Abstract
Microplot experiments were conducted over two years (four growing seasons) to evaluate Meloidogyne incognita resistance in rootstocks used for grafted tomato (Solanum lycopersicum), muskmelon (Cucumis melo), and watermelon (Citrullus lanatus). Three tomato rootstocks; ‘TX301’, ‘Multifort’, and ‘Aloha’, were tested in addition to the nongrafted scion, ‘Florida-47’. Two muskmelon rootstocks; Cucumis metuliferus and ‘Tetsukabuto’ (Cucurbita maxima × Cucurbita moschata) were evaluated with the nongrafted scion ‘Athena’. Two watermelon rootstocks included ‘Emphasis’, a lagenaria-type, and an interspecific squash hybrid ‘StrongTosa’, which were grafted to the scion ‘TriX Palomar’ and planted only in the second year. Microplots were infested with M. incognita eggs in September each year. Tomatoes were planted in September followed by melons in March. In both years of the study, M. incognita juveniles (J2) in soil were similar among all tomato rootstocks, but numbers in roots were higher in the nongrafted Florida 47 than in all grafted rootstocks. In muskmelon only C. metuliferus rootstock reduced galling in nematode infested soil. Tetsukabuto did not reduce numbers of M. incognita J2 in either soil or roots either year. There were no differences in nematode numbers, galling, or plant growth parameters among the watermelon rootstocks tested. The use of resistant rootstocks has great potential for improving nematode control in the absence of soil fumigants.
Keywords: Citrullus lanatus, Cucumis melo, Cucumis metuliferus, Cucurbita moschata, grafting, Meloidogyne incognita, muskmelon, root-knot nematode, Solanum lycopersicum, ‘Tetsukabuto’, tomato, watermelon
Grafting of herbaceous seedlings, including many vegetable crops, to increase yield and control plant diseases has been practiced in East Asian countries for centuries, and has been shown to increase plant growth and yield, control pests, increase tolerance for low temperatures, and improve fruit quality (Kubota et al., 2008). Since the early 1990’s, grafting has been commonly used for tomato production in the Mediterranean region to reduce losses due to nematodes and soilborne pathogens including Meloidogyne spp., Fusarium oxysporum f.sp. lycopersici, and Verticillium dahliae (Besri, 2005).
In the U.S., many fruit and vegetable crops including watermelon (Citrullus lanatus), cucumber (Cucumis sativus), eggplant (Solanum melongena), and tomato (Solanum lycopersicum) have potential for improvement in pathogen resistance using grafting technology. Grafting horticulturally desirable scions onto resistant rootstocks can provide pathogen control, while retaining existing fruit production and quality traits that meet market standards for characteristics such as shape, size, flavor, and epiphytic disease resistance. This approach to providing multiple pathogen resistance in commercial cultivars via rootstocks may also be faster than traditional plant breeding approaches for multiple pests. Sources of resistant rootstocks are wide-ranging and include closely related species, genera, hybrids, and weeds. Sources of nematode resistant rootstocks for grafting in tomato typically have been selected from the family Solanaceae or hybrids. Root-knot nematode resistance has not yet been identified in muskmelons, Cucumis melo. However, Cucumis metuliferus and hybrids of C. melo and C. metuliferus have proven to be good candidates for root-knot nematode resistant rootstocks suitable for grafting onto commercial muskmelon cultivars (Igarashi et al., 1987, cited in Kubota et al., 2008; Sigũenza et al., 2005). Attempts to incorporate this nematode resistance into C. melo using traditional plant breeding approaches have not been successful (Chen and Adelberg, 2000). Thies et al., (2008) found that wild watermelon (Citrullus lanatus var. citroides) germplasm lines and commercial wild watermelon rootstock (C. lanatus) had significantly less galling than ‘Fiesta’ a diploid seeded watermelon, the squash hybrid rootstock Cucurbita moschata × C. maxima, and bottlegourd rootstocks. Further work by Thies et al., (2009, 2010) confirms that wild watermelon germplasm derived from C. lanatus var. citroides may be useful as rootstocks for managing root-knot nematodes in watermelon. Other commercial rootstocks available for use with seedless watermelons include a lagenaria-type ‘Emphasis’, and an interspecific squash hybrid type ‘Strong Tosa’ (Syngenta Seeds, Inc., Rogers Brand Vegetable Seeds).
Combining grafting with other approaches for nematode and pathogen control and yield enhancement is practiced in some European countries. For example in Spain and Morocco, grafted plants are used in conjunction with other strategies including alternative fumigants, solarization, and biofumigation (Besri, 2005). Previous research has shown that grafting is effective for control of Meloidogyne incognita on pepper in greenhouse trials (Kokalis-Burelle, et al., 2009). The objective of this project was to determine if different grafted rootstocks reduced M. incognita damage to tomato, muskmelon, and watermelon under field microplot conditions in Florida.
Materials and Methods
Experimental design: Microplot experiments were conducted from 2007-2009 at the USDA, ARS U.S. Horticultural Research Lab's farm in Ft. Pierce, FL. Experiments were designed to evaluate southern root-knot nematode (M. incognita) resistance in rootstocks for production of grafted tomato, muskmelon, and watermelon. Microplots were constructed of plastic drums 58 cm in diameter and 90 cm deep which were buried in soil. The bottoms of the drums were removed before burial to allow for drainage and drums were filled with existing soil (Oldsmar sand) from the site. The three tomato rootstocks ‘TX301’ (Syngenta Seeds, Wilmington, DE), ‘Multifort’ (De Ruiter Seeds, Lakewood, CO), and ‘Aloha’ (American Takii Seed, Salinas, CA) were grafted with ‘Florida 47’(Asgrow Seed, Monsanto Co., St. Louis, MO) as a scion, and were compared to nongrafted plants of Florida 47. The two muskmelon rootstocks, C. metuliferus (Trade Winds Fruit, Windsor, CA) and ‘Tetsukabuto’ (Cucurbita maxima × C. moschata) (American Takaii Seed) were grafted with ‘Athena’ (Syngenta Seeds, Inc., Rogers Brand Vegetable Seeds, Boise, ID) and compared with nongrafted plants of Athena. In 2009, two grafted watermelon rootstocks were planted to replace muskmelon in the fourth season after a freeze. The watermelon scion cultivar was ‘TriX Brand Palomar’ (Syngenta Seeds, Inc., Rogers Brand Vegetable Seeds), which was tested on its own rootstock as well as grafted onto ‘Emphasis’ (Syngenta Seeds, Inc., Rogers Brand Vegetable Seeds), and ‘Strong Tosa’ rootstocks (Syngenta Seeds, Inc., Rogers Brand Vegetable Seeds). All plants were tested with and without the addition of Meloidogyne incognita inoculum. Treatments for all tests were replicated eight times and plots were arranged in a completely randomized design.
Grafted tomato and muskmelon plants were purchased from commercial production houses for the first year of trials (Speedling, Inc., Alamo, TX). Transplants for the second year of trials for both tomato and muskmelon were produced at the USDA facility in Ft. Pierce, FL. Grafted watermelon transplants, were supplied by Rogers® Brand Vegetable Seeds (Syngenta Seeds, Inc., Boise, ID).
Nematode inoculum: Meloidogyne incognita eggs were extracted from tomato roots from greenhouse cultures with the NaOCl method (Hussey and Barker, 1973) and applied at approximately 23,000 eggs/microplot. In late September 2007, inoculum was applied to microplot soil as an egg suspension drench in 500 ml of water. Immediately following the inoculum application, each plot was lightly watered and covered with a thin layer of fresh soil. Tomatoes were then transplanted September 12, 2007 followed by muskmelons March 12, 2008 without additional nematode inoculum. The test sequence, including re-application of M. incognita inoculum, was repeated with tomatoes planted September 25, 2008 and muskmelon planted February 25, 2009. Watermelons were planted March 16, 2009. Watermelon transplants were used in addition to muskmelon in March 2009 due to a freeze that threatened muskmelon plants, and a lack of grafted muskmelon transplants available for replanting. Plots previously planted with Florida 47 tomatoes were excluded from the melon trial and the three melon rootstocks tested were randomly distributed among the remaining plots with half of each rootstock type planted in plots previously inoculated with nematodes (before tomato) and half in non-inoculated plots.
Assessments: At the end of each crop, nematode populations were assessed in soil, and plants were evaluated for root weight, stem weight, root galling, root condition, and nematodes/g root. Three soil cores were taken in each plot using a 1.75-cm internal diameter soil probe and composited. A 100-cm3 subsample was used for all soil nematode extractions. At the end of experiments, plants with roots were removed from soil, brought to the lab and evaluated. A 10 g subsample of roots was used for nematode extraction. Nematodes were extracted from both soil and roots using the Baermann funnel technique (Hooper, 1986), and second-stage juveniles (J2) were counted after approximately 72 hrs. Root condition was used as a general indicator of root disease and was assessed using a subjective scale of 0 to 4 with 0 = 0% to 20% discolored roots, 1 = 21% to 40%, 2 = 41% to 60%, 3 = 61% to 80%, and 4 = 81% to 100%. Root galling was assessed using a root gall index (Bridge and Page, 1980) based on a scale of 0 to 10, with zero representing no galls and 10 representing severe (100%) galling.
Statistical Analysis: Data were statistically analyzed using analysis of variance procedures (ANOVA), and means were separated using least significant difference (LSD) or Tukey's honestly significant difference (MSD) tests (SAS 9.1, SAS Institute, Cary, NC). Unless otherwise stated, all differences referred to in the text were significant at the 5% -level of probability.
Results
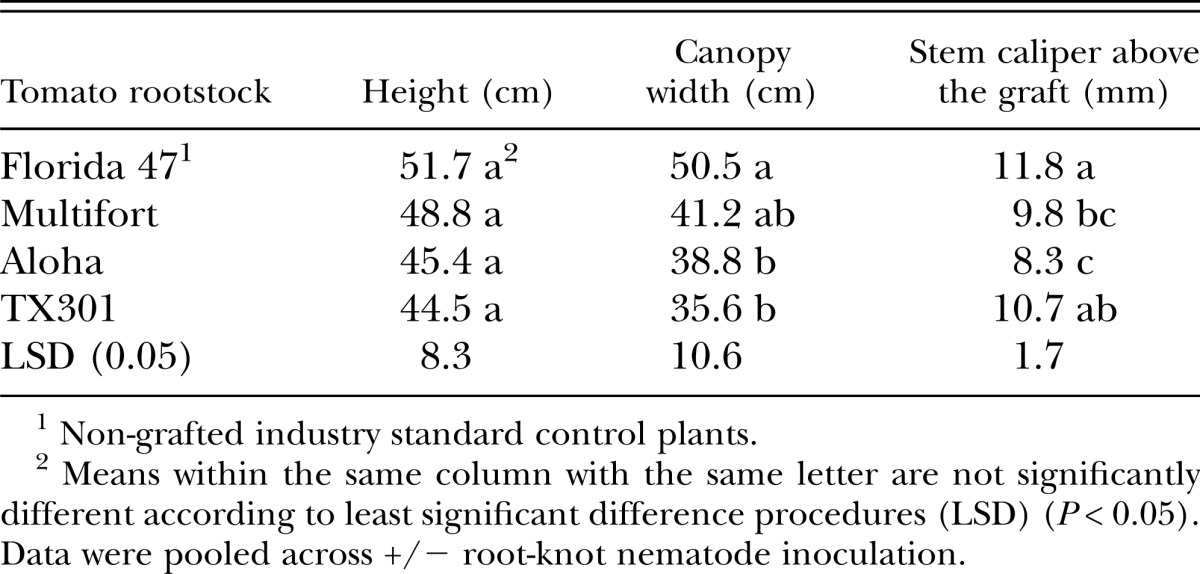
In the first tomato trial in fall of 2007, the rootstock treatments did not affect plant height, but both Aloha and TX301 rootstocks reduced plant canopy width compared to the nongrafted Florida 47 control. Tomato stems above the graft union were thinner with both Multifort and Aloha rootstocks than those of the nongrafted Florida 47 control, and plants grafted onto TX301 as a rootstock (Table 1).
Table 1.
Effect of grafted and nongrafted rootstock on tomato plant height, canopy width, and stem caliper at midseason, November 2007, 60 days after transplanting.
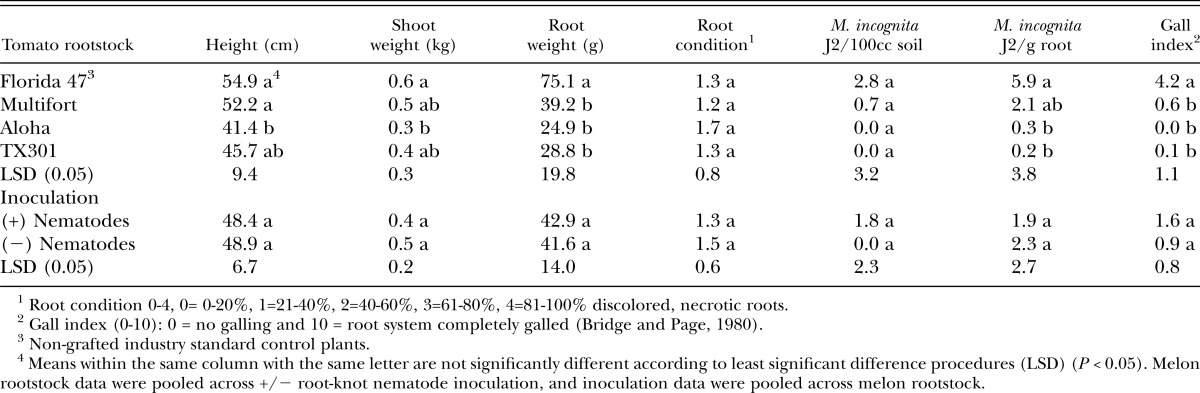
At the end of the season, nongrafted Florida 47 plants were taller and heavier than those grafted onto Aloha rootstock and had greater root mass than all grafted tomato plants. The number of M. incognita J2 in soil did not differ among plant types, but more J2 were isolated per gram of root tissue of Florida 47 than Aloha and TX301 rootstocks. Galling was greater in the nongrafted Florida 47 controls than in all grafted rootstocks (Table 2).
Table 2.
Effect of grafted and nongrafted rootstock on tomato plant growth, Meloidogyne incognita populations and root disease at the end of the season, November 2007, 76 days after transplanting.
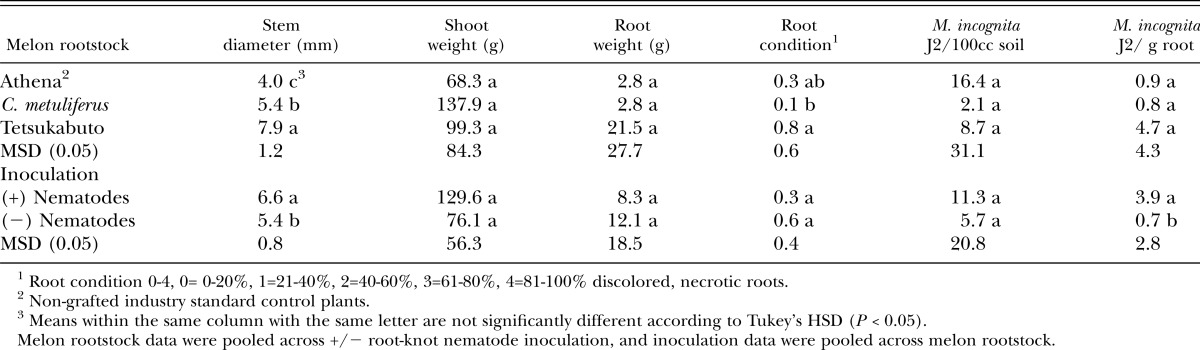
Nematodes isolated from microplot soil during the fallow period between experiments (March 10, 2008) did not differ among plots that received nematode inoculum before tomatoes were planted (data not shown). No M. incognita J2 were isolated between crops from plots that did not receive nematode inoculum. When muskmelons were planted in the spring following tomato, stem diameter was greatest with Tetsukabuto rootstock and lowest in the nongrafted Athena control (Table 3). In plots receiving nematode inoculum, stem diameter increased across all melon rootstocks compared to non-inoculated (Table 3). At harvest, shoot weight, root weight, and nematodes in soil and roots did not differ among melon rootstocks, or between inoculated and non-inoculated plants regardless of rootstock (Table 3). However, nematode inoculated soil had roots with higher numbers of J2 than non-inoculated soil. Root condition was better on C. metuliferus rootstock than Tetsukabuto and nongrafted Athena (Table 3). Expectedly, interactions occurred between nematode inoculation and galling. No differences in galling occurred among rootstocks in non-inoculated plots. However, in nematode inoculated plots, C. metuliferus had less galling than both the nongrafted Athena control and the Tetsukabuto rootstock (data not shown).
Table 3.
Effect of grafted and nongrafted rootstock on muskmelon growth, Meloidogyne incognita populations and root disease at the end of the season, May 2008, 75 days after transplanting.
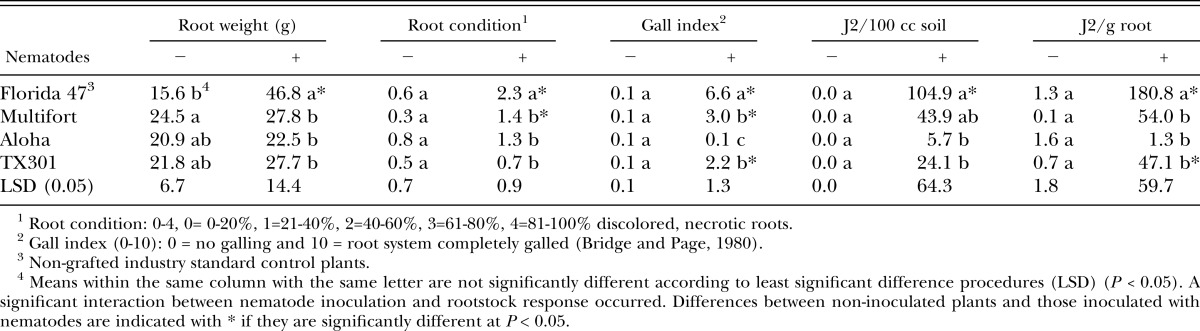
In the fall of 2008, all grafted tomato plants were taller than the nongrafted Florida 47 control (Table 4). Stem diameter was greater in Multifort, and shoot weight was higher in both TX301 and Multifort than the nongrafted control and Aloha (Table 4). Plant growth during the season did not differ between nematode-inoculated and non-inoculated plants regardless of rootstock (data not shown). However, by the end of the season, a nematode by tomato rootstock interaction occurred and consequently, data are presented separately for rootstocks from inoculated and noninoculated plots (Table 5). Without the addition of root-knot nematodes, Multifort had higher root weights than the non-grafted Florida 47 plants, however, no differences occurred in root condition, gall rate, or nematodes isolated from roots or soil in any plant types when nematodes were not added to plots (Table 5). In plots inoculated with nematodes, non-grafted Florida 47 plants had higher root weights but less healthy roots, more galling, and more M. incognita J2 extracted from both soil and roots than all other rootstocks, with the exception of Multifort with regard to nematodes isolated from soil, which did not differ from the non-grafted control (Tables 5). Rootstocks responded differently to the addition of nematode inoculum; for example in Aloha, plant growth, root disease, and nematodes isolated from roots and soil did not differ between inoculated and non-inoculated plants (Table 5). This indicates strong resistance to M. incognita in Aloha rootstock, whereas Florida 47 and TX301 had significantly more nematodes in either roots and soil, or roots in inoculated plots (Table 5).
Table 4.
Effect of grafted and nongrafted rootstock on tomato plant height, stem diameter, and shoot weight at the end of the season, December 2008, 71 days after transplanting.
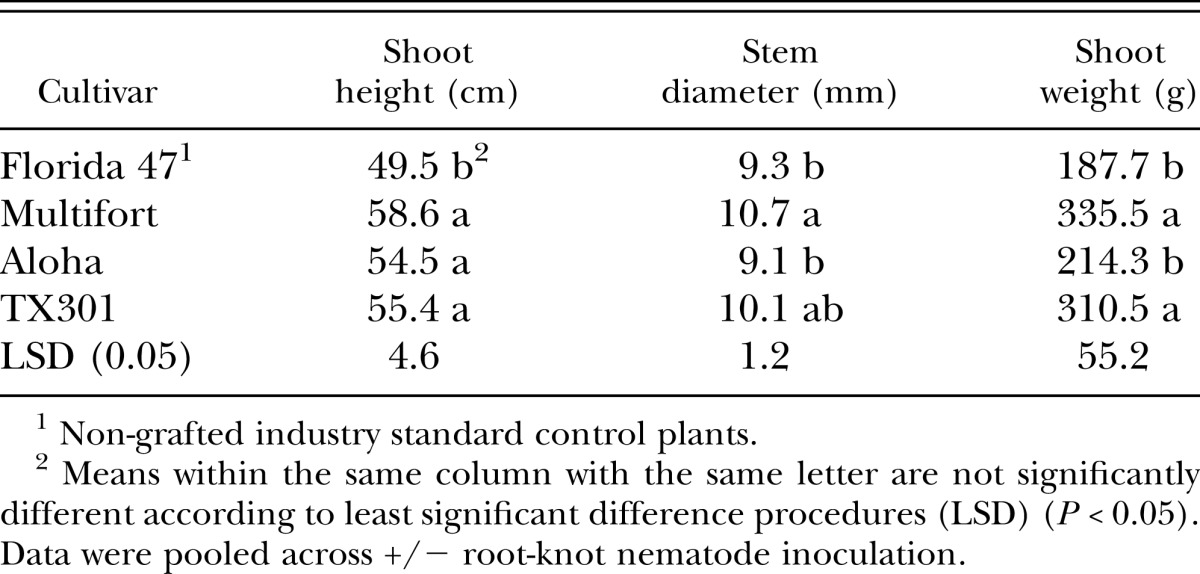
Table 5.
Effects of nematode inoculation and tomato rootstock on root growth and disease, and on Meloidogyne incognita extracted from soil and tomato roots at the end of the season, December 2008, 71 days after transplanting.
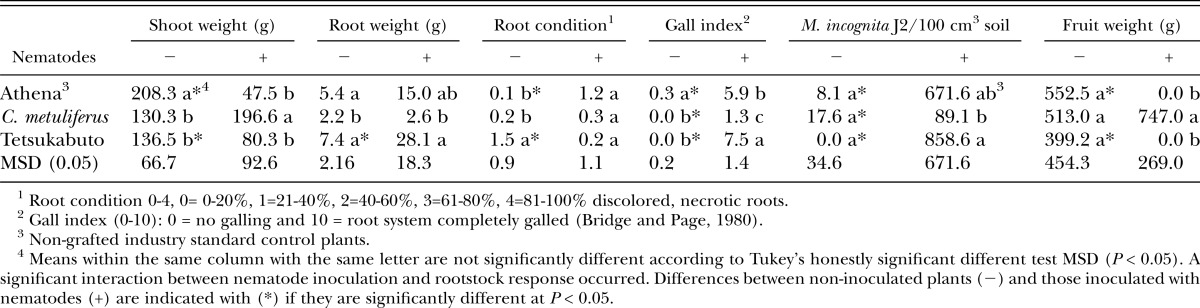
In the spring muskmelon trial of 2009, inoculation with nematodes prior to growing tomato in fall 2008 negatively impacted almost all growth and disease parameters measured, increasing disease and reducing plant growth in the nongrafted Athena and Tetsukabuto rootstock (Table 6). For the C. metuliferus rootstock, inoculation with nematodes increased isolation of nematodes from soil and galling, but did not affect any other disease, plant growth, or yield parameters measured (Table 6). Results for Tetsukabuto were mixed, with nematode inoculation increasing soil populations, galling, and root weight, while non-inoculated plants had higher shoot and fruit weight (Table 6). C. metuliferus provided a higher level of resistance to nematode population development and galling than the other rootstocks tested (Table 6). In plots not inoculated with nematodes, no differences occurred in the number of nematodes in soil, but, a low level of galling was noted on the nongrafted plants (Table 6). Root condition of Tetsukabuto rootstock was not as healthy as C. metuliferus and the nongrafted Athena control, while C. metuliferus had the lowest root weights (Table 6). Fruit weight did not differ in plots not receiving nematodes, however, in nematode inoculated plots, only C. metuliferus plots had any yield (Table 6). Shoot weight was highest in the nongrafted Athena control (Table 6). No differences occurred in root-knot nematode J2 isolated from muskmelon rootstocks at the end of the season (data not shown).
Table 6.
Nematode populations extracted from soil, and muskmelon plant growth, disease, and yield in microplots at the end of the season, May 2009, 89 days after transplanting.
Root-knot nematodes were only isolated in significant numbers from watermelon roots that were grown in inoculated plots, and galling only occurred in significant amounts in inoculated plots (data not shown). However, watermelon rootstocks did not affect plant growth or root disease. There were no differences in nematode numbers, galling, or plant growth parameters measured among the watermelon rootstocks tested (data not shown).
Discussion
These trials provide important data on the performance of several rootstocks being developed for use in grafted tomato, muskmelon, and watermelon production in soil with and without significant M. incognita population levels in Florida. In tomato, all the rootstocks tested provided good control of galling and reduced numbers of J2 of M. incognita in soil and roots to some extent. Tomato plant-growth on all rootstocks tested was comparable to the nongrafted control plants, without any noticeable reduction in growth or development. In general, grafted tomato plants resisted nematodes when they were present, and in the absence of nematodes, plant growth was similar to the nongrafted tomato cultivar. This is highlighted by the fact that, although M. incognita juvenile numbers in soil were similar, numbers in roots were higher in the non-grafted Florida 47 roots than in two of the grafted tomato rootstocks. The information presented here for tomato rootstock responses to root-knot nematodes is valuable because of the lack of data on rootstocks with potential to provide resistance for tomato production in tropical or subtropical regions where the Mi gene for root-knot nematode resistance may not function well in traditionally bred cultivars (Williamson, 1998).
In muskmelon trials, only C. metuliferus rootstock reduced galling in nematode infested soil. Under cold stress conditions in 2009, inoculation with nematodes negatively impacted almost all growth and disease parameters measured, increasing disease and reducing plant growth. Tetsukabuto did not reduce numbers of M. incognita J2 in either soil or roots in either year of the study, and should not be considered as a good rootstock for fields infested with M. incognita. These results are consistent with previous research (Igarashi et al., 1987) that evaluated several wild Cucumis species for root-knot nematode resistance and that identified Cucumis metuliferus Naud., used in our studies, as the most suitable rootstock for melon.
The watermelon rootstocks included in this study were selected based on their commercial availability, and while two of them have genetic resistance to Fusarium wilt, they had not been evaluated for nematode resistance. Unfortunately, none of the rootstocks tested provided good resistance to M. incognita under Florida conditions. However, it should be noted that watermelon was only included in one year of the study. Additional work has been conducted on watermelon with good success in identifying rootstocks for resistance to M. incognita (Thies et al., 2008, 2009, 2010).
Development of grafted scion/rootstock combinations for tomato and melons that are resistant to nematodes and also possess desirable horticultural traits can be accomplished more quickly than breeding for multiple resistance traits, including nematode resistance. Growers can be also assured that use of established cultivars as scions will meet industry standards for horticultural characteristics that meet market demands in addition to possessing nematode resistance. Overall, grafting vegetable cultivars onto resistant rootstocks appears to have potential as a practical component of a systems approach for root-knot nematode control under Florida field conditions.
Literature Cited
- Besri M. 2005. Current situation of tomato grafting as alternative to methyl bromide for tomato production in the Mediterranean region. Proceedings of the Annual International Research Conference on Methyl Bromide Alternatives and Emissions Reductions. San Diego, CA, 47-.1–47.3. [Google Scholar]
- Besri M. 2007. Current situation of tomato grafting as alternative to methyl bromide for tomato production in Morocco. Proceedings of the Annual International Research Conference on Methyl Bromide Alternatives and Emissions Reductions. San Diego, CA, 62.1–62.5. [Google Scholar]
- Bridge J, Page SLJ. Estimation of root-knot infestation levels in roots using a rating chart. Tropical Pest Management. 1980;26:296–298. [Google Scholar]
- Chen JF, Adelberg J. Interspecific hybridization in Cucumis—progress, problems, and perspectives. HortScience. 2000;35:11–15. [Google Scholar]
- Hooper DJ. Extraction of free-living stages from soil. In: Southey JF, editor. Laboratory methods for work with plant and soil nematodes. London, UK: HMSO; 1986. pp. 5–30. [Google Scholar]
- Hussey RS, Barker KR. A comparison of methods for collection inocula of Meloidogyne spp., including a new technique. Plant Disease Reporter. 1973;57:1025–1028. [Google Scholar]
- Igarashi I, Kano T, Kawabe T. Disease and pest resistance of wild Cucumis species and their compatibility as rootstock for melon, cucumber, and watermelon. Bull. Natl. Res. Inst. Veg. Ornam. Plants and Tea Japan. 1987;A1:173–185. [Google Scholar]
- Kokalis-Burelle N, Bausher MG, Rosskopf EN. Greenhouse evaluation of Capsicum rootstocks for management of Meloidogyne incognita on grafted bell pepper. Nematropica. 2009;39:121–132. [Google Scholar]
- Kubota C, McClure MA, Kokalis-Burelle N, Bausher MG, Rosskopf EN. Vegetable grafting: History, use and current technology status in North America. HortScience. 2008;43(6):1664–1669. [Google Scholar]
- Sigũenza C, Schochow M, Turini T, Ploeg A. Use of Cucumis metuliferus as a rootstock for melon to manage Meloidogyne incognita. J. Nematol. 2005;37:276–280. [PMC free article] [PubMed] [Google Scholar]
- Thies JA, Ariss J, Kousik CS, Hassell R. Grafting – a tool for managing root-knot nematodes in watermelon? Phytopathology. 2008;98:S156. [Google Scholar]
- Thies JA, Ariss J, Hassell R, Olson S. Grafting for Management of Root-Knot Nematodes in Watermelon. HortScience. 2009;44:576. [Google Scholar]
- Thies J.A, Ariss J, Hassell R, Kousik C.S, Olson S, Levi A. 2010. Grafting for Managing Southern Root-Knot Nematode, Meloidogyne incognita, in Watermelon. Plant Disease 94:1195–1199. [Google Scholar]
- Williamson VM. Root-knot nematode resistance genes in tomato and their potential for future use. Annu. Rev. Phytopathol. 1998;36:277–293. doi: 10.1146/annurev.phyto.36.1.277. [DOI] [PubMed] [Google Scholar]


