Introduction
Heart disease is both common and deadly. Cardiovascular disease is a global epidemic as it is the number one cause of death worldwide and it is estimated that one in three adults in the United States have cardiovascular disease1. While a number of pioneering initiatives have transformed our treatment of cardiovascular disease, new therapies are required to further address the growing incidence of this deadly disease. Intense interest has focused on regenerative medicine as an emerging strategy for chronic diseases such as cardiovascular disease.
A number of human tissues including skin2, gut, liver3–6 and skeletal muscle3, 7 have a tremendous regenerative capacity. For example, skeletal muscle is able to completely restore its cellular architecture and function following an injury that destroys more than 80% of the muscle7, 8. This regenerative response lacks a fibroproliferative response (i.e. formation of scar) and is associated with restoration of the vasculature, myofibers and extracellular matrix. Unlike skeletal muscle, the regenerative capacity of the adult heart is more limited.
Recent studies suggest that the adult heart is capable of cellular turnover and limited regeneration following injury although the networks that govern this process are ill defined. The use of genetic mouse models and molecular biological techniques are unveiling cell populations, pathways and extracellular cues that may direct cardiac regeneration and provide a platform for further investigation. The goal of this review is to examine the endogenous regenerative capacity of the adult heart and highlight new experimental regenerative therapies aimed at restoring myocardial architecture and function.
Endogenous repair and regeneration of the metazoan heart
Previous studies have demonstrated that metazoans such as the newt and zebrafish are capable of cardiac regeneration in response to a significant injury9–12. This myocardial regenerative response is complex and occurs over a two month period. In response to a myocardial injury (amputation of 30–40% of the ventricular chamber), there is formation of a fibrin clot, subsequent dedifferentiation of cardiomyocytes and recruitment of specialized cell populations including epicardial and ventricular myocardial cell populations11–13. Importantly, the regenerative response observed in both the newt and zebrafish lacks the formation of scar13. These results support the notion that there is an inverse relationship between scar formation and myocardial regeneration (Figure 1). Moreover, the studies in these regenerative models have defined the role of Notch14, 15, fibroblast growth factor215, 16 and retinoic acid17 signaling pathways in myocardial regeneration. Examination of these regenerative organisms as well as mammalian tissues that have an enhanced regenerative capacity are instructive regarding the mechanisms and pathways that govern this repair process in response to injury.
Figure 1.
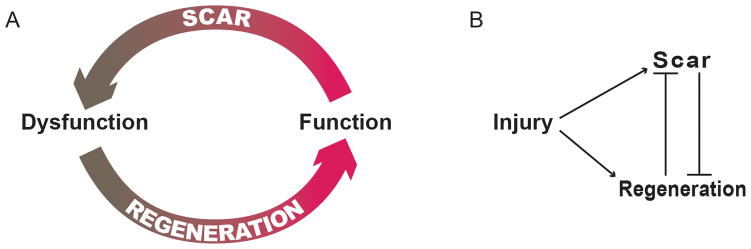
An emerging hypothesis is that the regeneration potential is linked to the fibroproliferative response of the injured tissue. A) The development of scar following injury results in tissue (cardiac) dysfunction. The ability of an injured heart to regenerate results in improvement of cardiac function. B) Models suggest there is a balance between scar formation and regeneration. These models support the notion that scar formation impairs regeneration and a regenerating tissue lacks scar.
Endogenous repair and regeneration of mammalian tissues
Every tissue is a product of stem cells and evidence suggests that essentially every adult mammalian tissue harbors a stem cell or progenitor cell population that participates in the maintenance or regeneration of their host tissue(s) in response to injury (Table 1)30. For example, the satellite cell population occupies a niche (satellite cells are sandwiched between the basal lamina and the plasmalemma in close association with the myofiber) and resides within adult skeletal muscle7, 31. The satellite cells represent the myogenic stem cell population that is quiescent in unperturbed muscle. In response to a severe injury, the quiescent satellite cells become activated (reenter the cell cycle), they proliferate and in response to cellular and extracellular cues they differentiate to form centronucleated myofibers (the hallmark of regenerated skeletal myofibers) thus restoring the cellular architecture of the injured tissue31. Importantly, the satellite cells are capable of self-renewal and reestablish their quiescent pool of myogenic stem cells32, 33. These studies emphasize the dynamic capacity of adult mammalian skeletal muscle to completely regenerate in response to injury. All striated muscle does not respond in a similar fashion to an injury.
Table 1.
Selected examples of somatic adult stem cell populations that reside in adult, mammalian tissues.
| Adult stem cell population | Niche (Tissue) | Derivatives | Reference |
|---|---|---|---|
| Bronchioalveolar Stem Cells | Lung | clara- and AT2-like cells | 18–19 |
| Bulge stem cells | Hair Follicle | hair follicles, sebaceous glands, and epidermis | 20–21 |
| Epithelial stem cells | Lining of Digestive Tract | villus columnar, mucous, entero-endocrine, and paneth cells | 22–23 |
| Hematopoietic stem cells | Bone Marrow | lymphoid (B cell, T cell, natural killer cell, and lymphoid dendritic cell lineage) and myeloid (all other lineages) blood cells | 24–25 |
| Neural stem cells | Ependymal Cell Layer | neurons, astrocytes, and oligodendrocytes | 26–27 |
| Oval cells | Liver | hepatocytes and biliary epithelium | 4–6 |
| Satellite cell | Skeletal muscle | skeletal muscle | 7, 28–29 |
Endogenous repair and regeneration of the mammalian heart
The neonatal mammalian heart is associated with considerable growth and cellular proliferation of myocardiocytes (Figure 2A). During the first week of life, the neonatal heart continues to proliferate and grow as measured by proliferative assays (BrdU pulse assays34 and tritiated thymidine assays35). This postnatal stage is also marked by increased apoptosis in the developing heart, which suggests an active modeling or sculpting process that is associated with growth and modulation by hemodynamic challenges, hormonal surges and changes occurring in extracardiac tissues. Following this postnatal period, the mammalian heart is associated with modest cellular turnover.
Figure 2.
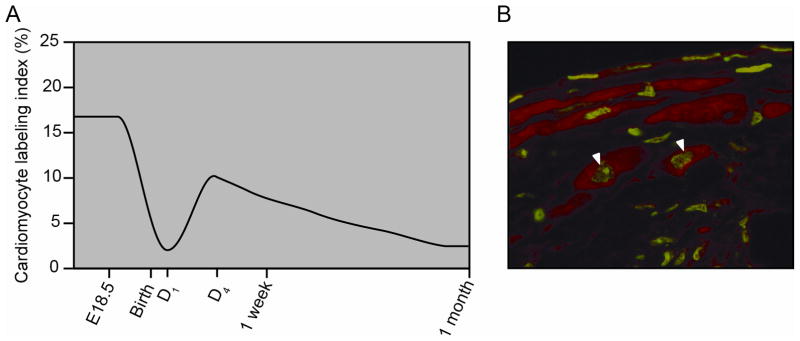
The neonatal and adult mammalian hearts are capable of cellular proliferation. A) Relative cardiomyocyte proliferation from BrdU labeling and tritiated thymidine incorporation studies of neonatal and adolescent mice support the notion that the heart’s proliferative capacity decreases with age (see references #34 and #35). B) BrdU labeling studies following injury reveal proliferation of murine cardiomyocytes in the border region of the adult mouse heart. Arrowheads mark BrdU labeled cardiomyocyte nuclei, which are green and are immunostained with α-actinin, which is red. Adapted from Naseem et al. Physiol Genomics, 2007.38 Am Physiol Soc, used with permission.
Recent studies utilizing labeling strategies and genetic mouse models suggest that the adult heart is capable of cellular replacement of cardiomyocytes, repair and limited regeneration in response to an ischemic or nonischemic injury. One study utilized radiocarbon cellular dating to examine cardiomyocyte turnover. Bergmann and colleagues relied upon the integration of carbon-14 into DNA as a measure of cardiomyocyte turnover in the adult heart36. The DNA of all organisms incorporated high concentrations of carbon-14 that were generated from nuclear bomb testing (which persisted until the 1963 Limited Nuclear Test Ban Treaty) into DNA as a measure of cardiomyocyte cellular kinetics or turnover in the adult heart. Therefore, the nuclear bomb testing and subsequent increase in atmospheric carbon-14 provided a pulse such that postnatal cellular turnover could be estimated by comparing the age of the DNA of the cardiomyocytes to the patients’ chronological age. This cellular dating technique and mathematical modeling support the notion that the adult heart is capable of cellular turnover (representing cardiomyocyte renewal) at the rate of 1% per year (although this rate decreased to 0.4% at age 75)36. The results further suggested that approximately 45% of the cardiomyocytes were generated or renewed in the 50 year old heart since birth. These studies provide an innovative strategy to examine the cardiomyocyte turnover of the adult human heart. Issues of polyploidy and origin of the cardiomyocytes remain active issues of investigation and further studies using similar strategies to examine the cardiomyocyte turnover in distinct populations are warranted. Nevertheless, these results provide further support that the adult human heart has ongoing turnover of the cardiomyocytes and support the notion that strategies to enhance this turnover may prevent the genesis of heart failure36.
A second study utilized genetic mouse models to evaluate cellular kinetics in the unperturbed and post-injured heart. Utilizing an inducible cardiomyocyte-specific transgenic fate mapping (MerCreMer) strategy in the mouse, cardiomyocytes were irreversibly labeled with the GFP reporter following the pulse of tamoxifen37. In contrast, cardiac stem cells or progenitors were not genetically labeled in response to tamoxifen as they did not express the cardiomyocyte specific marker (myosin heavy chain 6)37. This fate mapping strategy allowed for the measure of cardiomyocyte turnover in the adult mouse heart. While little to no cardiomyocyte turnover was observed in the unperturbed heart, the genetic labeling strategy following myocardial injury was associated with approximately 15% of unlabeled cardiomyocytes within the border region of injured myocardium suggesting that these cardiomyocytes had undergone cellular turnover (presumably from a progenitor or stem cell population). These genetic studies are further supported by BrdU incorporation studies, which further support the hypothesis that cardiomyocyte renewal occurs following myocardial injury (Figure 2B)38.
Collectively, these and other studies support the notion that the postnatal mammalian heart is associated with cardiomyocyte renewal (cellular turnover) which is increased following injury. These results further suggest that the amplification of resident or recruited stem cells or progenitors and regenerative myocardial pathways could increase endogenous myocardial repair of the acutely injured heart.
Resident cardiac stem and progenitor cell populations
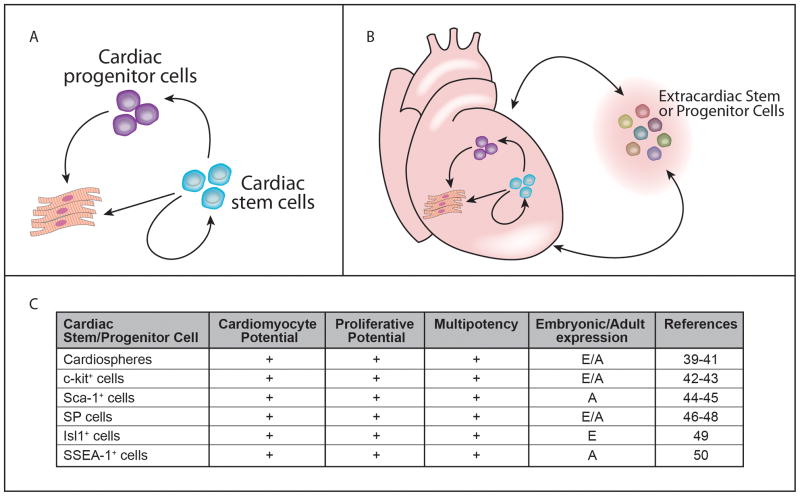
Previous studies have identified the contribution of stem cell and progenitor cell populations that are resident in the postnatal heart that are capable of generating cardiomyocytes (Figure 339–50). While a hierarchy of stem and progenitor cell populations have not been defined, a clonal c-kit+ cell population has been shown to generate all lineages of the heart, increases in number following myocardial injury, undergoes self renewal and generates cardiomyocytes in a number of mammalian models (rat, mouse, dog, etc.) including human42, 43. These c-kit expressing cells have been shown to occupy a niche within the adult heart. Using immunohistochemical studies, subpopulations of c-kit expressing cells have been identified and include those that express c-kit only versus those that coexpress c-kit with cardiac transcription factors and sarcomeric proteins. This continuum of c-kit expressing cells (with and without cardiac specific markers) has been proposed to represent a progression of cell stages from cardiac progenitor cell (CPC) to the committed progenitor cell to the immature cardiomyocyte43.
Figure 3.
The adult heart is capable of limited regeneration. A) Schematic highlighting the potential of stem cells for self renewal and their capacity to generate cardiac progenitors or cardiomyocytes. B) Schematic highlighting the possibility that extracardiac stem or progenitors can be recruited from the bone marrow, the endothelial lineage, skeletal muscle or other sources to further participate in myocardial regeneration and repair. C) Table outlining several cardiac stem or progenitors that have been identified and their characterization. E: embryonic expression; A: adult expression.
An additional cell population includes the cardiac side population (SP) cells that express multidrug resistance proteins (members of the ATP-binding cassette transporter family) and are isolated by FACS analysis based on their ability to efflux Hoechst 33342 dye46–48. These SP cells populate the heart early during development, are resident in the adult heart and increase in number within three days of cardiac injury. Using immunohistochemical techniques, these cardiac SP cells, following injury, have been shown to coexpress cardiomyocyte specific sarcomeric proteins (suggesting that the cardiac SP cells are capable of differentiating to fetal cardiomyocytes)47. Previous studies have defined that members of the ABC transporter family serve not only to mark the SP cells but also play an important cytoprotective role for these stem/progenitor cells in response to oxidative stress46. Transcriptome analysis has been useful in defining the molecular signature of cardiac SP cells that are isolated from the adult heart compared to other embryonic and somatic stem cell populations46, 47.
Studies have shown that Sca1+ cells can differentiate into cardiomyocytes44. Resident Sca1+/CD31− cardiac progenitors have been reported to increase in number and more than double fourteen days following acute myocardial infarction. These cardiac Sca1+/CD31− cells were capable of differentiation to endothelial and cardiomyocyte lineages in vitro and in vivo following the delivery into the post-injured heart. The transdifferentiation of the engrafted Sca1+/CD31−cells were accompanied by a significant improvement of left ventricular systolic function compared to controls45.
Further studies are warranted using a genetic labeling strategy (Cre-loxP technology) to fate map the c-kit, SP and Sca-1 expressing cell population during development, aging and following perturbations (including coronary artery ligation and pressure overload) using conventional and inducible genetic technologies. These fate mapping techniques will enhance our understanding of the degree to which these cell populations contribute to the renewal of cardiomyocytes and the vasculature following injury or aging.
Further cell populations that may represent a cardiac stem cell pool, progenitors (such as transit amplifying cells) or other stem cell populations that are capable of generating cardiomyocytes include EPC (endothelial progenitors)51, 52, MSC (mesenchymal stem cells)53, 54, CD34+ cells55, 56, myofibroblasts and others that have been reported to participate in cardiomyocyte renewal and regeneration. Studies support the notion that these cell populations form cardiomyocytes under permissive conditions.
Cardiac progenitors that are obtained from adult hearts (following an endomyocardial biopsy) coalesce in culture to form a three dimensional spherical structure termed a cardiosphere. Two independent laboratories have generated cardiospheres from both mouse and human biopsy specimens of adult hearts. These cardiospheres (up to 150 microns in size) have a tremendous proliferative capacity (generating more than one million cardiospheres in a one month period) and are capable of forming differentiated, contractile cardiomyocytes39, 40. Cardiospheres also represent a heterogeneous cell population with a cortex of c-kit expressing, proliferating cells and a mantle of differentiated cardiomyocytes39,40. Delivery of cardiospheres as a graft following myocardial injury resulted in improved cardiac function in rodent models and limited clinical trials are in progress to evaluate these autologous cell preparations in patients. It is unclear whether cardiospheres are derivatives of a resident cardiac stem/progenitor cell population or whether they represent reprogramming of cardiomyocytes (dedifferentiation) or progenitor cell populations. Moreover, a recent study demonstrated that transplantation of cardiosphere derived cells into the post-injured pig model further induced repair and regeneration by endogenous cardiac progenitors41.
A complementary cell population to the resident cardiac stem/progenitor cell population is the reprogrammed, induced pluripotent stem cell population (iPSC). These iPSCs have been derived from somatic cells such as skin fibroblasts through forced expression of gene expression (Oct3/4, Sox2, c-Myc and Klf4 vs. OCT4, SOX2, NANOG and LIN28) in mouse (2006)57, 58 and human (2008)59, 60. These iPSCs have been shown to mirror embryonic stem cells with regards to their proliferative capacity, pluripotency, chimera formation, teratoma formation and capacity to differentiate to all germ layer derivatives. Recent studies suggest that reprogramming is possible without genetic alteration of the somatic cell. In addition to these initiatives, the use of chemical genetics and exposure of cells to small molecules may be sufficient to direct somatic or stem cells to a cardiovascular fate61, 62. An alternative reprogramming strategy is to decipher the pathways or factors that will directly convert somatic cells (fibroblasts) to cardiomyocytes, therefore, bypassing the pluripotent state. An example of this strategy included the forced expression of three developmental transcription factors (Tbx5, Gata4 and Mef2c) to reprogram murine cardiac fibroblasts into cardiomyocyte-like cells that were similar to neonatal cardiomyocytes63. These results provide a proof of concept and rationale for future studies aimed at reprogramming human cardiac fibroblasts to a cardiomyocyte fate. This field is rapidly evolving and will provide a platform for disease specific stem cell populations, personalized stem cell populations and provide cell sources for pharmacogenetics studies.
Cell therapy for chronic diseases
The delivery of allogeneic or autologous cellular populations for the treatment of chronic diseases has been utilized for more than forty years. Since the world’s first and second successful bone marrow transplantation in 1968 at the University of Minnesota, this cellular therapy has been used to treat an array of diseases including solid tumors, mucopolysacharidoses, and hematological cancers64. In total more than 50,000 patients worldwide receive this life-saving therapy each year65. Bone marrow transplantation provides the rationale and the feasibility for using cellular therapeutic strategies for the treatment of terminal diseases such as cardiovascular disease and heart failure.
Cell therapy for cardiovascular disease
While bone marrow transplantation has been used successfully to treat terminal diseases, certain challenges need to be overcome in order to translate the use of cellular therapy to other tissues such as the heart. For example, the heart is unlike the bone marrow in that it has a highly structured cellular architecture that is electrically and functionally synchronized to produce more than 2 billion heart beats in a lifetime. Further, the working load of the heart is constantly changing and responding to local and systemic stimuli. These hemodynamic challenges provide both permissive and repressive challenges for the use of cell therapy. Third, the geometrical shape of the heart adapts in response to injury, scar, hemodynamic demand, etc. as remodeling promotes the change from a prolate ellipse to a spherical shape due to hemodynamic load. Collectively, these challenges are balanced with increasing prevalence of cardiovascular disease, decreasing donors for heart transplantation (the only definitive therapy for advanced heart failure) and a need to develop new therapies for this patient population.
As bone marrow transplantation has been an effective therapy for human diseases, studies were undertaken to examine the capacity of unfractionated bone marrow mononuclear cells (which contain hematopoietic stem cells) to transdifferentiate to a cardiomyocyte fate and improve the functional performance of the injured heart. Using genetic labeling strategies, studies demonstrated significant, limited or an absence of labeled bone marrow mononunucleated cells or stem cells to generate cardiomyocytes following the delivery into the post-injured rodent heart66–69. Despite differences in reported differentiation potential, several studies demonstrated a functional improvement in response to the delivery of hematopoietic stem cells, unfractionated bone marrow mononuclear cells and other cell populations (including fibroblasts, skeletal myoblasts, mesenchymal stem cells, endothelial progenitors, cord stem cells, etc.)69–71. Despite the variability of the results, the preclinical studies demonstrating positive results fueled the design of clinical trials using cell therapy for the treatment of cardiovascular disease (Table 2).
Table 2.
Results of selected cell therapy trials in patients with ischemic cardiomyopathies. AMI: acute myocardial infarction, STEMI: ST elevation myocardial infarction, CABG: coronary artery bypass surgery, BM: bone marrow, IC: intracoronary, IM: intramuscular, LV: left ventricle, LVESV: left ventricular end systolic volume, LVEDV: left ventricular end diastolic volume, MRI: magnetic resonance imaging, ECHO: echocardiography
| Study | No of Subjects | Design | Patients | Type of Cells | Mode of Delivery | Follow -up | Parameters measured | Modality | Improvement | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| TOP CARE- AMI | 59 | Randomized | AMI | Circulating progenitor cells Vs. BM mononuclear cells | IC | 12 months | LV Function LV ESV |
LV Angiogram MRI |
All parameters improved | 72 |
| BOOST | 60 | Randomized | STEMI | BM mononuclear cells | IC | 18 months | Infarct Size LVEF |
Stress ECHO MRI |
No change | 73 |
| REPAIR-AMI | 57 | Randomized | STEMI | BM mononuclear cells | IC | 12 months | LVEF LV EDV LV ESV |
MRI | Improvement in LVEF, LV EDV and LV ESV | 74 |
| ASTAMI | 100 | Randomized | STEMI | BM mononuclear cells | IC | 3 years | LVEF Infarct Size Exercise Capacity |
ECHO MRI |
No change in LVEF or infarct size. Slight improvement in exercise capacity. | 75 |
| MAGIC | 120 | Randomized | CABG | Autologous skeletal myoblasts | IM | 6 months | LVEF LV EDV LV ESV |
ECHO | No change in LVEF. Improvement in LV EDV and LV ESV with high dose. | 76 |
A majority of the cardiovascular clinical cell therapy trials have utilized autologous cell populations (the exception is the use of allogeneic mesenchymal stem cells), which obviate immunological mediated cellular rejection. The mode of delivery has been primarily intracoronary but has also included intramyocardial and endocardial routes. The initial nonrandomized studies delivered bone marrow mononuclear cells (intracoronary) seven days following percutaneous revascularization and observed no significant change in function (LVEF) compared to controls three months following delivery. TOPCARE-AMI was the first randomized clinical trial and compared two different cell populations (peripheral derived stem cells vs. mononucleated bone marrow cells) five days following PCI. At one year following cell delivery there was a decrease in infarct area in both patient populations72. The first randomized, controlled study (n = 60) was the BOOST trial which delivered bone marrow mononucleated cells (intracoronary) five days following PCI. While LVEF improved in those patients that received cell therapy at six months compared to controls77, no significant differences were noted at eighteen months post-delivery73. In contrast, the larger REPAIR-AMI trial (n = 204) was a randomized, controlled study and reported a modest improvement of LVEF as measured by ventriculography in patients receiving intracoronary delivery of bone marrow mononucleated cells five days post-PCI at four and twelve months follow-up compared to controls74, 78. The ASTAMI trial (n = 100) also examined the efficacy of intracoronary delivery of autologous bone marrow mononuclear cells six days post-PCI and observed no significant change in LVEF between experimental and control groups at three years post-intervention75.
Potential side effects associated with cell therapy include: arrythmyogenesis, tumorigenesis, myocardial injury, infection (bacterial or viral pathogens) or immunological responses. One example of the side effects associated with the delivery of autologous human skeletal myoblasts includes the genesis of ventricular arrhythmias and the need for implantable cardioverter defibrillator support76, 79. Another possible side effect is the immunological response to the cardiac delivery of allogeneic cell sources. While these complications are possible, very few side effects have been observed in clinical trials performed in the US and in Europe.
Collectively, these clinical trials support the conclusion that cell therapy for cardiovascular disease is relatively safe; it may modulate remodeling and may have a modest improvement in cardiac function (Table 2). Many of these clinical studies utilized ejection fraction as the only endpoint for the efficacy of these cell transfer studies which may be insufficient. Other primary or secondary endpoints for future studies may include exercise tolerance, infarct size, five year survival rate or progression to heart failure (NYHA Stages I–IV). Furthermore, future studies aimed at a mechanistic understanding of cell therapy for heart disease will be important for the advancement of this field.
Paracrine hypothesis and myocardial regeneration
An increasing number of studies suggest that cell therapy may also be associated with a bystander effect through the release of cytokines, antiapoptotic factors or growth factors which may improve cardiac function. For example, studies suggest that transplanted cells release paracrine factors that decrease program cell death (thereby limiting the remodeling process), promote angiogenesis or enhance myocardial regeneration mediated by the endogenous cardiac stem/progenitors that are resident in the adult heart80, 81. Alternatively, small molecules or growth factors such as neuregulin1 may induce cell cycle reentry and division of differentiated cardiomyocytes82. Increasing evidence supports the hypothesis that paracrine factors or the delivery of small molecules may promote myocardial repair and regeneration.
Gaps in knowledge and challenges for the future
To further examine these mechanistic questions and accelerate cell based therapies for cardiovascular disease, the National Heart, Lung and Blood Institute funded the Cardiovascular Cell Therapy Research Network (CCTRN) that includes investigators at five institutions across the US83. This network and other ongoing trials will need to collaborate with bench investigators to define the patient population that benefits from cell therapy (i.e. ischemic vs. nonischemic dilated cardiomyopathy), the optimal cell population (autologous vs. allogeneic vs. EPCs, skeletal muscle satellite cells, bone marrow mononuclear cells, CD34+ cells, MSCs, cardiospheres, etc.), mode of delivery (intracoronary, intravenous, intramyocardial, etc.), cell preparation (cultured and possibly reprogrammed vs. freshly isolated cells), numbers of cells delivered, site of delivery (infarct related artery, border region of injured myocardium, distant ventricular delivery, atrium, etc.), mechanisms of action of cell therapy (paracrine effect to limit apoptosis, promote neovascularization, promote myocardial regeneration, limit fibroproliferative response) and the role of multiple or serial interventions with cell delivery. These studies will further benefit from the design of FDA approved cell labeling strategies that will allow for the detection of single cells using imaging technologies. Moreover, cell therapy studies performed in combination with patches or scaffolds and ventricular assist devices used as a bridge to heart transplantation will allow histological analyses of the explanted heart at the time of transplant. These technologies provide new mechanistic insight regarding the use of cellular therapy for treatment of cardiac failure.
To complement these clinical trials, the National Heart, Lung and Blood Institute established the NHLBI Progenitor Cell Biology Consortium, which is a collaborative network for the exchange of reagents and acceleration of discoveries related to stem cell and progenitor cell biology84. One of the goals of this consortium is to gain an understanding of the mechanisms that direct stem and progenitor cells to a cardiac fate. Importantly, this network will provide an infrastructure for the field and will address issues including but not limited to:
The definition of a hierarchy of somatic stem and progenitor cells that reside in the adult heart.
The definition of transcriptional networks, epigenetic networks and microRNA networks that direct stem cells toward a cardiac fate.
To provide protocols for stem/progenitor cellular characterization and cardiomyocyte differentiation pathways.
To establish nonviral strategies to reprogram somatic cells to cardiomyocytes or iPSCs.
To establish fate mapping strategies to define the contribution of selected stem/progenitor cell populations to the cardiac lineage during development and following myocardial injury.
To compare specific cardiac and hematopoietic stem/progenitor cells using FACS, transcriptional, microRNA, functional or epigenetic analyses.
Together, these translational and basic science networks of investigators facilitate communication and collaborations. They further provide an infrastructure that supports ongoing and future discoveries that are intended to lead to new therapies for heart disease.
In summary, the field of cardiac regeneration has exploded with interest and opportunities. While significant advances have energized the field, further studies will be necessary to provide additional mechanisms and insights into the possibility of identifying the key(s) that will promote myocardial repair whether the strategy relies on the endogenous repair program of the heart or the use of a cell delivery program. While neither the endogenous repair program nor the cell therapy programs are ready for prime time, they will serve as a platform that will launch the field forward. Collaboration and exchange of data through professional networks should amplify and accelerate the science to move the field towards effective therapies.
Acknowledgments
Funding Sources
The authors acknowledge funding support from the NIH, National Heart Lung and Blood Institute and American Heart Association. DJG is an Established Investigator of the American Heart Association.
Footnotes
Disclosures
None
References
- 1.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Stafford R, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 2.Giangreco A, Jensen KB, Takai Y, Miyoshi J, Watt FM. Necl2 regulates epidermal adhesion and wound repair. Development. 2009;136:3505–3514. doi: 10.1242/dev.038232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goetsch SC, Hawke TJ, Gallardo TD, Richardson JA, Garry DJ. Transcriptional profiling and regulation of the extracellular matrix during muscle regeneration. Physiol Genomics. 2003;14:261–271. doi: 10.1152/physiolgenomics.00056.2003. [DOI] [PubMed] [Google Scholar]
- 4.Gennero L, Roos MA, Sperber K, Denysenko T, Bernabei P, Calisti GF, Papotti M, Cappia S, Pagni R, Aimo G, Mengozzi G, Cavallo G, Reguzzi S, Pescarmona GP, Ponzetto A. Pluripotent plasticity of stem cells and liver repopulation. Cell Biochem Funct. 2010;28:178–189. doi: 10.1002/cbf.1630. [DOI] [PubMed] [Google Scholar]
- 5.Menthena A, Deb N, Oertel M, Grozdanov PN, Sandhu J, Shah S, Guha C, Shafritz DA, Dabeva MD. Bone marrow progenitors are not the source of expanding oval cells in injured liver. Stem Cells. 2004;22:1049–1061. doi: 10.1634/stemcells.22-6-1049. [DOI] [PubMed] [Google Scholar]
- 6.Vig P, Russo FP, Edwards RJ, Tadrous PJ, Wright NA, Thomas HC, Alison MR, Forbes SJ. The sources of parenchymal regeneration after chronic hepatocellular liver injury in mice. Hepatology. 2006;43:316–324. doi: 10.1002/hep.21018. [DOI] [PubMed] [Google Scholar]
- 7.Shi X, Garry DJ. Muscle stem cells in development, regeneration, and disease. Genes Dev. 2006;20:1692–1708. doi: 10.1101/gad.1419406. [DOI] [PubMed] [Google Scholar]
- 8.Garry DJ, Meeson A, Elterman J, Zhao Y, Yang P, Bassel-Duby R, Williams RS. Myogenic stem cell function is impaired in mice lacking the forkhead/winged helix protein MNF. Proc Natl Acad Sci U S A. 2000;97:5416–5421. doi: 10.1073/pnas.100501197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bettencourt-Dias M, Mittnacht S, Brockes JP. Heterogeneous proliferative potential in regenerative adult newt cardiomyocytes. J Cell Sci. 2003;116:4001–4009. doi: 10.1242/jcs.00698. [DOI] [PubMed] [Google Scholar]
- 10.Sanchez Alvarado A. Regeneration in the metazoans: why does it happen? Bioessays. 2000;22:578–590. doi: 10.1002/(SICI)1521-1878(200006)22:6<578::AID-BIES11>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 11.Jopling C, Sleep E, Raya M, Marti M, Raya A, Belmonte JC. Zebrafish heart regeneration occurs by cardiomyocyte dedifferentiation and proliferation. Nature. 2010;464:606–609. doi: 10.1038/nature08899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kikuchi K, Holdway JE, Werdich AA, Anderson RM, Fang Y, Egnaczyk GF, Evans T, Macrae CA, Stainier DY, Poss KD. Primary contribution to zebrafish heart regeneration by gata4(+) cardiomyocytes. Nature. 2010;464:601–605. doi: 10.1038/nature08804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poss KD, Wilson LG, Keating MT. Heart regeneration in zebrafish. Science. 2002;298:2188–2190. doi: 10.1126/science.1077857. [DOI] [PubMed] [Google Scholar]
- 14.Raya A, Koth CM, Buscher D, Kawakami Y, Itoh T, Raya RM, Sternik G, Tsai HJ, Rodriguez-Esteban C, Izpisua-Belmonte JC. Activation of Notch signaling pathway precedes heart regeneration in zebrafish. Proc Natl Acad Sci U S A. 2003;100 (Suppl 1):11889–11895. doi: 10.1073/pnas.1834204100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lepilina A, Coon AN, Kikuchi K, Holdway JE, Roberts RW, Burns CG, Poss KD. A dynamic epicardial injury response supports progenitor cell activity during zebrafish heart regeneration. Cell. 2006;127:607–619. doi: 10.1016/j.cell.2006.08.052. [DOI] [PubMed] [Google Scholar]
- 16.Rosenblatt-Velin N, Lepore MG, Cartoni C, Beermann F, Pedrazzini T. FGF-2 controls the differentiation of resident cardiac precursors into functional cardiomyocytes. J Clin Invest. 2005;115:1724–1733. doi: 10.1172/JCI23418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mathew LK, Sengupta S, Franzosa JA, Perry J, La Du J, Andreasen EA, Tanguay RL. Comparative expression profiling reveals an essential role for raldh2 in epimorphic regeneration. J Biol Chem. 2009;284:33642–33653. doi: 10.1074/jbc.M109.011668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoffman AM, Shifren A, Mazan MR, Gruntman AM, Lascola KM, Nolen-Walston RD, Kim CF, Tsai L, Pierce RA, Mecham RP, Ingenito EP. Matrix modulation of compensatory lung regrowth and progenitor cell proliferation in mice. Am J Physiol Lung Cell Mol Physiol. 2010;298:L158–168. doi: 10.1152/ajplung.90594.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S, Crowley D, Bronson RT, Jacks T. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121:823–835. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 20.Sotiropoulou PA, Candi A, Mascre G, De Clercq S, Youssef KK, Lapouge G, Dahl E, Semeraro C, Denecker G, Marine JC, Blanpain C. Bcl-2 and accelerated DNA repair mediates resistance of hair follicle bulge stem cells to DNA-damage-induced cell death. Nat Cell Biol. 2010;12:572–582. doi: 10.1038/ncb2059. [DOI] [PubMed] [Google Scholar]
- 21.Wilson C, Cotsarelis G, Wei ZG, Fryer E, Margolis-Fryer J, Ostead M, Tokarek R, Sun TT, Lavker RM. Cells within the bulge region of mouse hair follicle transiently proliferate during early anagen: heterogeneity and functional differences of various hair cycles. Differentiation. 1994;55:127–136. doi: 10.1046/j.1432-0436.1994.5520127.x. [DOI] [PubMed] [Google Scholar]
- 22.Cheng H, Leblond CP. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian Theory of the origin of the four epithelial cell types. Am J Anat. 1974;141:537–561. doi: 10.1002/aja.1001410407. [DOI] [PubMed] [Google Scholar]
- 23.Gracz AD, Ramalingam S, Magness ST. Sox9 expression marks a subset of CD24-expressing small intestine epithelial stem cells that form organoids in vitro. Am J Physiol Gastrointest Liver Physiol. 2010;298:G590–600. doi: 10.1152/ajpgi.00470.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCulloch EA, Till JE. Regulatory mechanisms acting on hemopoietic stem cells. Some clinical implications. Am J Pathol. 1971;65:601–619. [PMC free article] [PubMed] [Google Scholar]
- 25.Osawa M, Hanada K, Hamada H, Nakauchi H. Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science. 1996;273:242–245. doi: 10.1126/science.273.5272.242. [DOI] [PubMed] [Google Scholar]
- 26.Johansson CB, Svensson M, Wallstedt L, Janson AM, Frisen J. Neural stem cells in the adult human brain. Exp Cell Res. 1999;253:733–736. doi: 10.1006/excr.1999.4678. [DOI] [PubMed] [Google Scholar]
- 27.Krampert M, Chirasani SR, Wachs FP, Aigner R, Bogdahn U, Yingling JM, Heldin CH, Aigner L, Heuchel R. Smad7 regulates the adult neural stem/progenitor cell pool in a TGF-{beta} and BMP-independent manner. Mol Cell Biol. 2010;30:3685–3694. doi: 10.1128/MCB.00434-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garry DJ, Yang Q, Bassel-Duby R, Williams RS. Persistent expression of MNF identifies myogenic stem cells in postnatal muscles. Dev Biol. 1997;188:280–294. doi: 10.1006/dbio.1997.8657. [DOI] [PubMed] [Google Scholar]
- 29.Mauro A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol. 1961;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weaver CV, Garry DJ. Regenerative biology: a historical perspective and modern applications. Regen Med. 2008;3:63–82. doi: 10.2217/17460751.3.1.63. [DOI] [PubMed] [Google Scholar]
- 31.Meeson AP, Shi X, Alexander MS, Williams RS, Allen RE, Jiang N, Adham IM, Goetsch SC, Hammer RE, Garry DJ. Sox15 and Fhl3 transcriptionally coactivate Foxk1 and regulate myogenic progenitor cells. EMBO J. 2007;26:1902–1912. doi: 10.1038/sj.emboj.7601635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olguin HC, Olwin BB. Pax-7 up-regulation inhibits myogenesis and cell cycle progression in satellite cells: a potential mechanism for self-renewal. Dev Biol. 2004;275:375–388. doi: 10.1016/j.ydbio.2004.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuang S, Kuroda K, Le Grand F, Rudnicki MA. Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell. 2007;129:999–1010. doi: 10.1016/j.cell.2007.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fernandez E, Siddiquee Z, Shohet RV. Apoptosis and proliferation in the neonatal murine heart. Dev Dyn. 2001;221:302–310. doi: 10.1002/dvdy.1139. [DOI] [PubMed] [Google Scholar]
- 35.Soonpaa MH, Kim KK, Pajak L, Franklin M, Field LJ. Cardiomyocyte DNA synthesis and binucleation during murine development. Am J Physiol. 1996;271:H2183–2189. doi: 10.1152/ajpheart.1996.271.5.H2183. [DOI] [PubMed] [Google Scholar]
- 36.Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabe-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, Jovinge S, Frisen J. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hsieh PC, Segers VF, Davis ME, MacGillivray C, Gannon J, Molkentin JD, Robbins J, Lee RT. Evidence from a genetic fate-mapping study that stem cells refresh adult mammalian cardiomyocytes after injury. Nat Med. 2007;13:970–974. doi: 10.1038/nm1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Naseem RH, Meeson AP, Michael Dimaio J, White MD, Kallhoff J, Humphries C, Goetsch SC, De Windt LJ, Williams MA, Garry MG, Garry DJ. Reparative myocardial mechanisms in adult C57BL/6 and MRL mice following injury. Physiol Genomics. 2007;30:44–52. doi: 10.1152/physiolgenomics.00070.2006. [DOI] [PubMed] [Google Scholar]
- 39.Messina E, De Angelis L, Frati G, Morrone S, Chimenti S, Fiordaliso F, Salio M, Battaglia M, Latronico MV, Coletta M, Vivarelli E, Frati L, Cossu G, Giacomello A. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ Res. 2004;95:911–921. doi: 10.1161/01.RES.0000147315.71699.51. [DOI] [PubMed] [Google Scholar]
- 40.Smith RR, Barile L, Cho HC, Leppo MK, Hare JM, Messina E, Giacomello A, Abraham MR, Marban E. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115:896–908. doi: 10.1161/CIRCULATIONAHA.106.655209. [DOI] [PubMed] [Google Scholar]
- 41.Johnston PV, Sasano T, Mills K, Evers R, Lee ST, Smith RR, Lardo AC, Lai S, Steenbergen C, Gerstenblith G, Lange R, Marban E. Engraftment, differentiation, and functional benefits of autologous cardiosphere-derived cells in porcine ischemic cardiomyopathy. Circulation. 2009;120:1075–1083. doi: 10.1161/CIRCULATIONAHA.108.816058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bearzi C, Rota M, Hosoda T, Tillmanns J, Nascimbene A, De Angelis A, Yasuzawa-Amano S, Trofimova I, Siggins RW, Lecapitaine N, Cascapera S, Beltrami AP, D’Alessandro DA, Zias E, Quaini F, Urbanek K, Michler RE, Bolli R, Kajstura J, Leri A, Anversa P. Human cardiac stem cells. Proc Natl Acad Sci U S A. 2007;104:14068–14073. doi: 10.1073/pnas.0706760104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 44.Oh H, Bradfute SB, Gallardo TD, Nakamura T, Gaussin V, Mishina Y, Pocius J, Michael LH, Behringer RR, Garry DJ, Entman ML, Schneider MD. Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proc Natl Acad Sci U S A. 2003;100:12313–12318. doi: 10.1073/pnas.2132126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang X, Hu Q, Nakamura Y, Lee J, Zhang G, From AH, Zhang J. The role of the sca-1+/CD31- cardiac progenitor cell population in postinfarction left ventricular remodeling. Stem Cells. 2006;24:1779–1788. doi: 10.1634/stemcells.2005-0386. [DOI] [PubMed] [Google Scholar]
- 46.Martin CM, Ferdous A, Gallardo T, Humphries C, Sadek H, Caprioli A, Garcia JA, Szweda LI, Garry MG, Garry DJ. Hypoxia-inducible factor-2alpha transactivates Abcg2 and promotes cytoprotection in cardiac side population cells. Circ Res. 2008;102:1075–1081. doi: 10.1161/CIRCRESAHA.107.161729. [DOI] [PubMed] [Google Scholar]
- 47.Martin CM, Meeson AP, Robertson SM, Hawke TJ, Richardson JA, Bates S, Goetsch SC, Gallardo TD, Garry DJ. Persistent expression of the ATP-binding cassette transporter, Abcg2, identifies cardiac SP cells in the developing and adult heart. Dev Biol. 2004;265:262–275. doi: 10.1016/j.ydbio.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 48.Pfister O, Mouquet F, Jain M, Summer R, Helmes M, Fine A, Colucci WS, Liao R. CD31- but Not CD31+ cardiac side population cells exhibit functional cardiomyogenic differentiation. Circ Res. 2005;97:52–61. doi: 10.1161/01.RES.0000173297.53793.fa. [DOI] [PubMed] [Google Scholar]
- 49.Moretti A, Caron L, Nakano A, Lam JT, Bernshausen A, Chen Y, Qyang Y, Bu L, Sasaki M, Martin-Puig S, Sun Y, Evans SM, Laugwitz KL, Chien KR. Multipotent embryonic isl1+ progenitor cells lead to cardiac, smooth muscle, and endothelial cell diversification. Cell. 2006;127:1151–1165. doi: 10.1016/j.cell.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 50.Ott HC, Matthiesen TS, Brechtken J, Grindle S, Goh SK, Nelson W, Taylor DA. The adult human heart as a source for stem cells: repair strategies with embryonic-like progenitor cells. Nat Clin Pract Cardiovasc Med. 2007;4 (Suppl 1):S27–39. doi: 10.1038/ncpcardio0771. [DOI] [PubMed] [Google Scholar]
- 51.Condorelli G, Borello U, De Angelis L, Latronico M, Sirabella D, Coletta M, Galli R, Balconi G, Follenzi A, Frati G, Cusella De Angelis MG, Gioglio L, Amuchastegui S, Adorini L, Naldini L, Vescovi A, Dejana E, Cossu G. Cardiomyocytes induce endothelial cells to trans-differentiate into cardiac muscle: implications for myocardium regeneration. Proc Natl Acad Sci U S A. 2001;98:10733–10738. doi: 10.1073/pnas.191217898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Badorff C, Brandes RP, Popp R, Rupp S, Urbich C, Aicher A, Fleming I, Busse R, Zeiher AM, Dimmeler S. Transdifferentiation of blood-derived human adult endothelial progenitor cells into functionally active cardiomyocytes. Circulation. 2003;107:1024–1032. doi: 10.1161/01.cir.0000051460.85800.bb. [DOI] [PubMed] [Google Scholar]
- 53.Makino S, Fukuda K, Miyoshi S, Konishi F, Kodama H, Pan J, Sano M, Takahashi T, Hori S, Abe H, Hata J, Umezawa A, Ogawa S. Cardiomyocytes can be generated from marrow stromal cells in vitro. J Clin Invest. 1999;103:697–705. doi: 10.1172/JCI5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang YJ, Qian HY, Huang J, Li JJ, Gao RL, Dou KF, Yang GS, Willerson JT, Geng YJ. Combined therapy with simvastatin and bone marrow-derived mesenchymal stem cells increases benefits in infarcted swine hearts. Arterioscler Thromb Vasc Biol. 2009;29:2076–2082. doi: 10.1161/ATVBAHA.109.189662. [DOI] [PubMed] [Google Scholar]
- 55.Zhang S, Wang D, Estrov Z, Raj S, Willerson JT, Yeh ET. Both cell fusion and transdifferentiation account for the transformation of human peripheral blood CD34-positive cells into cardiomyocytes in vivo. Circulation. 2004;110:3803–3807. doi: 10.1161/01.CIR.0000150796.18473.8E. [DOI] [PubMed] [Google Scholar]
- 56.Losordo DW, Schatz RA, White CJ, Udelson JE, Veereshwarayya V, Durgin M, Poh KK, Weinstein R, Kearney M, Chaudhry M, Burg A, Eaton L, Heyd L, Thorne T, Shturman L, Hoffmeister P, Story K, Zak V, Dowling D, Traverse JH, Olson RE, Flanagan J, Sodano D, Murayama T, Kawamoto A, Kusano KF, Wollins J, Welt F, Shah P, Soukas P, Asahara T, Henry TD. Intramyocardial transplantation of autologous CD34+ stem cells for intractable angina: a phase I/IIa double-blind, randomized controlled trial. Circulation. 2007;115:3165–3172. doi: 10.1161/CIRCULATIONAHA.106.687376. [DOI] [PubMed] [Google Scholar]
- 57.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 58.Meissner A, Wernig M, Jaenisch R. Direct reprogramming of genetically unmodified fibroblasts into pluripotent stem cells. Nat Biotechnol. 2007;25:1177–1181. doi: 10.1038/nbt1335. [DOI] [PubMed] [Google Scholar]
- 59.Park IH, Zhao R, West JA, Yabuuchi A, Huo H, Ince TA, Lerou PH, Lensch MW, Daley GQ. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141–146. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- 60.Zhang J, Wilson GF, Soerens AG, Koonce CH, Yu J, Palecek SP, Thomson JA, Kamp TJ. Functional cardiomyocytes derived from human induced pluripotent stem cells. Circ Res. 2009;104:e30–41. doi: 10.1161/CIRCRESAHA.108.192237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sadek H, Hannack B, Choe E, Wang J, Latif S, Garry MG, Garry DJ, Longgood J, Frantz DE, Olson EN, Hsieh J, Schneider JW. Cardiogenic small molecules that enhance myocardial repair by stem cells. Proc Natl Acad Sci U S A. 2008;105:6063–6068. doi: 10.1073/pnas.0711507105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Willems E, Bushway PJ, Mercola M. Natural and synthetic regulators of embryonic stem cell cardiogenesis. Pediatr Cardiol. 2009;30:635–642. doi: 10.1007/s00246-009-9409-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ieda M, Fu JD, Delgado-Olguin P, Vedantham V, Hayashi Y, Bruneau BG, Srivastava D. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. 2010;142:375–386. doi: 10.1016/j.cell.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Good RA. Cellular immunology in a historical perspective. Immunol Rev. 2002;185:136–158. doi: 10.1034/j.1600-065x.2002.18513.x. [DOI] [PubMed] [Google Scholar]
- 65.Goldman JM, Horowitz MM. The international bone marrow transplant registry. Int J Hematol. 2002;76 (Suppl 1):393–397. doi: 10.1007/BF03165291. [DOI] [PubMed] [Google Scholar]
- 66.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 67.Balsam LB, Wagers AJ, Christensen JL, Kofidis T, Weissman IL, Robbins RC. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004;428:668–673. doi: 10.1038/nature02460. [DOI] [PubMed] [Google Scholar]
- 68.Murry CE, Soonpaa MH, Reinecke H, Nakajima H, Nakajima HO, Rubart M, Pasumarthi KB, Virag JI, Bartelmez SH, Poppa V, Bradford G, Dowell JD, Williams DA, Field LJ. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428:664–668. doi: 10.1038/nature02446. [DOI] [PubMed] [Google Scholar]
- 69.Yoon YS, Wecker A, Heyd L, Park JS, Tkebuchava T, Kusano K, Hanley A, Scadova H, Qin G, Cha DH, Johnson KL, Aikawa R, Asahara T, Losordo DW. Clonally expanded novel multipotent stem cells from human bone marrow regenerate myocardium after myocardial infarction. J Clin Invest. 2005;115:326–338. doi: 10.1172/JCI22326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Perin EC, Dohmann HF, Borojevic R, Silva SA, Sousa AL, Silva GV, Mesquita CT, Belem L, Vaughn WK, Rangel FO, Assad JA, Carvalho AC, Branco RV, Rossi MI, Dohmann HJ, Willerson JT. Improved exercise capacity and ischemia 6 and 12 months after transendocardial injection of autologous bone marrow mononuclear cells for ischemic cardiomyopathy. Circulation. 2004;110:II213–218. doi: 10.1161/01.CIR.0000138398.77550.62. [DOI] [PubMed] [Google Scholar]
- 71.Hutcheson KA, Atkins BZ, Hueman MT, Hopkins MB, Glower DD, Taylor DA. Comparison of benefits on myocardial performance of cellular cardiomyoplasty with skeletal myoblasts and fibroblasts. Cell Transplant. 2000;9:359–368. doi: 10.1177/096368970000900307. [DOI] [PubMed] [Google Scholar]
- 72.Schachinger V, Assmus B, Britten MB, Honold J, Lehmann R, Teupe C, Abolmaali ND, Vogl TJ, Hofmann WK, Martin H, Dimmeler S, Zeiher AM. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction: final one-year results of the TOPCARE-AMI Trial. J Am Coll Cardiol. 2004;44:1690–1699. doi: 10.1016/j.jacc.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 73.Meyer GP, Wollert KC, Lotz J, Steffens J, Lippolt P, Fichtner S, Hecker H, Schaefer A, Arseniev L, Hertenstein B, Ganser A, Drexler H. Intracoronary bone marrow cell transfer after myocardial infarction: eighteen months’ follow-up data from the randomized, controlled BOOST (BOne marrOw transfer to enhance ST-elevation infarct regeneration) trial. Circulation. 2006;113:1287–1294. doi: 10.1161/CIRCULATIONAHA.105.575118. [DOI] [PubMed] [Google Scholar]
- 74.Schachinger V, Assmus B, Erbs S, Elsasser A, Haberbosch W, Hambrecht R, Yu J, Corti R, Mathey DG, Hamm CW, Tonn T, Dimmeler S, Zeiher AM. Intracoronary infusion of bone marrow-derived mononuclear cells abrogates adverse left ventricular remodelling post-acute myocardial infarction: insights from the reinfusion of enriched progenitor cells and infarct remodelling in acute myocardial infarction (REPAIR-AMI) trial. Eur J Heart Fail. 2009;11:973–979. doi: 10.1093/eurjhf/hfp113. [DOI] [PubMed] [Google Scholar]
- 75.Beitnes JO, Hopp E, Lunde K, Solheim S, Arnesen H, Brinchmann JE, Forfang K, Aakhus S. Long-term results after intracoronary injection of autologous mononuclear bone marrow cells in acute myocardial infarction: the ASTAMI randomised, controlled study. Heart. 2009;95:1983–1989. doi: 10.1136/hrt.2009.178913. [DOI] [PubMed] [Google Scholar]
- 76.Menasche P, Alfieri O, Janssens S, McKenna W, Reichenspurner H, Trinquart L, Vilquin JT, Marolleau JP, Seymour B, Larghero J, Lake S, Chatellier G, Solomon S, Desnos M, Hagege AA. The Myoblast Autologous Grafting in Ischemic Cardiomyopathy (MAGIC) trial: first randomized placebo-controlled study of myoblast transplantation. Circulation. 2008;117:1189–1200. doi: 10.1161/CIRCULATIONAHA.107.734103. [DOI] [PubMed] [Google Scholar]
- 77.Wollert KC, Meyer GP, Lotz J, Ringes-Lichtenberg S, Lippolt P, Breidenbach C, Fichtner S, Korte T, Hornig B, Messinger D, Arseniev L, Hertenstein B, Ganser A, Drexler H. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet. 2004;364:141–148. doi: 10.1016/S0140-6736(04)16626-9. [DOI] [PubMed] [Google Scholar]
- 78.Dill T, Schachinger V, Rolf A, Mollmann S, Thiele H, Tillmanns H, Assmus B, Dimmeler S, Zeiher AM, Hamm C. Intracoronary administration of bone marrow-derived progenitor cells improves left ventricular function in patients at risk for adverse remodeling after acute ST-segment elevation myocardial infarction: results of the Reinfusion of Enriched Progenitor cells And Infarct Remodeling in Acute Myocardial Infarction study (REPAIR-AMI) cardiac magnetic resonance imaging substudy. Am Heart J. 2009;157:541–547. doi: 10.1016/j.ahj.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 79.Menasche P, Hagege AA, Vilquin JT, Desnos M, Abergel E, Pouzet B, Bel A, Sarateanu S, Scorsin M, Schwartz K, Bruneval P, Benbunan M, Marolleau JP, Duboc D. Autologous skeletal myoblast transplantation for severe postinfarction left ventricular dysfunction. J Am Coll Cardiol. 2003;41:1078–1083. doi: 10.1016/s0735-1097(03)00092-5. [DOI] [PubMed] [Google Scholar]
- 80.Chimenti I, Smith RR, Li TS, Gerstenblith G, Messina E, Giacomello A, Marban E. Relative roles of direct regeneration versus paracrine effects of human cardiosphere-derived cells transplanted into infarcted mice. Circ Res. 2010;106:971–980. doi: 10.1161/CIRCRESAHA.109.210682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Maxeiner H, Krehbiehl N, Muller A, Woitasky N, Akinturk H, Muller M, Weigand MA, Abdallah Y, Kasseckert S, Schreckenberg R, Schluter KD, Wenzel S. New insights into paracrine mechanisms of human cardiac progenitor cells. Eur J Heart Fail. 2010;12:730–737. doi: 10.1093/eurjhf/hfq063. [DOI] [PubMed] [Google Scholar]
- 82.Bersell K, Arab S, Haring B, Kuhn B. Neuregulin1/ErbB4 signaling induces cardiomyocyte proliferation and repair of heart injury. Cell. 2009;138:257–270. doi: 10.1016/j.cell.2009.04.060. [DOI] [PubMed] [Google Scholar]
- 83.Traverse JH, Henry TD, Vaughan DE, Ellis SG, Pepine CJ, Willerson JT, Zhao DX, Piller LB, Penn MS, Byrne BJ, Perin EC, Gee AP, Hatzopoulos AK, McKenna DH, Forder JR, Taylor DA, Cogle CR, Olson RE, Jorgenson BC, Sayre SL, Vojvodic RW, Gordon DJ, Skarlatos SI, Moye LA, Simari RD. Rationale and design for TIME: A phase II, randomized, double-blind, placebo-controlled pilot trial evaluating the safety and effect of timing of administration of bone marrow mononuclear cells after acute myocardial infarction. Am Heart J. 2009;158:356–363. doi: 10.1016/j.ahj.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.NHLBI Awards $170 Million to Fund Stem Cell Research. NIH News. Available at: http://public.nhlbi.nih.gov/newsroom/home/GetPressRelease.aspx?id=2664.