora (Oswego) treatment for Round 1 WV soil, and the S. feltiae (SN) and S. rarum (17C&E) treatments in Round 3 WV soil. In a number of treatments, survival at 18°C was lower than 12°C: H. megidis (UK211) in WV soil Round 1; H. indica (HOM1), S. kraussei, and S. rarum (17C&E) in WV soil Round 2; H. georgiana (Kesha), S. carpocapsae (Sal), and S. riobrave (355) in NH soil Round 2; and S. riobrave (355) in WV soil Round 3. In one case C. nenuphar survival (at 12 d post-treatment) was higher at 18°C than at 12°C, i.e., in the S. riobrave (TP) treatment Round 1 WV soil.
Discussion
Substantial differences in virulence to C. nenuphar larvae were observed among nematode species. Similar to our study, diverse virulence responses have been observed among nematode species and strains in laboratory screening studies targeting other weevil species such as the sweetpotato weevil, Cylas formicarius (F.), (Mannion and Jansson, 1992), Diaprepes root weevil, Diaprepes abbreviatus (L.), (Shapiro and McCoy, 2000), and the guava weevil, Conotrachelus psidii Marshall, (Dolinski et al., 2006). Our results indicated that S. feltiae (SN), S. riobrave (355) and S. rarum (17C&E) possess particularly high levels of virulence because these nematodes distinguished themselves relative to other nematodes in a number of comparisons including Round 3 (the “best candidate” assay). These findings are in corroboration with those of Shapiro-Ilan et al. (2002) in that S. feltiae (SN), S. riobrave (355) also exhibited superior laboratory virulence to C nenuphar larvae in the prior study (S. rarum was not tested in the earlier study). However, in contrast to the results of Shapiro-Ilan et al. (2002), several species exhibited pathogenicity in the present but not the former, i.e., H. bacteriophora, H. megidis, and S. carpocapsae; the discrepancy is likely due to the exposure period in the former study being limited to 5 d (the species were also not pathogenic at 5 d post-treatment in the present study).
The present study expands substantially on previous laboratory screenings for C. nenuphar virulence. Our study included four previously untested nematode species (H. indica, H. georgiana, S. kraussei, and S. rarum) as well as a number of previously untested strains, e.g., H. bacteriophora (Oswego and Vs strains), S. riobrave (7-12 and TP strains), and S. carpocapsae (Sal strain). In addition to S. rarum (17C&E), a number of the other previously untested nematodes exhibited promising levels of virulence and may warrant further study including H. indica (HOM1 strain), H. bacteriophora (Oswego strain), S. kraussei, and S. carpocapsae (Sal strain).
Temperature affected nematode virulence to C. nenuphar larvae. In the assays that contained independent main effects (and allowed for statistical analysis of temperature across treatments), C. nenuphar survival decreased as temperature increased. Additionally, when temperature effects were analyzed by treatment C. nenuphar survival was also lowest in the highest temperature tested (25°C). The impact of temperature on C. nenuphar survival was not surprising as temperature is known to affect entomopathogenic nematode infectivity, virulence and reproductive capacity and most species are most active at 20 to 30°C (Kaya 1990, Grewal et al., 1994; Shapiro-Ilan et al., 2006). Also as expected, nematodes that have been observed to be cold tolerant species, i.e., S. feltiae and S. kraussei (Grewal et al., 1994; Mráček et al., 1999; Haukeland and Lola-Luz, 2010) exhibited relatively higher virulence to C. nenuphar than most other nematode treatments at the lower temperatures tested. However, several nematodes that have not been previously reported as cold tolerant also caused C. nenuphar suppression at the lower temperatures e.g., H. bacteriophora (VS), H. indica (HOM1), and S. riobrave (TP). The potential activity of these nematodes at cooler temperatures may be applicable for control of other target pests in other cropping systems.
Soil parameters such as texture, organic matter, and electrical conductivity can influence nematode virulence (Kaya, 1990; Shapiro-Ilan et al., 2006; Kaspi et al., 2010). Although not compared directly, our results indicate that relative virulence among nematode treatments varied in the two different soils tested. Several of the treatments that exhibited high levels of virulence were common to both soils, i.e., H. indica (HOM1), S. feltiae (SN), S. kraussei, and S. riobrave (355), whereas others were not, e.g., H. bacteriophora (Oswego) and S. rarum (17C&E) showed high virulence in the WV soil but not the NH soil, and S. carpocapsae (Sal) exhibited the opposite association. Generally, compared with lighter soils, soils with higher clay content restrict nematode movement and have potential for reduced aeration, which can result in reduced nematode survival and efficacy (Georgis and Poinar, 1983; Kung et al., 1990; Molyneux and Bedding, 1984). Thus, in this study, one might have expected lower virulence in the WV clay loam than the NH loam because the former contains lower levels of sand and more clay. However, in all assay rounds, more nematode treatments were separated from the control in the WV soil than the NH soil, and therefore the premise based on soil texture does not appear to have been supported. Indeed, even when soils of differing textures have been directly compared exceptions have been observed, i.e., the soil with higher sand (and lower clay) is not always most conducive to nematode infection (Georgis and Gaugler, 1991; Shapiro et al., 2000). Given the diversity of soil textures in which entomopathogenic nematodes have caused high levels of pest suppression (Miklasiewicz et al., 2002; Grewal et al., 2005; Shapiro-Ilan et al., 2004, 2008), and based on the results of our assays, we expect there is potential for significant control of C. nenuphar in the soils tested herein.
Our results may have predictive value in determining which nematode species or strains are most suitable for C. nenuphar suppression in different regions of North America. For example, based on our results S. feltiae may be particularly suitable to C. nenuphar control in the Northern US and Canada. However, despite being considered a warm-adapted nematode (Grewal et al., 1994), S. riobrave caused significant suppression of C. nenuphar in Michigan (Pereault et al., 2009), and therefore may be also suitable for northern regions. Predictions based on laboratory results do not always turn out as expected in the field, e.g., S. feltiae caused the highest C. nenuphar mortality in an earlier laboratory study (Shapiro-Ilan et al., 2002) but was ineffective in the field (Shapiro-Ilan et al., 2004). On the other hand numerous laboratory screening studies have led to the selection of entomopathogenic nematodes that proved successful in the field, e.g., H. indica for control of D. abbreviatus (Shapiro et al., 1999; Shapiro and McCoy, 2000; Shapiro-Ilan et al., 2005), and S. carpocapsae for control of the lesser peachtree borer, Synanthedon pictipes (Grote and Robinson) (Shapiro-Ilan and Cottrell, 2006; Shapiro-Ilan et al., 2010) and pecan weevil, Curculio caryae (Horn) (Shapiro-Ilan et al., 2003; Shapiro-Ilan and Gardner, 2012).
Conceivably, entomopathogenic nematodes might be used as a stand-alone tactic for reducing C. nenuphar populations, e.g., in organic orchards. Alternatively, we propose a multi-stage integrated management program that includes insect attractants deployed in sentinel trees (Leskey et al. 2008), selective use of chemical insecticides for adult C. nenuphar control, and soil applications of nematodes to suppress ground-dwelling stages. We are currently conducting research toward implementation of the integrated plan, and based on the results of this study, have initiated field studies for optimization of nematode treatments in the Northeastern US.
Literature Cited
- Abbott WS. A method for computing the effectiveness of an insecticide. J. Journal of Economic Entomology. 1925;18:265–267. [Google Scholar]
- Amis A, Snow JW. 1985 Conotrachelus nenuphar. Pp. 227–236 in P. Singh and R. F. Moore, eds. Handbook of insect rearing, Vol. 1. New York: Elsevier Science Publishing. [Google Scholar]
- Cochran WG, Cox GM. 1957 Experimental designs. New York: John Wiley & Sons. [Google Scholar]
- Dolinski C, Del Valle E, Stuart RJ. Virulence of entomopathogenic nematodes to larvae of the guava weevil, Conotrachelus psidii (Coleoptera: Curculionidae), in laboratory and greenhouse experiments. Biological Control. 2006;38:422–427. [Google Scholar]
- Dowds BCA, Peters A. 2002 Virulence mechanisms. Pp. 79–98 in R. Gaugler, ed. Entomopathogenic Nematology. New York: CABI. [Google Scholar]
- Georgis R, Poinar GO., Jr Effect of soil texture on the distribution and infectivity of Neoaplectana carpocapsae (Nematoda: Steinernematidae) Journal of Nematology. 1983;15:308–311. [PMC free article] [PubMed] [Google Scholar]
- Georgis R, Kaya HK, Gaugler R. Effects of steinernematid and heterorhabditid nematodes (Rhabditida: Steinernematidae and Heterorhabditidae) on nontarget arthropods. Environmental Entomology. 1991;20:815–822. [Google Scholar]
- Grewal PS, Selvan S, Gaugler R. Thermal adaptation of entomopathogenic nematodes – niche breadth for infection, establishment and reproduction. Journal of Thermal. Biology. 1994;19:245–253. [Google Scholar]
- Grewal PS, Ehlers R-U, Shapiro-Ilan DI. 2005 Nematodes as biocontrol agents. Wallingford, UK: CABI Publishing. [Google Scholar]
- Haukeland S, Lola-Luz T. Efficacy of the entomopathogenic nematodes Steinernema kraussei and Heterorhabditis megidis against the black vine weevil Otiorhynchus sulcatus in open field-grown strawberry plants. Agricultural and Forest Entomology. 2010;12:363–369. [Google Scholar]
- Horton D, Johnson D. 2005 Southeastern peach grower’s handbook. Univ. GA Coop. Ext. Serv., G.E.S. Handbook No. 1. [Google Scholar]
- Horton D, Brannen P, Bellinger B, Ritchie D. 2011 2011 Southeastern peach, nectarine, and plum pest management and culture guide, Bulletin 1171. Athens, GA: University of Georgia. [Google Scholar]
- Kaspi R, Ross A, Hodson AK, Stevens GN, Kaya HK, Lewis EE. Foraging efficacy of the entomopathogenic nematode Steinernema riobrave in different soil types from California citrus groves. Applied Soil Ecology. 2010;45:243–253. [Google Scholar]
- Kaya HK. 1990 Soil ecology. Pp. 93–116 in R. Gaugler and H. K. Kaya, eds. Entomopathogenic nematodes in biological control. Boca Raton, FL: CRC Press. [Google Scholar]
- Kaya HK, Gaugler R. Entomopathogenic nematodes. Annual Review of Entomology. 1993;38:181–206. [Google Scholar]
- Kaya HK, Stock SP. 1997 Techniques in insect nematology. Pp. 281–324 in L. A. Lacey ed. Manual of techniques in insect pathology. San Diego: Academic Press. [Google Scholar]
- Kung S, Gaugler R, Kaya HK. Soil type and entomopathogenic nematode persistence. Journal of Invertebrate Pathology. 1990;55:401–406. [Google Scholar]
- Leskey TC, Piñero JC, Wood S, Prokopy RJ. Odor baited trap trees: a potential management tool for the plum curculio. Journal of Economic Entomology. 2008;101:1302–1309. doi: 10.1603/0022-0493(2008)101[1302:ottanm]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Mannion CM, Jansson RK. Comparison of ten entomopathogenic nematodes for control of sweetpotato weevil (Coleoptera: Apionidae) Journal of Economic Entomology. 1992;85:1642–1650. [Google Scholar]
- Miklasiewicz TJ, Grewal PS, Hoy CW, Malik VS. Evaluation of entomopathogenic nematodes for suppression of carrot weevil. BioControl. 2002;47:545–561. [Google Scholar]
- Molyneux AS, Bedding RA. Influence of soil texture and moisture on the infectivity of Heterorhabditis sp. D1 and Steinernema glaseri for larvae of the sheep blowfly Lucilia cuprina. Nematologica. 1984;30:358–365. [Google Scholar]
- Mráček Z, Bečvár S, Kindlmann P, Webster JM. Factors influencing the infectivity of a Canadian isolate of Steinernema kraussei (Nematoda: Steinernematidae) at low temperature. Journal of Invertebrate Pathology. 1999;73:243–247. doi: 10.1006/jipa.1998.4833. [DOI] [PubMed] [Google Scholar]
- Olthof TH, Hagley EC. Laboratory studies of the efficacy of steinernematid nematodes against the plum curculio (Coleoptera: Curculionidae). Journal of Economic. 1993 Entomology 86:1078–1082. [Google Scholar]
- Pereault RJ, Whalon ME, Alston DG. Field efficacy of entomopathogenic fungi and nematodes targeting caged last-instar plum curculio (Coleoptera: Curculionidae) in Michigan cherry and apple orchards. Environmental Entomology. 2009;38:1126–1134. doi: 10.1603/022.038.0420. [DOI] [PubMed] [Google Scholar]
- Poinar GO. 1990 Biology and taxonomy of Steinernematidae and Heterorhabditidae. Pp. 23–62 in R. Gaugler and H. K. Kaya eds. Entomopathogenic nematodes in biological control. Boca Raton, FL: CRC Press. [Google Scholar]
- Racette G, Chouinard G, Vincent C, Hill SB. Ecology and management of plum curculio, Conotrachelus nenuphar [Coleoptera: Curculionidae], in apple orchards. Phytoprotection. 1992;73:85–100. [Google Scholar]
- Shapiro-Ilan DI, Cottrell TE. Susceptibility of the lesser peachtree borer (Lepidoptera: Sesiidae) to entomopathogenic nematodes under laboratory conditions. Environmental Entomology. 2006;35:358–365. [Google Scholar]
- Shapiro-Ilan DI, Gardner WA. 2012. Improved Control of Curculio caryae (Coleoptera: Curculionidae) through Multi-Stage Pre-Emergence Applications of Steinernema carpocapsae. Journal of Entomological Science. In Press. [Google Scholar]
- Shapiro DI, McCoy CW. Virulence of entomopathogenic nematodes to Diaprepes abbreviatus (Coleoptera: Curculionidae) in the laboratory. Journal of Economic Entomology. 2000;93:1090–1095. doi: 10.1603/0022-0493-93.4.1090. [DOI] [PubMed] [Google Scholar]
- Shapiro DI, Cate JR, Pena J, Hunsberger A, McCoy CW. Effects of temperature and host age on suppression of Diaprepes abbreviatus (Coleoptera: Curculionidae) by entomopathogenic nematodes. Journal of Economic Entomology. 1999;92:1086–1092. [Google Scholar]
- Shapiro DI, McCoy CW, Fares A, Obreza T, Dou H. Effects of soil type on virulence and persistence of entomopathogenic nematodes in relation to control of Diaprepes abbreviatus. Environmental Entomology. 2000;29:1083–1087. [Google Scholar]
- Shapiro-Ilan DI, Mizell RF, III, Campbell JF. Susceptibility of the Plum Curculio, Conotrachelus nenuphar, to Entomopathogenic Nematodes. Journal of Nematology. 2002;34:246–249. [PMC free article] [PubMed] [Google Scholar]
- Shapiro-Ilan DI, Stuart R, McCoy CW. Comparison of beneficial traits among strains of the entomopathogenic nematode, Steinernema carpocapsae, for control of Curculio caryae (Coleoptera: Curculionidae) Biological Control. 2003;28:129–136. [Google Scholar]
- Shapiro-Ilan DI, Mizell RF, III, Cottrell TE, Horton DL. Measuring field efficacy of Steinernema feltiae and Steinernema riobrave for suppression of plum curculio, Conotrachelus nenuphar, larvae. Biological Control. 2004;30:496–503. [Google Scholar]
- Shapiro-Ilan DI, Duncan LW, Lacey LA, Han R. 2005 Orchard crops. Pp. 215–229 in P. S. Grewal, R.-U. Ehlers, and D. I. Shapiro-Ilan, eds. Nematodes as biological control agents. Wallingford, UK: CABI Publishing. [Google Scholar]
- Shapiro-Ilan DI, Gouge DH, Piggott SJ, Patterson Fife J. Application technology and environmental considerations for use of entomopathogenic nematodes in biological control. Biological Control. 2006;38:124–133. [Google Scholar]
- Shapiro-Ilan DI, Mizell RF, III, Cottrell TE, Horton DL. Control of plum curculio, Conotrachelus nenuphar with entomopathogenic nematodes: effects of application timing, alternate host plant, and nematode strain. Biological Control. 2008;44:207–215. [Google Scholar]
- Shapiro-Ilan DI, Cottrell TE, Mizell RF, III, Horton DL, Behle RW, Dunlap CA. Efficacy of Steinernema carpocapsae for control of the lesser peachtree borer, Synanthedon pictipes: Improved aboveground suppression with a novel gel application. Biological Control. 2010;54:23–28. [Google Scholar]
- Southwood TRE. 1978 Ecological methods. London: Chapman and Hall. [Google Scholar]
- Steel RGD, Torrie JH. 1980 Principles and procedures of statistics. New York: McGraw-Hill Book Company. [Google Scholar]
- Tedders WL, Weaver DJ, Wehunt EJ, Gentry CR. Bioassay of Metarhizium anisopliae, Beauveria bassiana, and Neoaplectana carpocapsae against larvae of the plum curculio, Conotrachelus nenuphar (Herbst) (Coleoptera: Curculionidae) Environmental Entomology. 1982;11:901–904. [Google Scholar]