Summary
A new microcatheter-delivered, highly-flexible, fully-retrievable intracranial stent has been developed in order to facilitate the endovascular treatment of wide-necked aneurysms, though it might also prove useful for other intracranial pathology.
The nitinol stent has radiopaque proximal and distal markers, is available in a wide range of sizes and is as flexible as a micro-guidewire. It is electrolytically detached, allowing retrieval even after full deployment. The stent is compatible with all currently available embolic agents and does not degrade MR images.
Key words: stent, aneurysm, coil, interventional neuroradiology, embolization
Introduction
Stents are now increasingly used as an adjunct to coil treatment for wide-necked intracranial aneurysms1.
The ideal stent for intracranial use would be flexible, precisely delivered, retrievable, able to be repositioned, atraumatic, available in various lengths and diameters, thin-walled and radiopaque. It should provide sufficient coverage to restrain coils, while having wide enough fenestrations to permit catheterisation with coil or other embolic agent delivery catheters. The currently available over-the-wire stents are not ideal. The balloon-expandable stents of sufficient length are too stiff to be reliably and safely deployed. While existing self-expanding stents offer some improvement in this respect2, there are still serious difficulties in deploying them in distal locations and the currently available or planned stents for intracranial use are not available in the small diameters necessary for distal intracranial use.
This new stent has been specifically designed to address the shortcomings of the available over-the-wire intracranial stents.
The stent is delivered through a microcatheter, allowing standard microcatheter/wire techniques to reach locations inaccessible to over-the-wire stents. It fulfils all the criteria mentioned above. A particularly appealing characteristic is its ability to be retrieved and repositioned after complete delivery, if its position is felt to be suboptimal or if the stent proves not to be necessary. The stent conforms completely to the normal vessel geometry and is not prone to strut opening on convexities. It is compatible with all currently used embolic agents for aneurysm occlusion and is MR compatible.
Technical Specifications
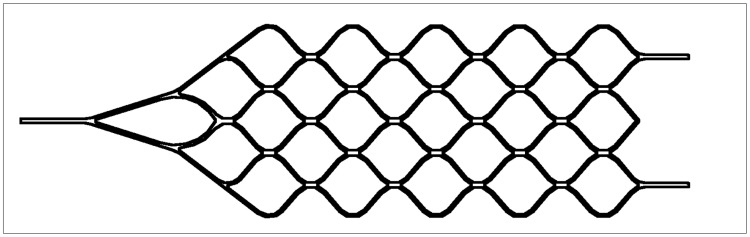
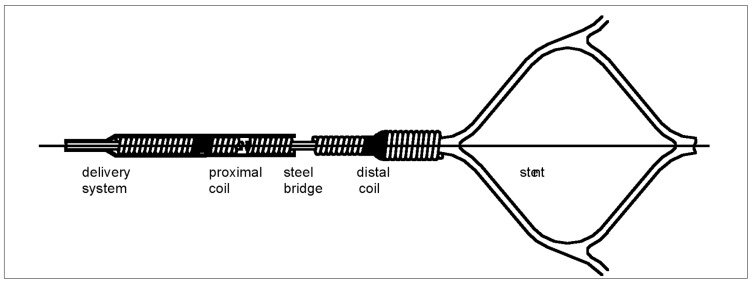
The stent is laser-cut from a nitinol plate into a honeycomb pattern (figure 1), folded, then attached to a stainless steel delivery system, from which it is electrolytically detachable using the EDC II coil-delivery system (figure 2). This system is of proven reliability and safety, with both failure of detachment and premature detachment extremely rare3.
Figure 1.
Schematic drawing of the honeycomb pattern of the self-expanding intracranial stent, laser-cut into a nitinol plate.
Figure 2.
Detachment zone (schematic drawing), used to connect the self-expanding stent to a delivery system. Two platinum coils are connected to each other through a stainless-steel-bridge. The other ends of these coils are connected to the delivery system and the stent. The steel bridge is firm enough to allow complete retrieval of the stent into the microcatheter if required. Stent detachment is accomplished by interrupting the steel bridge by the application of direct current (2 mA, 4-6 V, 30-60 sec).
The stent can be manufactured in any diameter from 2.0-7.0 mm and any length from 10-35 mm (figure 3).The wall thickness is 50-70 µm, creating minimal intrusion on the lumen of the vessel, especially important in small vessels, and potentially permitting placement of overlapping or concentric stents. The outside diameter of the stent before deployment is small enough to allow delivery through a 0.021-0.027 inch inner diameter microcatheter. Any appropriate micro-guidewire may be used to position the delivery microcatheter. The stent has radiopaque proximal and distal markers.
Figure 3.
Photograph of the self-expanding intracranial nitinol stent. Note the radiopaque distal marker.
Applications
The stent was designed with intracranial aneurysm treatment in mind. Its relatively low radial force, high flexibility and thin wall will permit its use in locations hazardous for, or inaccessible to, currently available stents. In particular, the smaller sizes will allow stent deployment across M1/M2 and A1/A2 junctions (common sites of wide-necked aneurysm, and sites where occlusion of the distal branches cannot be tolerated) without excessive deformation of the vessels, that would be extremely hazardous in the presence of an aneurysm. The larger sizes will be suitable for aneurysms of the internal carotid, vertebral and basilar arteries.
As the stent is available in small sizes, and is extremely compliant, it should also be possible to deploy “kissing stents” at bifurcation aneurysms, allowing filling of the aneurysm sac to recreate a flow divider, re-directing flow into the distal vessels rather than the aneurysm base, hopefully discouraging recurrence4.
In addition, as the stent is deployed without a wire, instead of being used to bridge the aneurysm neck, it might also be exploited to construct an artificial neck in terminal aneurysms. In this case the stent would be deployed along the long axis of the vessel of origin, into the aneurysm itself, providing a boundary between parent vessel and aneurysm sac.
While application of this technology to stenotic lesions such as dissection, atheroma or non-atheromatous focal narrowing has not been tested, there is no reason to assume that a version with modified radial force will not be effective in these settings. Preliminary testing of post-deployment angioplasty indicates that no specific difficulties should be anticipated.
Since the stent is introduced through a microcatheter and is firmly attached to its delivery system, a modification of this design might permit thrombus or foreign body retrieval, in this instance with the distal end closed to form a conical pouch and the proximal end left open. It would also be easily adapted to a filter device for procedures such as carotid or even intracranial angioplasty or stenting by producing a similar conical pouch with fine fenestrations. A detachable version of this conical device could also be used to restrain coils or balloons from distal migration when used for parent artery occlusion.
As the stent is cut from a nitinol sheet, it is possible to have non-uniform distribution of fenestrations. This offers possibilities in terms of both longitudinal and radial alteration of fenestration density to facilitate exclusion of aneurysms, without increasing unexpanded stent delivery volume. A combination of narrow fenestrations in the middle segment of the stent and two or even three such stents deployed coaxially into each other might be especially useful for the treatment of wide-necked intracranial aneurysms with liquid embolic agents5,6.
Conclusions
All currently available stents have serious limitations for intracranial use. This new, radically different stent addresses many of these deficiencies and also has several new potential applications.
References
- 1.Higashida RT, Smith W, et al. Intravascular stent and endovascular coil placement for a ruptured fusiform aneurysm of the basilar artery. Case report and review of the literature. J Neurosurg. 1997;87:944–949. doi: 10.3171/jns.1997.87.6.0944. [DOI] [PubMed] [Google Scholar]
- 2.Henkes H, Bose A, et al. Endovascular coil occlusion of intracranial aneurysms assisted by a novel self-expandable nitinol microstent (Neuroform) Interventional Neuroradiology. 2002;8:107–119. doi: 10.1177/159101990200800202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Henkes H, Drepper P, et al. Technical note on VDS. A system for the endovascular electrolytical detachment of platinum coils at variable length. Interventional Neuroradiology. 2002;8:197–200. doi: 10.1177/159101990200800212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoganathan AP, Chatzimavroudis GP. Haemodynamics. In: Lanzer P, Topol EJ, editors. PanVascular Medicine. New York - Berlin Heidelberg: Springer Verlag; 2002. pp. 138–150. [Google Scholar]
- 5.Mawad M, Cekirge S, et al. Endovascular treatment of giant and large intracranial aneurysms by using a combination of stent placement and liquid polymer injection. J Neurosurg. 2002;96:474–482. doi: 10.3171/jns.2002.96.3.0474. [DOI] [PubMed] [Google Scholar]
- 6.Klisch J, Schellhammer F, et al. Combined stent implantation and embolization with liquid 2-polyhydroxyethyl methacrylate for treatment of experimental canine widenecked aneurysms. Neuroradiology. 2002;44:503–512. doi: 10.1007/s00234-001-0761-z. [DOI] [PubMed] [Google Scholar]