Abstract
Observations in three Australian sugarcane fields suggested that the soil just under the trash blanket (the covering of crop residue that remains on the soil surface after crops are harvested) was suppressive to plant-parasitic nematodes. Roots were concentrated in this upper layer of soil but plant-parasitic nematode populations were relatively low and roots showed few signs of nematode damage. Root biomass was much lower 15 cm further down the soil profile, where root health was poor and populations of plant-parasitic nematodes were 3-5 times higher than near the soil surface. A bioassay in which Radopholus similis (a nematode that does not occur in sugarcane soils) was inoculated into heat-sterilized and untreated soils, confirmed that biological factors were limiting nematode populations in some of the soils, with soil from 0-2 cm much more suppressive than soil from 15-17 cm. Surface soil from one site was highly suppressive, as only 16% of R. similis recoverable from heated soil were retrieved from this soil after 8 days. Numerous soil chemical, biochemical, and biological properties were measured, and non-linear regression analysis identified two major groups of factors that were significantly associated with suppressiveness. One group reflected the amount of organic matter in soil (total C, total N, and labile C) and the other was associated with the size of the free-living nematode community (total numbers of free-living nematodes, and numbers of plant associates, bacterial feeders, fungal feeders, and carnivores). These results suggested that suppressiveness was biologically mediated and was sustained by C inputs from crop residues and roots. Since nematode-trapping fungi in the test soils could not be quantified using traditional dilution plating methods, their possible role as suppressive agents was assessed by generating TRFLP profiles with Orbiliales-specific primers, and by sequencing cloned PCR products. Although the molecular data were obtained from a limited number of samples, the level of suppression was significantly correlated to the number of Orbiliales clone groups and was also related to the number of Orbiliales species and TRFs, suggesting that this group of fungi may have been one of the suppressive factors operating in the test soils.
Keywords: biological control, clone library, mulch, nematode community analysis, nematode-trapping fungi, Orbiliales, predatory nematodes, organic matter-mediated suppression, TRFLP, sugarcane, suppressive soil
Green cane trash blanketing (GCTB), the term used to describe the process of harvesting sugarcane while it is still growing and retaining the crop residues on the soil surface as mulch, was introduced into the Australian sugar industry in the mid 1980s. Previously, the crop was burnt to facilitate the harvest operation and in the process, crop residues were destroyed. Under GCTB, about 15 t dry matter/ha remains in the field when a crop yielding 100 t/ha of millable cane (fresh weight basis) is harvested, and higher-yielding crops leave behind even more organic matter (Mitchell and Larsen, 2000). Since sugarcane is a perennial crop, additional crop residues are added every year, and so the trash blanket remains undisturbed for about five years before the soil is cultivated to destroy old stools and plant the next crop. During the replanting process, the trash blanket is incorporated and the soil surface then remains bare until a new trash blanket is laid down when the plant crop is harvested. However, with the recent introduction of a new sugarcane farming system based on minimum tillage, controlled traffic, legume break crops, and residue retention (Garside et al., 2005), an increasing proportion of Australia’s cane-growing soils is now permanently covered with crop residues.
Improved soil health is one of the benefits obtained from the new sugarcane farming system and GCTB contributes through its impact on soil physical and chemical properties (Garside et al., 2005; Stirling, 2008). However, the trash blanket also has important biological effects. Plant-parasitic nematodes cause serious damage to sugarcane (Cadet and Spaull, 2005; Blair and Stirling, 2007), but studies in microcosms have shown that multiplication of the two most damaging nematode pests, Pratylenchus zeae and Meloidogyne javanica, is much lower in soil mulched with sugarcane residue than in non-mulched soil (Stirling et al., 2011). Additional studies have shown that roots just under the trash blanket are healthier than those only 5-10 cm further down the profile and harbour fewer plant-parasitic nematodes (Stirling et al., 2011). These observations suggest that the trash blanket is involved in some way with maintaining root health near the soil surface. Given that organic matter-mediated suppression of soilborne pathogens is a well-recognized phenomenon (Stirling, 1991; Hoitink and Boehm, 1999; Stone et al., 2004; Oka, 2010; Stirling, 2011), the most likely scenario is that C inputs from crop residues and roots are responsible for sustaining an active and diverse soil food web that is responsible for suppressing nematodes and perhaps other root pathogens.
A common way of confirming that a soil is biologically suppressive to plant-parasitic nematodes is to compare nematode multiplication rates in sterilized and non-sterilized soil, or in sterilized soil to which small quantities of the test soil have been added (Westphal, 2005). However, one weakness of such assays is that plants often do not grow as well in the test soil as in partially or fully-sterilized soil and a reduction in the number of feeding sites available to the nematode may confound detection of suppressiveness. Thus in this study, we modified suppression assays that have been used by others (Jaffee et al., 1998; Pyrowolakis et al., 2002; Sanchez-Moreno and Ferris, 2007). Soil from trash blanketed sugarcane fields was collected from different depths in the profile and suppressiveness was assessed by inoculating heat-treated and untreated soil with a plant-parasitic nematode (Radopholus similis) that is not naturally present in the test soils. The number of nematodes recovered from treated and untreated soils was determined following a short incubation period in the laboratory.
Having assessed soils for suppressiveness to plant-parasitic nematodes, the next step was to better understand the regulatory mechanisms operating in those soils. Organic matter-mediated suppression is a complex phenomenon, as it involves multiple interactions within the soil food web and numerous modes of action, including parasitism, predation, nutrient depletion, lytic activity, and antibiosis (Stirling, 2011). Since many different organisms are likely to be contributing to suppressiveness in sugarcane soils, we chose to focus on predatory nematodes and the nematophagous fungi. Sanchez-Moreno and Ferris (2007) recently showed that the ratio of predators to prey and the prevalence of omnivore and predator species was associated with suppressiveness; while fungi capable of parasitising nematodes were observed in a sugarcane soil that had been rendered suppressive by amending it with sugarcane residue (Stirling et al., 2005).
In our studies of the nematophagous fungi, we concentrated on species in the Orbiliales (Ascomycota) that are usually referred to as nematode-trapping fungi. One reason for focusing on this group of fungi is that molecular detection techniques are now available to complement the inadequate culture-based methods that have been used in the past. In recent years, studies with restriction fragment length polymorphisms (RFLP) and DNA sequences have shown that the trapping device should be used to delimit genera of Orbiliales, and these results have led to a complete revision of the taxonomy of the Orbiliales (Persson et al., 1996; Ahrén et al., 1998; Hagedorn and Scholler, 1999; Scholler et al., 1999; Li et al., 2005; Chen et al., 2007). This work enabled the development of Orbiliales-specific PCR primers that amplify 5.8s rDNA, ITS2, and part of the 28s rDNA gene, and they have recently been used to detect nematode-trapping fungi without culturing (Smith and Jaffee, 2009). Thus one of the objectives of this study was to confirm the specificity of these primers and determine whether they could be used in terminal-RFLP (TRFLP) and molecular cloning to provide ecological information on nematode-suppressive soils.
Materials and Methods
Sites and soil sampling: The sugarcane crops used in this study were located near Bundaberg, Queensland (24°54’S, 152°22’E) on three farms with different soil types but a long history (>50 years) of sugar production. Site B was a deep, fertile clay loam of volcanic origin; site H was a fine sand with about 7% clay in the upper layers of the soil profile; while the surface soil at site C was a fine sandy clay loam. The soils were classified as a Red Ferrosol, Red Dermosol and Yellow Dermosol, respectively, according to the Australian Soil Classification system of Isbell (1996). Crops at the three sites were grown using standard management practices for irrigated sugarcane in Australia, and since all had been harvested green, the soil surface had been covered with a trash blanket for the previous 3-5 years (depending on the time elapsed since replanting). On 25 February 2010, soil and roots were collected at two depths (0-2 cm and 15-17 cm) from three points about 25 m apart in each of these fields to provide a total of 18 samples (3 sites × 2 depths × 3 replicates). All samples were taken in the non-compacted zone in the centre of a sugarcane row. A composite sample of roots from each site and depth was photographed, a sub-sample of the soil was used for chemical analyses and the remaining soil was used for the assays listed below.
To enable a more comprehensive assessment of root distribution with depth, the same sites were re-sampled one-month later. Three holes 17.5 × 17.5 × 20 cm were dug with a spade and during that process, all the soil from five depth intervals (0-2, 2-5, 5-10, 10-15, and 15-20 cm) was collected.
Chemical and biochemical properties: A 300 g subsample of soil was air-dried (400C for 7 days), sieved (< 2 mm), and then pH and EC (1:5 water) were determined. Total organic C and total N were measured by the Dumas dry combustion method in a combustion analyser and labile C (33 mM permanganate-oxidisable C) was determined using the method of Moody et al. (1997). Microbial activity was estimated by allowing soil enzymes to hydrolyze fluorescein diacetate (FDA) to water-soluble fluorescein, and measuring the end product with a spectrophotometer (Schnürer and Rosswall, 1982). Readings were corrected for background absorbance and appropriate standard curves (Chen et al., 1988) were used to calculate microbial activity (expressed as μg FDA hydrolysed/g dry soil/min).
Root distribution with depth: The soil collected at each depth interval was weighed and then sieved to retrieve sugarcane roots. After fresh weight was determined, roots were floated in water and rated for the presence and functionality of fine roots using the following scale: 1= severely diseased, with no fine roots; 2= highly diseased, with fine roots erratically distributed and contributing <20% of total root length; 3= intermediate levels of disease, with fine roots contributing 20-50% of total root length; 4= mainly healthy, with large numbers of functional fine roots; 5= very healthy, with a uniform spread of functional fine roots contributing >90% of total root length. Total root length was determined by measuring the length of a weighed sub-sample of fresh roots and then all roots were dried and weighed.
Nematode community analysis: A 200 mL sample of field-moist soil was spread on a standard extraction tray (Whitehead and Hemming, 1965) and after 2 days, nematodes were recovered by sieving twice on a 38-μm-sieve. Total plant-parasitic nematodes (PPN), total free-living nematodes (FLN), and numbers of plant-parasitic nematodes (identified to species level) were counted in fresh samples at a magnification of 40X. Nematodes were then fixed in formalin-acetic acid and about 100 randomly- selected specimens were identified at a magnification of 400X. Each nematode was assigned a trophic group and colonizer-persister (cp) value, and several indices that are often used to summarize nematode assemblages were calculated (Bongers, 1990; Yeates and Bongers, 1999; Ferris et al., 2001; Yeates, 2003).
Radopholus similis suppression assay: Duplicate 100 g samples of field-moist soil were added to 250 mL screw-capped containers and one of each pair was heated at 65°C for 1 hr. Radopholus similis was retrieved from cultures maintained on sterile carrot tissue and 2,000 nematodes were added to heated and untreated soil. After 8 days at 25°C, nematodes were extracted using the method described above. The number of R. similis retrieved from heated and untreated samples of each soil (NH and NU, respectively) was used to calculate the percentage suppressiveness [100 × (NH -NU)/NH].
Egg-parasitic fungi: Glasshouse-grown tomatoes infected with root-knot nematode (Meloidogyne incognita) were harvested at a time when most of the eggs were in early stages of development. Samples of 5 g soil were placed in 30 mL vials and then about 20,000 eggs retrieved from roots using 0.5% NaOCl were pipetted into each vial. After 4 and 6 days at 25°C, the soil was suspended in 750 ml water, eggs and second-stage juveniles were recovered from the suspension on a 38-μm-sieve, separated from debris by centrifugation in sugar solution (484 g sugar/liter water) and counted. A sample of eggs and juveniles was then spread on water agar + streptomycin and 2 days later, eggs were checked at a magnification of 400 X for signs of fungal parasitism.
Nematophagous fungi: Standard methods (Jaffee et al., 1998; Jaffee, 2003) were used to detect nematode-trapping fungi in each soil sample and estimate their population density using a most probable number technique. For each of the 18 soil samples, three dilutions (1, 0.1 and 0.001 g soil) were plated onto five replicate plates of ¼-strength corn meal agar. The first dilution was obtained by spreading 1 g soil on a plate, while the other dilutions were achieved by shaking 5 g soil vigorously in 10 ml water, diluting it 10-fold and then placing 5 drops (i.e. 0.2 ml) of each dilution on the surface of each plate. About 500 bacterial-feeding rhabditid nematodes were then added and 10, 14, and 22 days later, the nematode-trapping and other nematophagous fungi observed on the plates were identified by their morphology.
Molecular studies: Soil collected on 25 February 2010 was air-dried at 25-30°C, passed through a 2-mm-sieve and then 10 g (dry weight equivalent) sub-samples (each a composite of the three replicate samples from each site and depth) were dispensed into vials and stored at -20°C until required. DNA was extracted from 10g soil using a PowerMax Soil DNA isolation kit (MO-BIO Laboratories, Solana Beach, CA, USA) and its integrity (from this extraction and all methods described below) was checked on a 1.5% agarose gel stained with ethidium bromide. The primers used in all PCR reactions were obtained from Sigma-Genosys, Australia. Other PCR reagents were from Bioline, Australia.
The presence of PCR amplifiable soil DNA was demonstrated using fungal specific ITS primers (IT1F forward primer and ITS4 reverse primer; White et al., 1990). Each 25 μl PCR reaction contained 5μl soil DNA, 0.5 μl 50μM ITS1F, 0.5 μl 50μM ITS4, 0.5 μl 40mM dNTP, 2.5 μl AccuSure 10X Buffer, 0.5 μl AccuSure Taq (2.5 U/μl) and 15.5 μl water. PCR cycling conditions consisted of 95oC 10 min; 32 cycles of 94 oC 1 min, 55 oC 1 min, 72 oC 2 min; and a final extension of 72 oC for 10 min.
TRFLP was used to analyse the structure of Orbiliales, fungal, and bacterial communities in soil samples. Orbiliales-specific PCR products were produced from soil DNA as described by Smith and Jaffee (2009) using 10 μl soil DNA, 2 μl 50mM MgCl2, 1 μl 40mM dNTP, 2 μl of each primer at 50μM (Orb5.8s1F 5’ labelled with FAM [6-carboxyfluorescin] and Orb28s2R), 5 μl 10X Sahara Buffer, 1 μl Sahara Taq (4 U/μl) and 27 μl H2O. Cycling conditions were 95oC 10 min; 35 cycles of 94 oC 1 min, 66 oC 1 min, 68 oC 4 min; and a final extension of 68 oC for 10 min. The specificity of the PCR was confirmed using DNA extracted from Orbiliales and non-Orbiliales isolates (data not shown).
Fungal ITS PCR products for TRFLP were produced using 10 μl soil DNA (1/10 dilution), 2 μl 50mM MgCl2, 1 μl 40mM dNTP, 1 μl of each primer at 50μM (FAM-ITS1F and ITS4), 5 μl 10X Sahara Buffer , 0.75 μl Sahara Taq (4 U/μL,) and 29.25 μl H2O. Cycling conditions were 95oC 10 min; 32 cycles of 94 oC 1 min, 55 oC 1 min, 68 oC 4 min; and a final extension of 68 oC for 10 min.
Bacterial 16S rDNA PCR products for TRFLP were produced as follows. PCR reactions consisted of 10 μl soil DNA (1/10 dilution), 1.5 μl 50mM MgCl2, 1 μl 40mM dNTP, 2 μl BSA, 0.4 μl of each primer at 50 μM (FAM-27F and 1492R; Weisburg et al., 1991), 5 μl 10X NH4 Buffer, 0.6 μl BioTaq (5 U/μl), and 29.1 μl H2O. Cycling conditions were 95oC 10 min; 27 cycles of 94 oC 1 min, 58 oC 1 min, 72 oC 1 min; and a final extension of 72 oC for 10 min.
For TRFLP analyses, 2 replicate 50 μl PCR reactions were pooled and PCR products were then purified using Wizard SV Gel and PCR Clean-Up System (Promega, Australia). Restriction digests were performed with 1 μl Hae III (10U/μl), 1.5 μL 10X Buffer C, 0.15 μl BSA, 10 μl PCR product and 2.35 μl H2O (all reagents were from Promega, Australia). Digests were incubated at 37 oC for 3 hr and then heat inactivated at 85 oC for 15 min. Capillary electrophoresis was performed by the Australian Genome Research Facility (Parkville, Australia) using an Applied Biosystems® 3730 DNA Analyzer. Samples were prepared for electrophoresis by adding 1 μl of digest to 10uL of Hi Di Formamide and LIZ 500 size standard mix (Applied Biosystems) and then heated to 95 oC for 5 min. GeneMapper® software version 3.7 was used to generate electropherograms and extract TRFLP data. T-REX (http://trex.biohpc.org/) was used for filtering of noise (1 standard deviation), alignment of peaks, calculation of number of peaks and production of matrices for statistical analysis.
To confirm that Orbiliales primers were specific; to gain further information on community composition; and to compare cloning results to TRFLP results, PCR products from the six soils were cloned and sequenced. PCR products produced as described above using unlabelled primers were cloned into a pUC vector and then 48 clones were sequenced with primer M13F. Cloning and sequencing were performed by Macrogen Inc, Korea. Sequences were analysed using BLAST (http://www.ncbi.nlm.nih.gov/) and multiple alignments were conducted using ClustalW2 (http://www.ebi.ac.uk/Tools/clustalw2/index.html) to determine relatedness of clones within a sample. Sequences with a clustal score of 98-100 were placed into ‘clone groups’. Genome Workbench (http://www.ncbi.nlm.nih.gov/projects/gbench/) was used to perform multiple sequence alignment using MUSCLE (Edgar 2004); for construction of phylogenetic trees using 5.8S rDNA (from Orb1F) and including ITS2; and to align cloned sequences and 114 Orbiliales sequences retrieved from Genbank. Clone identity was determined by considering BLAST maximum identity and maximum scores, BLAST Distance tree of results and phylogenetic trees produced using Genome Workbench.
Statistical analyses: Data from a bioassay comparing the suppressiveness of various soils to R. similis were analysed by ANOVA. Relationships between chemical, biochemical, and biological parameters, and between those parameters and suppressiveness, were assessed using non-linear regression analysis (n = 18), with the strength of those relationships determined by the correlation coefficient (r) and its level of significance (*, **, and *** for P<0.05, 0.01, and 0.001, respectively). Due to cost considerations, the molecular data were obtained using composite samples from each site and depth (n = 6). Relationships between molecular parameters and the mean % suppression for each site and depth in the R. similis bioassay were examined using linear regression.
Results
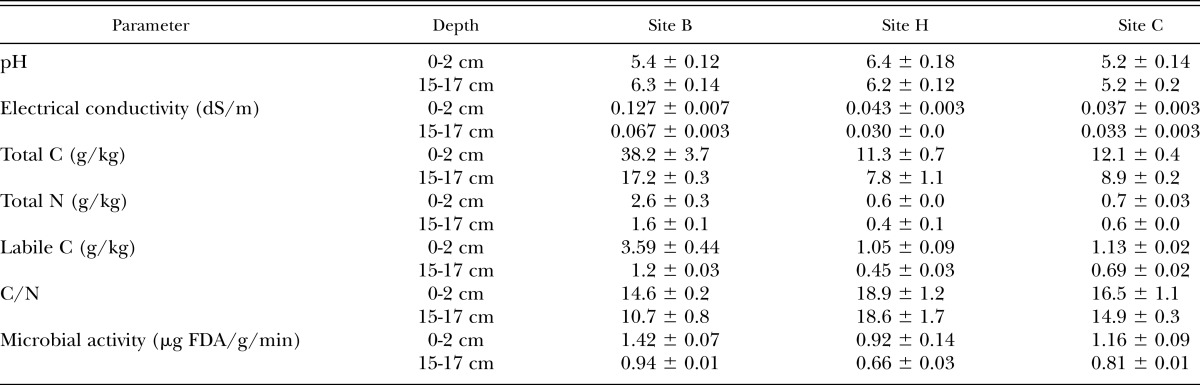
Chemical and biochemical properties: Chemical analyses indicated that soil from site B had much more C, N, and labile C and greater electrical conductivity than soil from the other sites. Also, the C and N status of soil from 15-17 cm was much poorer than soil from 0-2 cm at all sites (Table 1). Despite a marked decline in the concentration of both C and N with depth at site B, soil from 15-17 cm at this site had a better C and N status than surface soils from the other two sites (Table 1). Levels of microbial activity (measured as the rate of hydrolysis of FDA) varied with site and depth (Table 1) and were correlated with the levels of total C (r = 0.77***) and labile C (r = 0.87***).
Table 1.
Chemical and biochemical properties of sugarcane soils from three sites at Bundaberg, Queensland, at two depths in the soil profile.
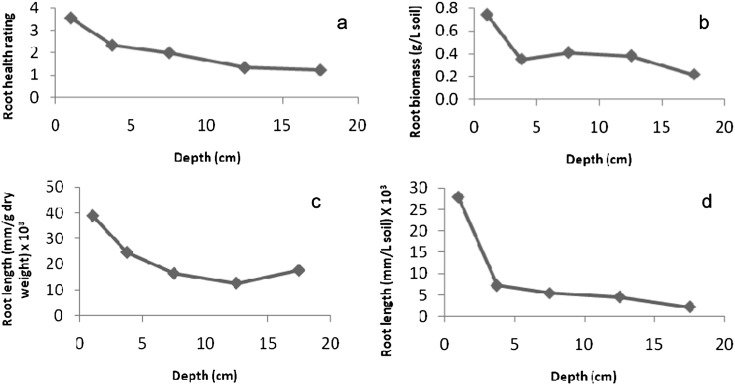
Root distribution with depth: There was a general trend across all sites for roots to be relatively healthy at a depth of 0-2 cm (i.e. many light brown fine roots with little discoloration and few lesions) and in very poor condition at a depth of 15-17 cm (Figure 1). Root health therefore declined with depth (Figure 2a), with regression analysis indicating that this effect was significant (P < 0.001). Root biomass was much higher just under the trash blanket than at depth (Figure 2b) and since there were many more fine roots in this region than further down the profile, root length was highest near the surface and declined with depth (Figures 2c, d). The depth effect was significant (P < 0.05) for all root biomass and root length parameters. Although root distribution patterns were similar at all sites, the root systems at site B had nearly four times more fine roots than the other two sites (54.6 × 103, 13.7× 103, and 15.5 × 103 mm roots/liter soil at sites B, C, and H, respectively).
Fig. 1.
Sugarcane roots collected in February 2010 from three sites at Bundaberg, Queensland. Roots from sites B, H and C are at the bottom, middle and top, respectively, with roots from 0-2 cm (left) and 15-17 cm (right).
Fig. 2.
Root health (a) and distribution of sugarcane roots (b, c, and d) in the upper 20 cm of the soil profile, averaged across three sites at Bundaberg, Queensland.
Nematode community analysis: Although the predominant plant-parasitic nematodes varied between sites, there were consistently more plant-parasitic nematodes at 15-17 cm than in soil near the surface (Table 2). In contrast, populations of free-living nematodes were highest near the surface at all sites (Table 2) and were strongly correlated with levels of labile C (r = 0.91***). There were also more plant associates, bacterial feeders, fungal feeders, omnivores, and carnivores at 0-2 cm than at 15-17 cm, except that sites H and C had very few nematodes in the latter trophic groups at either depth. When averaged across sites, plant-parasitic nematodes comprised 67% of the nematode community at 15-17 cm and only 11% of the community at 0-2 cm. The ratio of free-living to plant-parasitic nematodes varied between sites and depths, but was particularly high in the 0-2 cm layer at site B (Table 2).
Table 2.
Parameters describing the nematode community in sugarcane soils from three sites at Bundaberg, Queensland, at two depths in the soil profile.
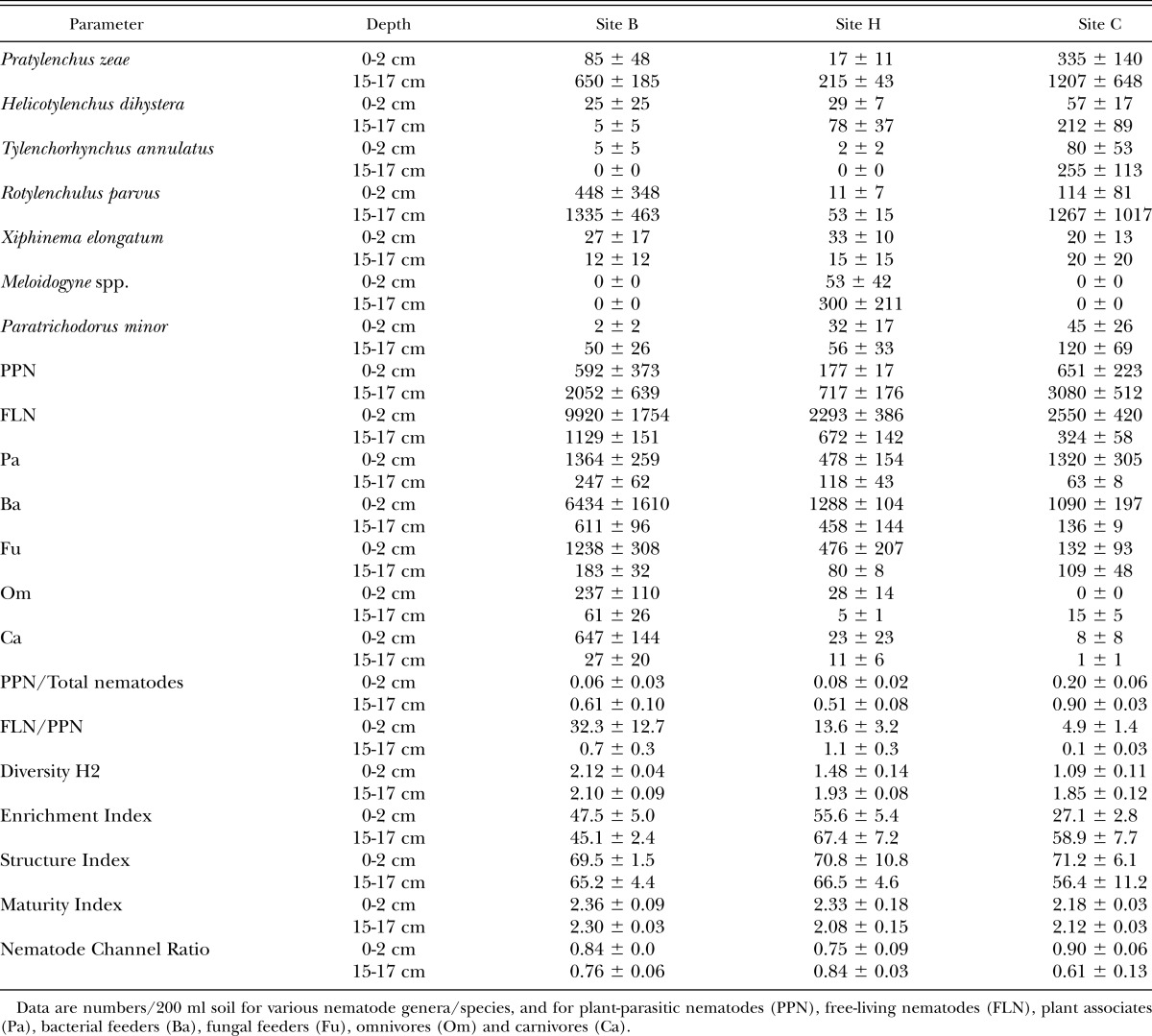
Indices derived from nematode community analyses suggested that there were both similarities and differences in the free-living nematode communities at the three sites (Table 2). All sites had relatively high nematode channel ratios, indicating that decomposition pathways in the detritus food web were predominantly bacterial rather than fungal. Site B had greater nematode diversity than the other sites, while enrichment and maturity indices sometimes differed between sites. Depth did not affect most indices, other than diversity and the nematode channel ratio at site C.
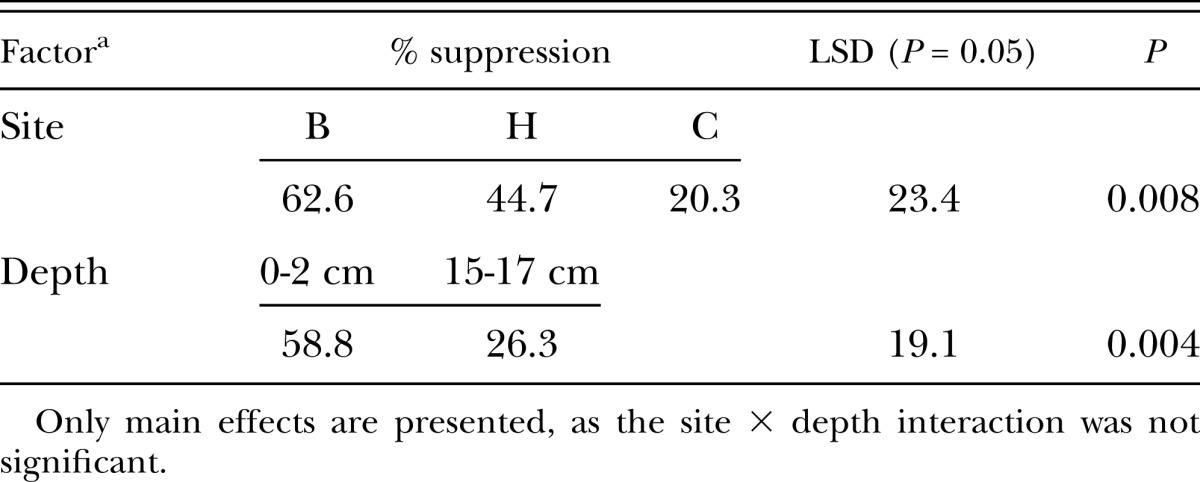
Radopholus similis suppression assay: R. similis was recovered readily from heated soil (an average of 1123 ± 58 nematodes across all soils and depths) whereas only 160 ± 20, 470 ± 115, and 706 ± 162 R. similis were recovered from the untreated surface soils at sites B, H, and C, respectively. Analysis of variance showed that site and depth significantly affected levels of suppressiveness, but the site × depth interaction was not significant. Soil from sites B and H was more suppressive than soil from site C, while soil from a depth of 0-2 cm was more suppressive than soil from 15-17 cm (Table 3). Surface soil from site B was highly suppressive, as only 16% of the R. similis recoverable from heated soil were retrieved from this soil after 8 days. The population of R. similis used in the bioassay consisted of 82% females, 14% males and 4% juveniles, but there was no evidence that one of these stages were more susceptible than the other to the suppressive agent/s (data not shown).
Table 3.
Suppressiveness to Radopholus similis in a laboratory bioassay with six sugarcane soils from Bundaberg, Queensland (3 sites × 2 depths in the soil profile)
Egg-parasitic fungi: Approximately 70% of the nematode eggs added to soil were recovered, with only about 10% having hatched. All eggs appeared to be relatively healthy and showed no signs of fungal parasitism when plated onto water agar.
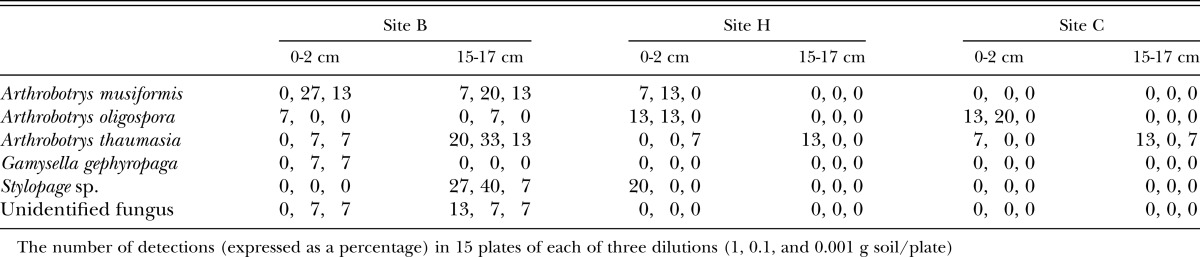
Nematophagous fungi: Nematophagous fungi were detected in all samples, but the frequency of occurrence was low and generally not related to dilution levels. Fungal populations could not be quantified due to the inconsistent nature of the data, and so results have been presented as the number of plates (in 3 replicate samples of 5 plates) in which a particular fungus was detected (Table 4). Four species of nematode-trapping fungi were observed (Arthrobotrys musiformis, A. oligospora, A. thaumasia and Gamysella gephyropaga), with site and depth in the profile having no obvious effect on occurrence. A species of Stylopage was seen at two sites and an unidentified fungus that captures nematodes on bulbous hyphal extensions (Stirling et al., 2005) was occasionally observed.
Table 4.
Frequency of occurrence of nematophagous fungi in sprinkle plates containing sugarcane soil from three sites at Bundaberg, Queensland, at two depths in the soil profile.
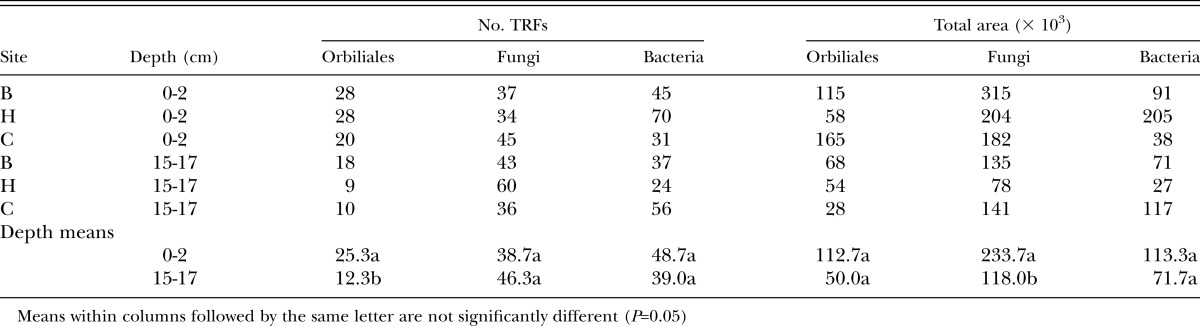
Molecular studies: PCR products were obtained from soil DNA using Orbiliales-, fungal- and bacterial-specific primers and TRFLP profiles were then generated from these products. The Orbiliales profiles differed in the number of terminal restriction fragments (TRFs) and the total area of peaks (Table 5), with analysis of variance indicating that the number of TRFs in the top layer of soil (0-2 cm) was significantly greater than in soil from 15-17 cm. The site effect was not significant for either parameter. For fungi, the number of TRFs was not affected by site or depth, but total peak area was significantly greater in the upper layer of soil than in the 15-17 cm layer. For bacteria, effects of site or depth were not significant for either of the measured parameters.
Table 5.
Number of terminal restriction fragments (TRFs) and the total area of peaks in TRFLP profiles of Orbiliales, fungi and bacteria in sugarcane soils from three sites at Bundaberg, Queensland at two depths in the soil profile.
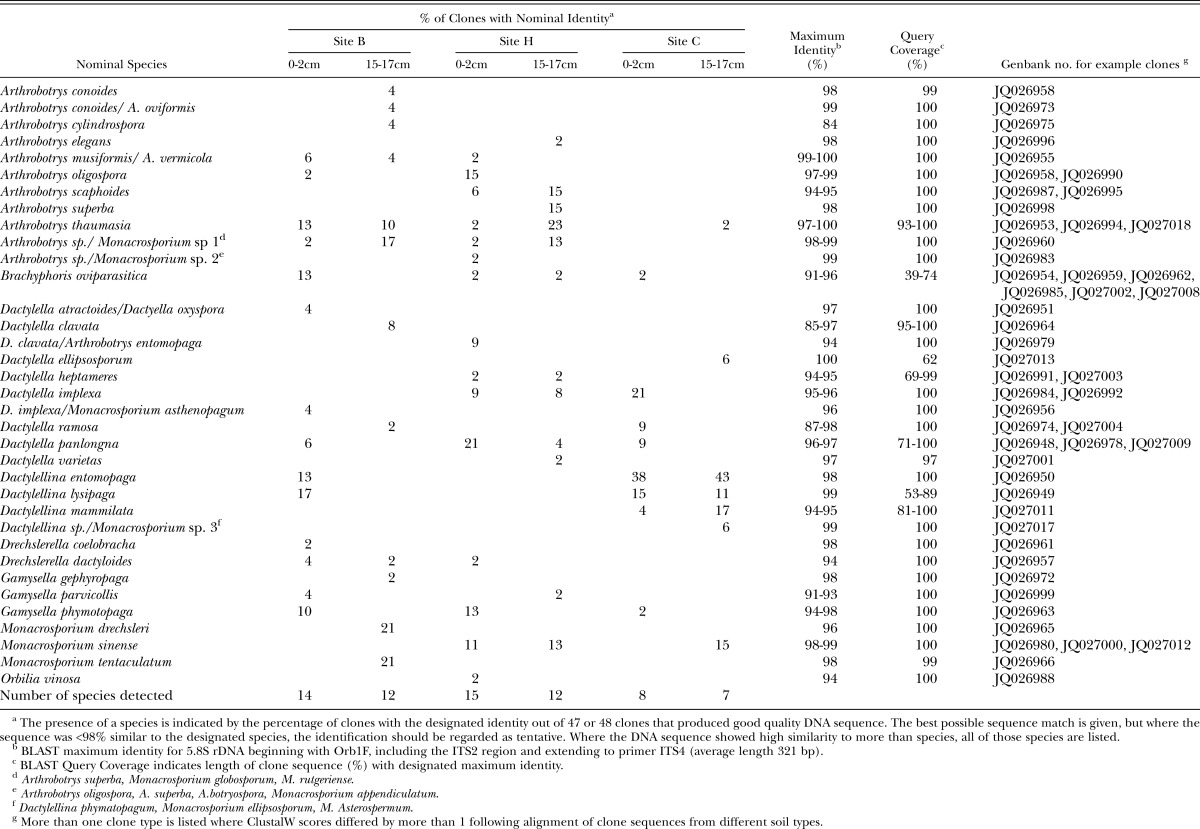
Sequencing of cloned PCR products confirmed that Orbiliales primers specifically amplified Orbiliales DNA from soil. Representative sequences from all soil types were submitted to Genbank (JQ026948 to JQ027018), with the locus and clone annotation details indicating site (B/H/C), depth [0-2 cm (S) or 15-17 cm (D)] and clone number. Thus clone BD16, for example, was clone number 16 from site B at a depth of 15-17 cm. All clones were most closely related to Orbilales and numerous phylotypes were detected in each sample. About 70% of the clones were identified with a reasonable degree of certainty (Table 6) on the basis that a sequence had high similarity to previously characterised species (i.e. 98-100% maximum identity as reported by BLAST together with a high maximum score). For about 30% of the clones, sequences did not closely match those of previously characterized species (84-97% similarity) and/or sequence data did not distinguish between closely related species. For most of these sequences, clone identities were assigned by analysis of phylogenetic trees produced using BLAST and Genome Workbench. Dactylellina entomopaga, Arthrobotrys thaumasia, Dactylellina lysipaga, Dactylella panlongna, Dactylella implexa, and Monacrosporium sinense were the most commonly detected species across all soils and depths, with sequences of at least 6 of the 48 clones examined (i.e. more than 12.5% of the clones) matching the designated species in at least one of the soil samples. However, there was no obvious pattern with regard to particular species being predominant at certain sites or depths (Table 6).
Table 6.
Species of Orbiliales identified by sequencing cloned PCR products (generated with Orbiliales-specific primers) from three sugarcane soils at two depths in the soil profile.
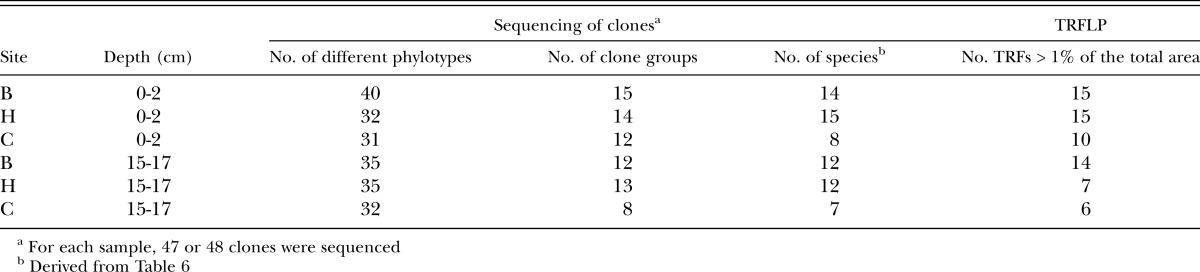
Orbiliales clones clustered into groups following ClustalW alignment, with the number of clone groups in each soil approximating the number of different species detected (Table 7). The number of groups in the Orbiliales community was also determined using peaks that accounted for greater than 1% of the total area in the TRFLP profile and those results indicated that the number of OTUs in each soil was of the same order of magnitude as the number obtained by sequencing cloned PCR products (Table 7). However, results from TRFLP and sequencing were not identical, either because more than one species can produce the same sized terminal restriction fragment or because the number of species will be underestimated when the sequences of two unrelated clones are similar to a given species in Genbank.
Table 7.
A comparison of the number of groups in the Orbiliales communities of six sugarcane soils (3 sites × 2 depths), as determined by sequencing cloned PCR products or TRFLP analysis
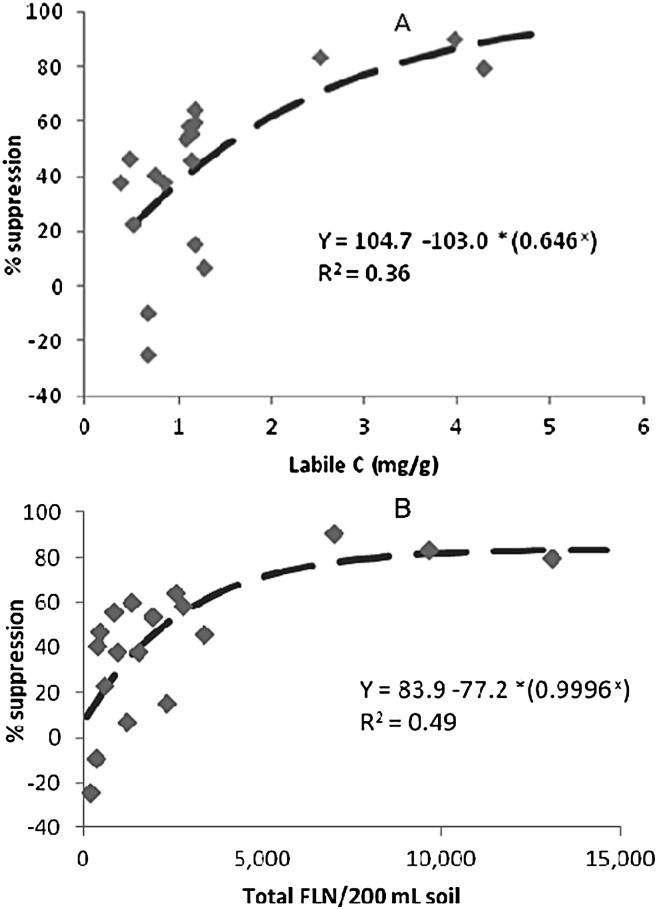
Relationships between various parameters and suppressiveness: The level of suppression in the bioassay with R. similis was significantly and positively correlated with four of the chemical and biochemical parameters listed in Table 1: electrical conductivity (r = 0.54*); total C (r = 0.57*); total N (r = 0.53*) and labile C (r = 0. 60*); and to five of the biological parameters listed in Table 2: total free-living nematodes (r = 0.70**); total plant associates (r = 0. 52*); total bacterial feeders (r = 0. 70**); total fungal feeders (r = 0. 63**), and total carnivores (r = 0.72**)]. Examples of some of those relationships are given in Figure 3. There was also a significant negative relationship between suppressiveness and the proportion of plant parasites in the nematode community (r = -0.64**). None of the indices derived from nematode community analysis, and none of the other parameters measured, were significantly correlated with suppressiveness.
Fig. 3.
Relationships between the suppressiveness of various soils to Radopholus similis in a laboratory assay and (A) the concentration of labile C and (B) total numbers of free-living nematodes.
Although the molecular data set was too small to draw firm conclusions, relationships between suppressiveness and the parameters listed in Tables 5 and 7 were examined. The number of Orbiliales clone groups was significantly correlated with suppressiveness (P = 0.006, r = 0.92**) and the relationship to the number of Orbiliales TRFs and the number of Orbiliales species was almost significant (P = 0.051, r = 0.75; P = 0.064, r = 0.72, respectively). A significant relationship was also observed between suppressiveness and the relative abundance of TRFs at 149 and 472 bp (P = 0.032, r = 0.85* and P = 0.036, r = 0.84*, respectively).
Discussion
Root distribution, root health and suppressiveness to plant-parasitic nematodes: Initial observations of root distribution in the field indicated that regardless of the soil texture, sugarcane roots were heavily concentrated in the upper layers of the soil profile, supporting previous observations on the importance of the zone immediately beneath the trash blanket as a medium for root growth (Stirling et al., 2011). Perhaps more importantly, the roots in this zone were healthier and had many more fine roots than those only a few centimetres further down the profile, indicating that the most functional part of the sugarcane root system is near the soil surface. Stratification with depth was particularly apparent in the clay loam soil at site B, with root length/liter soil 7 times greater at 0-2 cm than at 2-5 cm. The surface roots at this site were healthier than the roots normally seen on sugarcane, as they formed a fine network of white to light brown roots that was readily visible when the trash blanket was removed. Since plant-parasitic nematodes normally debilitate sugarcane root systems and reduce yield (Cadet and Spaull, 2005; Blair and Stirling, 2007), the absence of lesions and other symptoms of nematode attack suggested that roots near the soil surface were not being damaged by nematodes.
Plant-parasitic nematodes were present at all sites, but the predominant species varied from site to site, probably because each nematode species has a preference for soils of a particular texture (Blair et al., 1999 a, b). However, all species (except X. elongatum) showed marked stratification with depth, with population densities much lower in surface soil than at 15-17 cm. Populations of P. zeae, for example, were 3-12 times lower at a depth of 0-2 cm than at 15-17 cm, confirming previous observations that this nematode does not reach high densities in surface soils, whether populations are measured in soil or roots (Stirling et al., 2011). Given that root biomass was much greater near the surface than at depth, this result was surprising, as plant-parasitic nematodes are obligate parasites of plants and their distribution was expected to mirror root distribution. Although it is possible that plant-parasitic nematodes prefer deeper soils because the moisture or temperature regime is in some way more amenable, it is unlikely that this was the reason for the observed distribution pattern, as all the crops were irrigated and the blanket of trash on the soil surface would have limited moisture loss and dampened temperature fluctuations at the soil surface. Given the high C levels in surface soils and its flow-on effects on biological activity, we hypothesized that nematode populations were being regulated by organisms in the soil food web and that their suppressive effects were greater near the surface than at depth.
The results of our bioassay with R. similis confirmed the suppressive nature of some of the soils. After an incubation period of only 8 days, more than 1,100 R. similis were recovered from heated soils and less than 700 R. similis from many of the untreated soils. The surface soil from site B was highly suppressive, as 84% fewer nematodes were recovered from this soil than from heated soil after 8 days. Soil from site C (15-17 cm) was not suppressive and the other four soils were moderately suppressive.
The main reason for undertaking this study was to better understand the factors associated with suppressiveness to plant-parasitic nematodes in sugarcane soils. Our choice of soils was deliberate, as we hoped that a group of soils with diverse properties (quite different textures and a range of soil carbon contents) would provide the greatest opportunity to determine whether particular properties (or groups of properties) were associated with suppressiveness. Numerous chemical, biochemical, and biological parameters were measured but an initial assessment of the data using Principal Component Analysis failed to identify any factor or combination of factors that explained the variability in suppressiveness that was apparent in the assay with R. similis. However, non-linear regression analysis identified two major groups of factors that were positively related to suppressiveness: one group reflecting the amount of organic matter in soil (total C, total N and labile C) and the other associated with the size of the free-living nematode community (numbers of free-living nematodes, and numbers of plant associates, bacterial feeders, fungal feeders and carnivores). Although the strength of these relationships may have been exaggerated by the fact that the data were obtained from a limited number of samples, such a result adds to the consensus (Stone et al., 2004; Ghorbani et al., 2008) that organic matter-mediated suppression is associated with regular organic inputs and high levels of biological activity.
The role of organic matter in enhancing suppressiveness to plant-parasitic nematodes is well recognized (see reviews by Muller and Gooch, 1982; Stirling, 1991; Akhtar and Malik, 2000; Oka, 2010; Thoden et al., 2011); while there is experimental evidence to indicate that crop residues enhance suppressiveness in sugarcane soils (Stirling et al., 2003; 2005). Our study adds to this body of knowledge by demonstrating that organic matter-mediated suppressiveness develops naturally in trash blanketed sugarcane soils and is sometimes effective enough near the soil surface to reduce populations of plant-parasitic nematodes to levels that cause no obvious damage to roots. The results also suggest that suppressiveness is associated with high levels of biological activity and that this activity is sustained by root exudates and soil organic matter, the energy sources that drive the soil food web. Fungi and bacteria utilise these resources; free-living nematodes respond to that food source by increasing their population densities; and in turn, they become a food source for a wide range of organisms that are parasitic, predacious or in some way antagonistic to nematodes.
Antagonists possibly associated with suppressiveness: In our attempts to determine which biological factors were associated with suppressiveness, predatory nematodes were assessed because previous work had shown that suppressiveness is related to the ratio of predators to prey and to the prevalence of omnivore and predator species (Sanchez-Moreno and Ferris, 2007). Predatory nematodes (predominantly Tripyla, with some actinolaims and mononchids) comprised between 0 and 6.9% of the nematode community in the test soils and we found a significant negative relationship between population densities of these nematodes and the number of R. similis recovered in our suppression assay. However, unlike Sanchez-Moreno and Ferris (2007), we failed to show that suppressiveness was related to numbers of omnivores, or to the two indices (structure index, SI, and maturity index, MI) that reflect the proportion of omnivores and predators in the nematode community. We suspect that the lack of a relationship with SI and MI is due to the fact that such indices do not provide information on the magnitude of ecosystem functions (Ferris, 2011). Soils with different nematode population densities may have the same proportion of predators (i.e. a similar SI and MI), but worthwhile levels of suppression are most likely to occur in soils with high numbers of predators.
We attempted to estimate the population densities of nematophagous fungi in the test soils using a standard dilution plating and most probable number technique (Persmark et al., 1996; Jaffee et al., 1998; Jaffee 2003). However, the results demonstrated the limitations of culture-based assays, as the data were of little value in exploring relationships between the presence or population density of predatory fungi and suppressiveness. Replicate plates from the same sample did not consistently yield the same fungi and the number of times a particular fungus was detected did not always decline as soil dilution rates increased. Four species of nematode-trapping fungi (A. musiformis, A. oligospora, A. thaumasia and G. gephyrophaga) were detected and the results in Table 4 suggested that they occurred more commonly at site B. Two members of the lower, non-septate fungi (Stylopage sp. and an unidentified predatory fungus observed previously by Stirling et al., (2005), were also observed, again most commonly in soil from site B. However, because of variability in the data, we could not conclude with any degree of confidence that the soil from site B had more nematophagous fungi than the other soils.
Practical considerations: Our observations indicate that organic matter-mediated suppression is occurring in Australian sugarcane soils, raising the question of whether it is possible to predict whether a soil is suppressive enough to substantially reduce the amount of damage caused by nematode pests. Our results suggest that general indicators of soil biological activity are likely to be better predictors of suppressiveness than the presence or population densities of certain groups of antagonists, and so we would argue that soil C is the simplest and most practical indicator currently available. This study included surface soils with much higher C levels than would have occurred in samples of bulk soil, so it provided some indication of the C contents of sugarcane soils that were highly suppressive to plant-parasitic nematodes. Our most suppressive soil was collected from just under the trash blanket at site B, and the C concentration in three replicate samples of this soil varied from 31 to 43 g/kg. Since this is much higher than the average C content of typical sugarcane soils in Australia (Stirling et al., 2010), most soils currently used for sugar production are not likely to be suppressive to plant-parasitic nematodes, except perhaps near the soil surface. However, data obtained from several of the sugarcane farms sampled by Stirling et al. (2010) showed that in non-tilled soils that are maintained under permanent grass pasture, soil C levels of 30-53 g/kg are achievable to a depth of 10 cm. Given the C benefits expected to accrue when the sugar industry moves to a minimum till, trash retained production system, soil C levels are expected to gradually improve over time, with suppressiveness to plant-parasitic nematodes eventually increasing to levels capable of protecting at least some part of the root system from nematode damage. The downside of this scenario is that most of the suppressive activity will be confined to upper soil layers, with roots further down the profile remaining vulnerable to nematode attack.
Molecular studies: Given the problems involved in detecting and quantifying nematode-trapping fungi using traditional dilution plating methods, we considered that TRFLP might be a suitable alternative, as it is one of several molecular methods that can be used to generate a fingerprint of an unknown microbial community. It is a relatively new, and increasingly popular, high-throughput technique for monitoring changes in the structure and composition of soil microbial communities (Thies, 2007; Schütte et al., 2008), and has recently been used to identify bacterial populations associated with plant disease suppression (Benítez et al., 2007; Benítez and McSpadden Gardener, 2009). However, we believe our study is the first to use TRFLP to investigate the structure of the Orbiliales community using specific primers. Although the principal aim of the work was to check the utility of the technique, our results were encouraging, as the number of Orbiliales TRFs and the total area of peaks in TRFLP profiles varied between sites and also between sampling depths. Since a TRF represents an operational taxonomic unit that signifies one or more species, while peak area is indicative of abundance (Schütte et al., 2008), these results suggest that there were differences in the diversity and abundance of Orbiliales between both sites and depths.
Although our TRFLP data set was too small to draw firm conclusions about relationships between the structure of the Orbiliales community and suppressiveness, the correlation between % suppression in our Radopholus bioassay and both the number of Orbiliales TRFs and the presence of certain TRFs was strong enough to suggest that further work with this technique is warranted. TRFLP is a relatively cost-effective procedure and should be utilized in a wider study of sugarcane soils to determine whether functionally important members of the Orbiliales community are consistently associated with suppressiveness to plant-parasitic nematodes.
In our study of Orbiliales-specific PCR products, we sequenced 48 clones from each site and depth because that number of clones is considered sufficient to detect the most common fungal species in soil DNA (Anderson et al., 2003; Smith and Jaffee, 2009). All of the clones were Orbiliales, confirming the specificity of the PCR primers designed by Smith and Jaffee (2009) and demonstrating that TRFLP profiles were representative of Orbiliales community structure. About 70% of the clones had sequences with high similarity to previously characterised Orbiliales species, but even within this group of clones it was sometimes difficult to assign a sequence to a particular species. The most likely reason for this is that there are many closely related species within the Orbiliales fungi and the ITS2 DNA fragment generated with Orbiliales-specific primers contains only a limited amount of genetic information, representing a relatively small region of the rDNA that is typically used in phylogenetic studies. Clearly there was a wide range of Orbiliales diversity within the samples processed, but our identifications should be regarded as tentative until the species present in sugarcane soils are better characterised using both morphological and molecular methods. A more comprehensive data base of Orbiliales sequences from diverse locations is also needed. Currently, most sequences deposited in Genbank have originated from China and North America, or have been derived from cultures held in international culture collections. This is the first contribution of molecular information on Orbiliales from Australia.
Clones from each site and depth separated into groups following ClustalW alignment, with the number of species in each sample approximating the number of peaks that accounted for most of the relative area in the corresponding TRFLP profile. Although the number of species would be expected to provide an indication of Orbiliales diversity within each sample, there was sequence variation within some groups (as indicated by the number of phylotypes), indicating that there may have been several Orbiliales strains within those groups. Further work is therefore required to confirm whether the phylotypes and groups generated from our analyses are taxonomically and biologically meaningful.
Collectively, the results of our sequencing work with cloned PCR products indicate that a diverse range of Orbiliales occur in sugarcane soils, and that they are particularly common in the soil just beneath the trash blanket. However, this does not necessarily mean that all the species that were detected are actively preying on nematodes, or that they are capable of suppressing populations of plant-parasitic species. Most predatory Orbiliales species are able to survive as saprophytes and are not therefore dependent on nematodes for nutrition, while other species can parasitise or prey on copepods, collembolans, rotifers, mites, fungi, and other soil organisms (Smith and Jaffee, 2009). It will therefore be a major task to determine whether nematodes are one of the primary food sources for these fungi in an environment where there are many potential modes of nutrition. The same applies to determining whether egg parasitism by fungi is contributing to suppressiveness. Egg-parasitic Orbiliales (e.g. Brachyphoris oviparasitica) were detected in some samples using molecular methods, and although they are known to parasitize eggs that are congregated within cysts or egg masses (Stirling and Mankau, 1978; Westphal and Becker, 2001), it is not known whether they have any impact on eggs that are laid singly in soil or root tissue. When we freed Meloidogyne eggs from the gelatinous matrix and added them to the test soils, our results suggested that individual eggs were not being parasitized.
Competitive, predacious and parasitic interactions are a feature of nematode-suppressive soils, and it will always be difficult to unravel the taxonomic and ecological complexities that exist in such an environment. DNA technologies provide new tools to assess this complexity (Borneman and Becker 2007; van Elsas et al., 2008; Buée et al., 2009 a, b), and may eventually enable us to determine whether particular groups of organisms are primarily responsible for regulating populations of free-living and plant-parasitic nematodes in a suppressive soil. Our preliminary results from sugarcane soils suggest that some nematophagous fungi may have a regulatory role, as there was a relationship between suppressiveness to plant-parasitic nematodes and the presence of a diverse Orbiliales community (as measured by the number of clone groups). This association must eventually be confirmed at a greater number of sites, but more comprehensive studies are now limited by the cost of cloning and sequencing. Also, interpretation of results from molecular studies is hampered by the dearth of Orbiliales sequence data archived in databases; the absence of information on the primary food sources of many Orbiliales, particularly species in Dactylella and related genera that do not trap nematodes; and the paucity of molecular information on non-orbiliaceous predators (e.g. fungal and oomycete endoparasites).
Literature Cited
- Ahrén D, Ursing BM, Tunlid A. Phylogeny of nematode trapping fungi based on 18S rDNA sequences. FEMS Microbiology Letters. 1998;158:179–184. doi: 10.1111/j.1574-6968.1998.tb12817.x. [DOI] [PubMed] [Google Scholar]
- Akhtar M, Malik A. Roles of organic soil amendments and soil organisms in the biological control of plant-parasitic nematodes: a review. Bioresource Technology. 2000;74:35–47. [Google Scholar]
- Anderson IC, Campbell CD, Prosser JI. Potential bias of fungal 18s rDNA and internal transcribed spacer polymerase chain reaction primers for estimating fungal biodiversity in soil. Environmental Microbiology. 2003;5:36–47. doi: 10.1046/j.1462-2920.2003.00383.x. [DOI] [PubMed] [Google Scholar]
- Benítez M- S, McSpadden-Gardener BB. Linking sequence to function in soil bacteria: sequence-directed isolation of novel bacteria contributing to soilborne disease suppression. Applied and Environmental Microbiology. 2009;75:915–924. doi: 10.1128/AEM.01296-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benítez M- S, Tustas FB, Rotenberg D, Kleinhenz MD, Cardina J, Stinner D, Miller SA, McSpadden-Gardener BB. Multiple statistical approaches of community fingerprint data reveal bacterial populations associated with general disease suppression arising from the application of different organic field management strategies. Soil Biology and Biochemistry. 2007;39:2289–2301. [Google Scholar]
- Blair BL, Stirling GR. The role of plant-parasitic nematodes in reducing yield of sugarcane in fine-textured soils in Queensland, Australia. Australian Journal of Experimental Agriculture. 2007;47:620–634. [Google Scholar]
- Blair BL, Stirling GR, Pattemore JA, Whittle PJL. Occurrence of pest nematodes in Burdekin and central Queensland sugarcane fields. Proceedings of the Australian Society of Sugarcane Technologists. 1999a;21:227–233. [Google Scholar]
- Blair BL, Stirling GR, Whittle PJL. Distribution of pest nematodes on sugarcane in south Queensland and relationship to soil texture, cultivar, crop age and region. Australian Journal of Experimental Agriculture. 1999b;39:43–49. [Google Scholar]
- Bongers T. The maturity index: an ecological measure of environmental disturbance based on nematode species composition. Oecologia. 1990;83:14–19. doi: 10.1007/BF00324627. [DOI] [PubMed] [Google Scholar]
- Borneman J, Becker JO. Identifying microorganisms involved in specific pathogen suppression in soil. Annual Review of Phytopathology. 2007;45:153–172. doi: 10.1146/annurev.phyto.45.062806.094354. [DOI] [PubMed] [Google Scholar]
- Buée M, de Boer W, Martin F, van Overbeek L, Jurkevitch E. The rhizosphere zoo: An overview of plant-associated communities of microorganisms, including phages, bacteria, archaea, and fungi, and some of their structural factors. Plant and Soil. 2009a;321:189–212. [Google Scholar]
- Buée M, Reich M, Murat C, Morin E, Nilsson RH, Uroz S, Martin F. 454 Pyrosequencing analyses of forest soils reveal an unexpectedly high fungal diversity. New Phytologist. 2009b;184:449–456. doi: 10.1111/j.1469-8137.2009.03003.x. [DOI] [PubMed] [Google Scholar]
- Cadet P, Spaull VW. 2005. Nematode parasites of sugarcane. Pp. 645–674 in M. Luc, R. A. Sikora, and J. Bridge, eds. ‘Plant Parasitic Nematodes in Subtropical and Tropical Agriculture’, 2nd edition. Wallingford: CAB International. [Google Scholar]
- Chen J, Xu L- L, Liu B, Liu X- Z. Taxonomy of Dactylella complex and Vermispora. II. The genus Dactylella. Fungal Diversity. 2007;26:85–126. [Google Scholar]
- Chen W, Hoitink HAJ, Schmitthenner AF, Tuovinen OH. The role of microbial activity in the suppression of damping-off caused by. Pythium ultimum. Phytopathology. 1988;78:314–322. [Google Scholar]
- Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris H. Form and function: metabolic footprints of nematodes in the soil food web. European Journal of Soil Biology. 2011;46:97–104. [Google Scholar]
- Ferris H, Bongers T, de Goede RGM. A framework for soil food web diagnostics: extension of the nematode faunal concept. Applied Soil Ecology. 2001;18:13–29. [Google Scholar]
- Garside AL, Bell MJ, Robotham BG, Magarey RC, Stirling GR. Managing yield decline in sugarcane cropping systems. International Sugar Journal. 2005;107:16–26. [Google Scholar]
- Ghorbani R, Wilcockson S, Koocheki A, Leifert C. Soil management for sustainable crop disease control. Environmental Chemistry Letters. 2008;6:149–162. [Google Scholar]
- Hagedorn G, Scholler M. A reevaluation of predatory orbiliaceous fungi. 1. Phylogenetic analysis using rDNA sequence data. Sydowia. 1999;51:27–48. [Google Scholar]
- Hoitink HAJ, Boehm MJ. Biocontrol within the context of soil microbial communities: a substrate-dependent phenomenon. Annual Review of Phytopathology. 1999;37:427–446. doi: 10.1146/annurev.phyto.37.1.427. [DOI] [PubMed] [Google Scholar]
- Isbell RF. 1996 The Australian Soil Classification. Melbourne: CSIRO Publishing. [Google Scholar]
- Jaffee BA. Correlations between most probable number and activity of nematode-trapping fungi. Phytopathology. 2003;93:1599–1605. doi: 10.1094/PHYTO.2003.93.12.1599. [DOI] [PubMed] [Google Scholar]
- Jaffee BA, Ferris H, Scow KM. Nematode-trapping fungi in organic and conventional cropping systems. Phytopathology. 1998;88:344–350. doi: 10.1094/PHYTO.1998.88.4.344. [DOI] [PubMed] [Google Scholar]
- Li Y, Hyde KD, Jeewon R, Cai L, Vijaykrishna D, Zhang K. Phylogenetics and evolution of nematode-trapping fungi (Orbiliales) estimated from nuclear and protein encoding genes. Mycologia. 2005;97:1034–1046. doi: 10.3852/mycologia.97.5.1034. [DOI] [PubMed] [Google Scholar]
- Mitchell RDJ, Larsen PJ. A simple method for estimating the return of nutrients in sugarcane trash. Proceedings of the Australian Society of Sugarcane Technologists. 2000;22:212–216. [Google Scholar]
- Moody PW, Yo SA, Aitken RL. Soil organic carbon, permanganate fractions and the chemical properties of acidic soils. Australian Journal of Soil Research. 1997;35:1301–1308. [Google Scholar]
- Muller R, Gooch PS. Organic amendments in nematode control. An examination of the literature. Nematropica. 1982;12:319–326. [Google Scholar]
- Oka Y. Mechanisms of nematode suppression by organic soil amendments- a review. Applied Soil Ecology. 2010;44:101–115. [Google Scholar]
- Persmark L, Banck A, Jansson H-B. Population dynamics of nematophagous fungi and nematodes in an arable soil: vertical and seasonal fluctuations. Soil Biology and Biochemistry. 1996;28:1005–1014. [Google Scholar]
- Persson YS, Erland S, Jansson H-B. Identification of nematode-trapping fungi using RFLP analysis of the PCR-amplified ITS region of ribosomal DNA. Mycological Research. 1996;100:531–534. [Google Scholar]
- Pyrowolakis A, Westphal A, Sikora RA, Becker JO. Identification of root-knot nematode suppressive soils. Applied Soil Ecology. 2002;19:51–56. [Google Scholar]
- Sánchez-Moreno S, Ferris H. Suppressive service of the soil food web: effects of environmental management. Agriculture. Ecosystems and Environment. 2007;119:75–87. [Google Scholar]
- Scholler M, Hagedorn G, Rubner A. A reevaluation of predatory orbiliaceous fungi. II. A new generic concept. Sydowia. 1999;51:89–113. [Google Scholar]
- Schnürer J, Rosswall T. Fluorescein diacetate hydrolysis as a measure of total microbial activity in soil and litter. Applied and Environmental Microbiology. 1982;43:1256–1261. doi: 10.1128/aem.43.6.1256-1261.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schütte UME, Abdo Z, Bent SJ, Shyu C, Willimas CJ, Pierson JD, Forney LJ. Advances in the use of terminal restriction fragment length polymorphism (T-RFLP) analysis of 16S rRNA genes to characterize microbial communities. Applied Microbiology and Biotechnology. 2008;80:365–380. doi: 10.1007/s00253-008-1565-4. [DOI] [PubMed] [Google Scholar]
- Smith ME, Jaffee BA. PCR primers with enhanced specificity for nematode-trapping fungi (Orbiliales) Environmental Microbiology. 2009;58:117–128. doi: 10.1007/s00248-008-9453-0. [DOI] [PubMed] [Google Scholar]
- Stirling GR. 1991 Biological control of plant-parasitic nematodes. Wallingford: CAB International. [Google Scholar]
- Stirling GR. The impact of farming systems on soil biology and soilborne diseases: examples from the Australian sugar and vegetable industries- the case for better integration of sugarcane and vegetable production and implications for future research. Australasian Plant Pathology. 2008;37:1–18. [Google Scholar]
- Stirling GR. 2011. Biological control of plant-parasitic nematodes: an ecological perspective, a review of progress and opportunities for further research. Pp. 1–38 in Y. Spiegel and K. Davies eds. Biological control of plant-parasitic nematodes: building coherence between microbial ecology and molecular mechanisms. Dordrecht: Springer. [Google Scholar]
- Stirling GR, Mankau R. Parasitism of Meloidogyne eggs by a new fungal parasite. Journal of Nematology. 1978;10:236–240. [PMC free article] [PubMed] [Google Scholar]
- Stirling GR, Halpin NV, Bell MJ. A surface mulch of crop residues enhances suppressiveness to plant-parasitic nematodes in sugarcane soils. Nematropica. 2011;41:107–119. [Google Scholar]
- Stirling GR, Moody PW, Stirling AM. The impact of an improved sugarcane farming system on chemical, biochemical and biological properties associated with soil health. Applied Soil Ecology. 2010;46:470–477. [Google Scholar]
- Stirling GR, Wilson EJ, Stirling AM, Pankhurst CE, Moody PW, Bell MJ. Organic amendments enhance biological suppression of plant-parasitic nematodes in sugarcane soils. Proceedings of the Australian Society of Sugarcane Technologists. 2003;25 CD ROM. [Google Scholar]
- Stirling GR, Wilson EJ, Stirling AM, Pankhurst CE, Moody PW, Bell MJ, Halpin N. Amendments of sugarcane trash induce suppressiveness to plant-parasitic nematodes in a sugarcane soil. Australasian Plant Pathology. 2005;34:203–211. [Google Scholar]
- Stone, A. G., Scheuerell, S. J, and Darby, H. M. 2004. Suppression of soilborne diseases in field agricultural systems: Organic matter management, cover cropping, and other cultural practices. Pp. 131–177 in F. Magdoff and R. R. Weil eds. Soil organic matter in sustainable agriculture. Boca Raton: CRC Press. [Google Scholar]
- Thies JE. Soil microbial community analysis using Terminal Restriction Fragment Length Polymorphisms. Soil Science Society of America Journal. 2007;71:579–59. [Google Scholar]
- Thoden TC, Korthals GW, Termorshuizen AJ. Organic amendments and their influences on plant-parasitic and free-living nematodes: a promising method for nematode management? Nematology. 2011;13:133–153. [Google Scholar]
- Van Elsas JD, Speksnijder AJ, van Overbeek LS. A procedure for the metagenomics exploration of disease-suppressive soils. Journal of Microbiological Methods. 2008;75:515–522. doi: 10.1016/j.mimet.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Weisburg W, Barns S, Pelletier D, Lane D. 16S ribosomal DNA amplification for phylogenetic study. Journal of Bacteriology. 1991;173:697–705. doi: 10.1128/jb.173.2.697-703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphal A. Detection and description of soils with specific nematode suppressiveness. Journal of Nematology. 2005;37:121–130. [PMC free article] [PubMed] [Google Scholar]
- Westphal A, Becker JO. Components of soil suppressiveness against Heterodera schachtii. Soil Biology and Biochemistry. 2001;33:9–16. [Google Scholar]
- White TJ, Bruns TD, Lee SB, Taylor JW. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. Pp. 315–322 in M. A. Innis, D. H Gelfand, J. J. Sninsky, and T. J. White eds. PCR protocols. A guide to methods and applications. San Diego: Academic Press. [Google Scholar]
- Whitehead AG, Hemming JR. A comparison of some quantitative methods of extracting small vermiform nematodes from soil. Annals Applied Biology. 1965;55:25–38. [Google Scholar]
- Yeates GW. Nematodes as soil indicators: functional and diversity aspects. Biology and Fertility of Soils. 2003;37:199–210. [Google Scholar]
- Yeates GW, Bongers T. Nematode diversity in agroecosystems. Agriculture. Ecosystems and Environment. 1999;74:113–135. [Google Scholar]