Abstract
Amnesic patients with early and seemingly isolated hippocampal injury show relatively normal recognition memory scores. The cognitive profile of these patients raises the possibility that this recognition performance is maintained mainly by stimulus familiarity in the absence of recollection of contextual information. Here we report electrophysiological data on the status of recognition memory in one of the patients, Jon. Jon's recognition of studied words lacks the event-related potential (ERP) index of recollection, viz., an increase in the late positive component (500–700 ms), under conditions that elicit it reliably in normal subjects. On the other hand, a decrease of the ERP amplitude between 300 and 500 ms, also reliably found in normal subjects, is well preserved. This so-called N400 effect has been linked to stimulus familiarity in previous ERP studies of recognition memory. In Jon, this link is supported by the finding that his recognized and unrecognized studied words evoked topographically distinct ERP effects in the N400 time window. These data suggest that recollection is more dependent on the hippocampal formation than is familiarity, consistent with the view that the hippocampal formation plays a special role in episodic memory, for which recollection is so critical.
Keywords: hippocampus, episodic memory, event-related potentials, amnesia
It is now clear from lesion studies in humans (1–3) and animals (4) that bilateral injury to selective components of the medial temporal lobe (MTL) is associated with limited forms of memory dysfunction. Understanding the nature of these limited memory impairments holds the promise of establishing selective structure/function relationships within the MTL. The key issue here is whether the hippocampal formation and the parahippocampal region of the MTL serve qualitatively distinct functions in cognitive memory or whether they act within a unitary MTL memory system (5–7). Progress on this issue is hampered by ambiguities regarding what the limited impairments tell us about the separable functions composing cognitive memory. Jon's case (1) is a striking example. He has sustained early and relatively isolated bilateral hippocampal injury and his parahippocampal region (comprising the entorhinal, perirhinal, and parahippocampal cortices) is seemingly intact. Although severely disabled in remembering daily experiences and events, he displays a relatively normal memory for facts that is apparent in a rich vocabulary as well as a high level of both abstract and concrete knowledge of the world, and, most importantly for the current investigation, he shows relatively normal recognition performance (1, 8).
Cognitive models of recognition, including Tulving's theory of episodic memory (9) and the dual-process model (10, 11), converge on the concept that normal recognition has two qualitatively different bases. Recollection-based recognition, or “remembering,” is accompanied by contextual information about the episode in which an item was encountered, whereas familiarity-based recognition, or “knowing,” is devoid of such information (12). There is abundant experimental evidence from cognitive studies that recollection (or remembering) and familiarity-based judgments (or knowing) cannot be reduced to a quantitative difference, but, instead, reflect two qualitatively different aspects of recognition memory (11, 13). Amnesic patients with MTL damage that is not restricted to the hippocampal formation show an impairment of recognition memory that extends to both its recollection and familiarity aspects (11). On the other hand, Jon's cognitive memory profile together with data from a recent metaanalysis of several studies (2) suggest that if MTL injury is limited to the hippocampal formation, recognition memory is relatively spared, although such relative sparing might be less visible in adult onset hippocampal injury (14) as opposed to early hippocampal injury. One interpretation of these findings is that, in isolated hippocampal injury, recollection is severely impaired but the parahippocampal region allows maintenance of recognition on the basis of familiarity. An alternative possibility is that both recollection and familiarity show equivalent but limited impairment that is not severe enough to affect recognition performance substantially. The first interpretation implies that the hippocampal formation and the parahippocampal region serve qualitatively distinct functions in cognitive memory; the former is critical only for recollection, whereas the latter is sufficient to sustain familiarity. The second interpretation is compatible instead with the view that the hippocampal formation and the parahippocampal region serve a unitary declarative memory function, which contributes equally to recollection and familiarity.
Here, we sought to assess these differing possibilities by measuring Jon's event-related potentials (ERPs) while he performed a visual word recognition task, with the goal of providing an electrophysiological basis for interpreting his memory problems. ERP evidence suggests that qualitatively distinct brain activity patterns are associated with recollection and familiarity. Recollecting recognized words, a form of retrieval that is enhanced by deep encoding during study (15), causes an increase in ERP positivity over left parietal scalp areas between 500 and 700 ms after the onset of word presentation (16–18), a shift that is sometimes referred to as the late positive component, or LPC, effect. On the other hand, familiarity-based recognition, commonly associated with shallow encoding (15), causes an earlier and more frontally distributed positive shift in the ERPs, between 300 and 500 ms (18), referred to as the N400 effect by some authors (19, 20). The temporal and topographical dissociation of the two ERP effects suggests that they are generated by different neuronal populations (18, 20). It is notable that in two investigations, these distinct ERP effects were found under conditions that required simple recognition judgments of studied words. Recollection and familiarity were manipulated by using deep and shallow processing during study in one investigation (18), and the repetition of the studied word or its plural in the other (21). Importantly, the link between the frontocentral N400 effect and familiarity could be established under conditions where studied but unrecognized words elicited an N400 effect that was topographically dissociable from the one elicited by correct recognition (18). This topographic dissociation suggests that different neural generators also underlie the ERP effects of familiarity and implicit memory. [It should be noted that implicit memory measured by studied but unrecognized words yields a somewhat different ERP effect from that measured by word-form priming, where the ERP modulation occurs slightly earlier and with a slightly different topography (22); whether these are two different types of implicit memory, and, if so, whether one is a better measure of implicit memory than the other, is currently unknown.]
A somewhat different picture concerning recollection and familiarity emerged in so-called source memory paradigms, where recollection is conceptualized as recognition with correct source information (for instance, the gender of the person who presented the word) and familiarity, as recognition with erroneous or absent source information (23). Thus defined, only quantitative amplitude differences between ERPs to recollection and familiarity have so far been reported (24). One explanation for this lack of a qualitative ERP difference might be that false source judgments can be associated with the recollection of events other than those inquired about in the source question (noncriterial recollection) (25). In this situation a recollective experience would be classified as familiarity-based. Another explanation might be that even erroneous source judgments can be accompanied by a recollective experience (“false memory”) that is electrophysiologically very similar to the recollection of correct source information (19, 26).
On the basis of the reported ERP evidence, we investigated Jon's recognition memory with a study–test paradigm that resembled the simple word recognition paradigms in which recollection, familiarity, and implicit memory were reported to have dissociable ERP correlates (18, 21). We refrained from using a Remember/Know procedure because it was difficult to make the distinction between remembering and knowing sufficiently clear to Jon (8). It should be noted that although we refer to the ERP modulations in the time window between 300 and 500 ms as N400 effects, we do not thereby mean to imply that processes related to recognition and nonrecognition of studied words account for all of the functional underpinnings of the N400 component. A large body of data obtained with language paradigms shows that processes in the N400 time window are also sensitive to the semantic context in which words are presented (for a review see ref. 27). However, the modulations of the N400 component evoked by semantic congruity have a right hemispheric scalp distribution that differs from the frontocentral ERP effects that have been related to familiarity (28). In amnesia, such modulations and the effects on them of repetition are preserved even in the absence of conscious recognition (28). The paradigms used to assess semantic congruity effects on the N400 are different from recognition memory paradigms used to assess familiarity (as used here), and it is currently unclear how the two effects are related to each other.
Methods
Participants.
Jon, who is 23 years old, a native speaker of English, and right-handed, and two right-handed healthy males (24 and 26 years old, native speakers of English), matched to Jon in IQ (Verbal, Performance, and Full Scale IQ scores of Jon are 108, 120, and 114, and those of the two controls are 107, 120, and 114 and 97, 108, and 101, respectively) and sociocultural background, participated in the investigation. The other participants were 10 healthy university students, all right-handed, and all native speakers of German (5 males and 5 females; mean age 22.4 years, range 19–32 years). For simplicity in the following description of the methods, the study of Jon and his two controls will be referred to as Study 1 (with experiments 1 and 2), and that of the university students, as Study 2 (with experiment 3).
Stimuli.
In Study 1, the stimuli were 1,360 English nouns, 4–9 letters in length (mean, 5.7), with a mean frequency of 46 per million (29). Of these words, 480 words representing “living” things (e.g., rabbit) and 480 representing “nonliving” things (e.g., sword) were selected for experiment 1. Of the residual 400 words, 200 were concrete nouns and 200 were abstract, and these were selected for experiment 2. In Study 2, the stimuli were 560 German nouns, 4–9 letters in length (mean, 5.4), with a mean frequency of 41 per million (29). Half of the selected words represented “living” things and half, “nonliving” (30). In both Study 1 and 2 the words were presented in white lowercase letters (10 mm high, 40–80 mm wide) at the center of a black computer screen located 50 cm from the subject in Study 1 and 80 cm in Study 2.
Recognition Task.
In the study phase of experiment 1 of Study 1, 20 words were presented one at a time, and the participants were asked to make living/nonliving judgments on each, a category judgment that encourages deep processing of the studied words (15). Half the words were in one category, and half in the other, randomly intermixed. After 30 s, the test phase followed in which the 20 studied (“old”) words were presented randomly intermixed with 20 unstudied (“new”) words, and the participants made old/new decisions on each. Jon received 10 such study–test blocks in each of two different sessions (separated by ≈3 months), and his controls received 12 such study–test blocks in one session. In experiment 2 of Study 1 (Jon's third session), Jon was asked to make an abstract/concrete judgment rather than a living/nonliving judgment during study. All other experimental parameters were as in experiment 1. In Study 2, the subjects received 7 study–test blocks with the same instructions as in experiment 1. The stimuli were presented in the same way as in experiments 1 and 2, except that each study phase consisted of 40 words, and each test phase consisted of the 40 studied old words and 40 unstudied new words; assignment of words to old and new was counterbalanced across subjects.
The words were presented for 1,700 ms during study and for 300 ms during test, with an interstimulus interval of 2,000 ms. Category judgments during study and recognition judgments during test were made by pressing one of two buttons (within 300-2000 ms after the onset of the word) with the index finger of either the left or the right hand (counterbalanced within subjects in Study 1 and across subjects in Study 2). Subjects were instructed that accuracy and speed (i.e., reaction time) were equally important. To remind Jon of the task and response requirements, the category words “living” and “nonliving” and, in experiment 2, “concrete” and “abstract” were taped on the appropriate sides of the computer screen during study, and the words “old” and “new” were taped on the appropriate sides of the screen during test. Earlier results show that the presence of such words does not interfere with the LPC effect (30). To reduce ERP artifacts (see below), 3-s blink pauses followed each test word in Study 1, whereas in Study 2, 4-s blink pauses were randomly interspersed every 3–10 test words.
ERPs.
Scalp ERPs (0.01–70 Hz bandpass, 250 digitization rate, off-line artifact rejection) were recorded from tin electrodes (64 in Study 1 and 27 in Study 2) mounted in an electro cap (Electro-Cap International, Eaton, OH). The electroencephalogram was referenced to both the left mastoid and the common average for comparison of Study 1 and 2, and to linked mastoids for the analyses of Study 1. The positions of the 64 electrodes on Jon's scalp were measured at the first session, and the electrodes were replaced at these same scalp locations in his two subsequent sessions. Eye blinks and eye movements were monitored through horizontal and vertical electro-oculogram electrodes. Artifacts due to blinks, saccades, excessive muscle activity, and amplifier blocking were rejected off-line.
ERP mean amplitudes were measured in the N400 (300–500 ms) and the LPC (500–700 ms) time windows after word onset in the test phase. Statistical analyses in Study 1, experiment 1, were performed after dividing the ERP data from each session into first and second halves, each containing 52–90 correctly recognized old words (“hits”) and the same number of correctly classified new words (correct rejections). For Jon, data from experiment 2 (his third session), which were not divided into halves, were added to his four data sets from experiment 1. Each of his resulting 5 data sets (four from experiment 1 and one from experiment 2) contained 25–30 incorrectly rejected studied words (“misses”). Amplitude differences were assessed by analysis of variance [ANOVA; degrees of freedom (df) corrected for nonsphericity (31)]. Differences in scalp topography were analyzed by df-corrected ANOVA from all electrodes after normalization (32).
Results
Behaviorally, Jon performed fairly well during encoding (81% correct living/nonliving judgments vs. 83% for the controls). During retrieval (Table 1), however, his performance fell below that of his controls (69.3% hits versus 88.3%, respectively; F1,3 = 40.7, P < 0.08). Also, his reaction times to hits and correct rejections were about 200 ms slower than those of the controls (Table 1). The 10 normal university students performed comparably to Jon's controls (Table 1), although their recognition paradigm was slightly more difficult (40-word lists instead of 20-word lists).
Table 1.
Recognition scores and reaction times
| Subjects | Hit rate, % | F.a. rate, % | RT hits, ms | RT misses, ms | RT c.rej., ms | RT f.a., ms |
|---|---|---|---|---|---|---|
| Ten normals | 88.0 ± 4.0 | 13.0 ± 3.0 | 760 ± 105 | 850 ± 110 | 827 ± 100 | 865 ± 104 |
| Two controls | 88.3 ± 5.0 | 11.1 ± 3.0 | 765 ± 100 | 840 ± 90 | 830 ± 108 | 870 ± 101 |
| Jon | 69.3 ± 2.3 | 11.9 ± 2.1 | 966 ± 52 | 1,118 ± 17 | 1,037 ± 97 | 1,211 ± 59 |
Hit rate, correctly identifying old word as old; F.a. rate, false-alarm rate—i.e., incorrectly identifying new word as old; RT, reaction time; miss, incorrectly identifying old word as new; c.rej., correct rejection—i.e., correctly identifying new word as new.
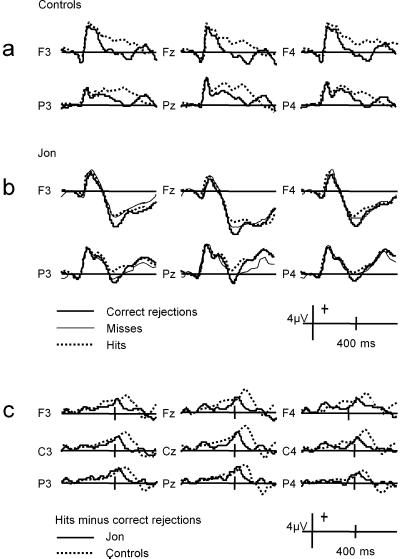
Jon's ERPs to visual word recognition and the ERPs of the two control subjects are shown in Fig. 1. For the control subjects, hits elicited more positive ERPs in both the N400 and LPC time windows compared with correct rejections (Fig. 1 a and c). By contrast, Jon's ERPs showed a prominent N400 effect only, the LPC effect being absent at nearly all electrode sites and inverted in polarity over left frontotemporal areas (Fig. 1b). ANOVA of the mean amplitudes (electrodes F3, F4, P3, P4) between 300 and 500 ms revealed a significant main effect of word type (hits vs. correct rejections) in the controls (F1,3 = 68.4, P < 0.005) and in Jon (F1,4 = 7.9, P < 0.05). Between 500 and 700 ms a significant main effect of word type (hits vs. correct rejections) was found for the controls (F1,3 = 21.7, P < 0.05), but for Jon no such effect emerged either between 500 and 700 ms (P = 0.6) or between 700 and 900 ms (P = 0.8). In Jon, unrecognized repetitions of studied words (misses) elicited ERPs that were more positive than correct rejections at frontocentral and left posterior temporal electrodes. ANOVA showed a significant interaction (F1,4 = 9.1, P < 0.05) between word type (misses vs. correct rejections) and position (Fz vs. Pz). The presence of an N400 effect and the concurrent lack of an LPC effect is highlighted in the difference waves between hits and correct rejections (Fig. 1c).
Figure 1.
ERP waveforms elicited at left, midline, and right frontal electrodes (F3, Fz, and F4 of the 10–20 system), and at left, midline, and right parietal electrodes (P3, Pz, and P4) by correctly recognized studied words (hits) and correctly classified new words (correct rejections). (a) In the two controls, averaged together. (b) In Jon. (c) ERP waveforms of the differences between hits and correct rejections for the controls (dotted lines) and Jon (solid lines) including midline (Fz, Cz, Pz) and central (C3 and C4) electrodes.
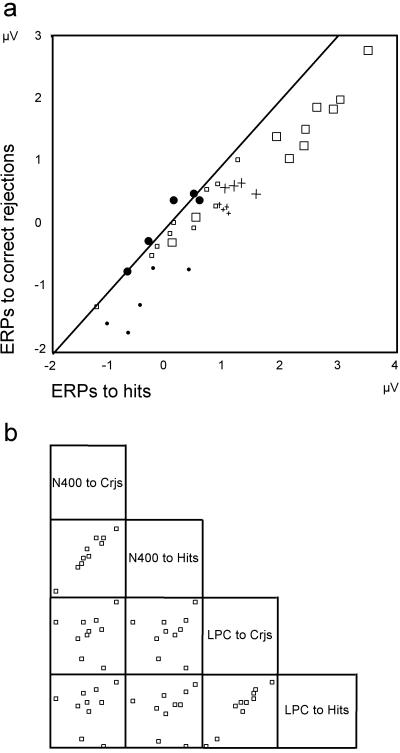
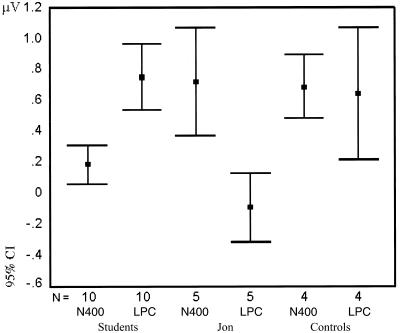
Scatter plots (Fig. 2) of the ERP amplitudes obtained from the 10 normal university students illustrate that each of these subjects had more positive ERPs to hits than to correct rejections in both the N400 time window (mean amplitudes collapsed over eight frontocentral electrodes) and the LPC time window (mean amplitudes collapsed over eight centroparietal electrodes). By contrast, although Jon too had more positive N400 amplitudes to hits than to correct rejections, his LPC amplitudes for these two stimulus classes lie close to the isoelectric line in all five of his data sets. These negligible LPC differences fall outside the 95% confidence interval of those of the students and his controls (Fig. 4). This is the case even when, in view of Jon's slower reaction times, his LPC is measured 200 ms later than that of the healthy subjects. In the 10 normals, there was no cross-correlation between the N400 and LPC effects (Fig. 2b).
Figure 2.
(a) ERPs elicited by hits and correct rejections are contrasted for the 10 university students (▫ and □), Jon's two controls (+ and +, four measurements), and Jon (• and ●, five measurements). In each group, large symbols indicate the mean amplitude of the LPC measured between 500 and 700 ms after word onset over eight centroparietal electrodes, and small symbols indicate the mean N400 amplitude measured over eight frontocentral electrodes between 300 and 500 ms after stimulus onset (all data sets are transformed into a common average reference). On the black diagonal line, ERPs to hits and correct rejections are isoelectric. Jon's LPCs to hits and correct rejections (●) are all close to the isoelectric line, whereas all those of his two controls and of the 10 university students are more positive for hits than for correct rejections. (b) Mean LPC and N400 amplitudes elicited by correct rejections (Crjs) and hits are contrasted for the 10 university students. Although there is a high correlation between ERPs elicited by correct rejections and hits within the N400 and the LPC, there is no cross-correlation between the two.
Figure 4.
The 95% confidence intervals of the ERP amplitude differences between hits and correct rejections are contrasted for the 10 university students, Jon's two controls, and Jon. The ERP differences in the N400 are measured in the 300- to 500-ms time window over eight frontocentral electrodes, and in the LPC they are measured in the 500- to 700-ms time window over eight centroparietal electrodes (all data derived from a common reference average). Jon's LPC difference lies outside the 95% confidence interval of that of both the university students and the controls. N, number of data sets.
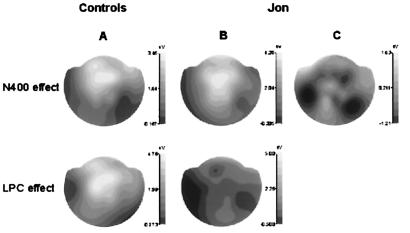
Topographically, the controls' N400 and LPC effects had different scalp distributions (Fig. 3A). ANOVA revealed a significant latency (N400 vs. LPC) by locus [midline frontal (Fz) versus midline parietal (Pz) electrode] interaction (F1,3 = 54.5, P < 0.05) for the ERP difference between hits and correct rejections. Jon's N400 effect was very similar in magnitude and scalp topography to that of his two controls (Fig. 3B). Repeated words that Jon recognized correctly (hits), and repeated words that he classified as new (misses) elicited N400 effects with different scalp topographies (Fig. 3C). ANOVA revealed a significant interaction (F1,4 = 9.8, P < 0.05) between word type (hits and misses) and locus (Fz and Pz). (The low number of misses in the 10 normals and 2 controls, fewer than 20 for each data set, precludes their analysis in these subjects.)
Figure 3.
Scalp distribution of the difference between misses and correct rejections in the N400 (300–500 ms) and the LPC (500–700 ms) time windows. (A) In the two controls, the N400 effect has a more anterior scalp distribution than the LPC effect. (B) In Jon, the N400 effect is very similar in magnitude and topography to that of the controls, whereas the LPC effect is absent. (c) Jon also showed an N400 effect for unrecognized repetitions of studied words (misses) compared with correct rejections, but with a scalp topography different from that for the N400 effect based on hits vs. correct rejections.
The absolute amplitude of Jon's N400 component was higher than in the two control subjects (Fig. 1b). Jon's ERPs over frontal sites starting ≈200 ms after the onset of both new and repeated words were also more negative than those of the controls. Finally, early ERP waveforms (P100, N180, P220, N270) were slightly delayed in Jon and, between 300 and 400 ms, the ERP difference between hits and correct rejections was particularly prominent over posterior sites in Jon, whereas they were distributed more anteriorly in the controls.
Discussion
These physiological data help elucidate Jon's cognitive memory impairment by showing that an ERP correlate of recollection (the LPC effect) is substantially impaired in Jon, whereas an ERP correlate of stimulus familiarity (the frontocentral N400 effect) appears to be relatively intact (Figs. 1 and 2). The topographic analyses of the two ERP effects in the normal subjects replicated previous demonstrations that the N400 and LPC effects of recognition have distinct scalp distributions reflecting separable neural generators (18, 21) (Fig. 3A). Additionally, topographic analyses showed that the neural generators underlying Jon's recognition memory in the N400 time window are separable also from those underlying his implicit memory for repeated words in the same time window (18) (Fig. 3B); unlike the frontocentral N400 effect elicited by recognized words, repeated words that Jon classified as new (misses) elicited a more posterior N400 effect, and this effect has been related to implicit memory in normals (18). As previously described (18), such a topographic difference is important in linking Jon's frontocentral N400 effect to familiarity-based recognition rather than to an unconscious form of memory. Thus, in the absence of a normal LPC effect of recognition, the finding of an N400 recognition effect that is (i) topographically dissociable from the N400 repetition effect for unrecognized words and (ii) similar in topography to the N400 effects so far related to familiarity and knowing (18, 19, 21) suggests that Jon's ERP correlate of familiarity is less affected than his ERP correlate of recollection. This dissociation, obtained in a paradigm that reliably elicits both ERP effects in normal subjects, suggests that recognition after selective bilateral hippocampal damage is qualitatively different from normal recognition. More specifically, the dissociation provides physiological support for the hypothesis that recognition memory may be largely preserved after such damage because the dual basis of recognition allows its maintenance by familiarity even though recollection is impaired (2). However, in situations such as the present one, where deep encoding facilitates recollection-based recognition in normal subjects, recognition impairment becomes evident in Jon, presumably because of his inability to benefit from deep encoding and its normal effect on recollection (15).
Jon's, as well as his two controls', mean differences in the amplitudes of N400 components elicited by hits and correct rejections were larger than those of the normal subjects (Fig. 4). This result is likely to be due to the 40-word lists used with the normals as compared with the 20-word lists used with Jon and his controls, and consequently, a mean delay of approximately 4 min between the first presentation of a word and its repetition, as compared with 2 min for the shorter lists. Increasing repetition lag reduces the magnitude of the N400 repetition effect (33) without affecting the LPC effect. The explanation for this difference is still unclear (see, e.g., ref. 34).
Jon's ERP results support the conclusion that MTL pathology limited to the hippocampal formation—i.e., without apparent damage to the parahippocampal region—impairs recollection differentially rather than causing equivalent quantitative losses across both recollection and familiarity. Animals also may show intact recognition after bilateral excitotoxic hippocampal damage (4). Jon's ERP data support the notion that in animals too hippocampal function may be more closely related to human recollection than to familiarity-based recognition (35). They are compatible also with recent functional magnetic resonance imaging (fMRI) data in which activation of the hippocampal formation by word list recognition in healthy subjects (36) was found to be related to recollection but not familiarity-based recognition (37). Importantly, familiarity was not associated simply with weaker responses than recollection; rather, hippocampal activity to familiarity was indistinguishable from that to misses and correct rejections of new words (37).
The ability to recollect events is a hallmark of episodic memory (38). The selective effect of hippocampal injury on Jon's recollection-based recognition thus indicates that the hippocampal formation plays a special role in episodic memory. To what extent semantic memory (7), the ability to memorize factual information, is physiologically intact in Jon can only be indirectly inferred from the present ERP data. According to Tulving's theory (7), knowing (or familiarity) is a property of the semantic memory system. Indeed, Jon's relatively preserved memory for facts together with his relatively normal N400 effect of familiarity supports this view. Recent accounts of data from single-unit recordings and lesion data in animal studies are also compatible with the notion that semantic memory and familiarity based-recognition might depend on the integrity of the same neuroanatomical areas (notably the perirhinal cortex) within the parahippocampal region (39). However, so far, an ERP index of semantic memory acquisition that would allow a more direct investigation of Jon's semantic memory has not been identified. The modulations of the N400 component by semantic congruity as well as the effect of repetition on this modulation are preserved in amnesic patients (28), suggesting that this type of N400 modulation might be more closely related to a short-term process that serves language comprehension (28) than to semantic memory acquisition.
It is possible that the clear dissociation of brain indices found here, which we have proposed are related to episodic and perhaps to semantic memory, is contingent on early injury to the hippocampal formation allowing for compensatory neural reorganization (1). Indeed, although Jon's ERPs in the N400 time window were similar to those of normal subjects in many respects, the absolute amplitude of his N400 component was much higher than in the two control subjects and the 10 university students (as illustrated in the scatter plots of the N400 amplitudes in Fig. 2). This finding suggests that, in Jon, there may have been developmental adaptation of the neural processes underlying the N400 familiarity effect. Jon's ERPs thus suggest some evidence of neural reorganization, providing grounds to investigate more extensively the adaptive capacity of the MTL after early partial injury.
Acknowledgments
We thank Jon and his parents for making this study possible. We also thank Ken Paller, Michael Rugg, and Thomas Münte for helpful comments on earlier versions of the manuscript and Hans-Jürgen Warmbold for technical support. This study was supported by grants from the Deutsche Forschungsgemeinschaft (DFG/SFB 426, TP C7).
Abbreviations
- MTL
medial temporal lobe
- ERP
event-related potential
- LPC
late positive component
References
- 1.Vargha-Khadem F, Gadian D G, Watkins K E, Connelly A, Van Paesschen W, Mishkin M. Science. 1997;277:376–380. doi: 10.1126/science.277.5324.376. [DOI] [PubMed] [Google Scholar]
- 2.Aggleton J P, Shaw C. Neuropsychologia. 1996;34:51–62. doi: 10.1016/0028-3932(95)00150-6. [DOI] [PubMed] [Google Scholar]
- 3.Rempel-Clower N L, Zola S M, Squire L R, Amaral D G. J Neurosci. 1996;16:5233–5255. doi: 10.1523/JNEUROSCI.16-16-05233.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murray E A, Mishkin M. J Neurosci. 1998;18:6568–6582. doi: 10.1523/JNEUROSCI.18-16-06568.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Squire L R, Zola S M. Hippocampus. 1998;8:205–211. doi: 10.1002/(SICI)1098-1063(1998)8:3<205::AID-HIPO3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 6.Mishkin M, Vargha-Khadem F, Gadian D G. Hippocampus. 1998;8:212–216. doi: 10.1002/(SICI)1098-1063(1998)8:3<212::AID-HIPO4>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 7.Tulving E, Markowitsch H J. Hippocampus. 1998;8:198–204. doi: 10.1002/(SICI)1098-1063(1998)8:3<198::AID-HIPO2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 8.Baddeley, A., Vargha-Khadem, F. & Mishkin, M., J. Cogn. Neurosci., in press. [DOI] [PubMed]
- 9.Tulving E. Can Psychol. 1985;26:1–12. [Google Scholar]
- 10.Jacoby L L. J Mem Lang. 1991;30:513–541. [Google Scholar]
- 11.Yonelinas A P, Kroll N E, Dobbins I, Lazzara M, Knight R T. Neuropsychology. 1998;12:323–339. doi: 10.1037//0894-4105.12.3.323. [DOI] [PubMed] [Google Scholar]
- 12.Gardiner J M, Java R I, Richardson-Klavehn A. Can J Exp Psychol. 1996;50:114–122. [Google Scholar]
- 13.Knowlton B J, Squire L R. J Exp Psychol Learn Mem Cogn. 1995;21:699–710. doi: 10.1037//0278-7393.21.3.699. [DOI] [PubMed] [Google Scholar]
- 14.Manns J R, Squire L R. Hippocampus. 1999;9:495–499. doi: 10.1002/(SICI)1098-1063(1999)9:5<495::AID-HIPO2>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 15.Brown S C, Craik F I M. In: The Oxford Handbook of Memory. Tulving E, Craik F I M, editors. Oxford: Oxford Univ. Press; 2000. pp. 93–107. [Google Scholar]
- 16.Paller K A, Kutas M. J Cogn Neurosci. 1992;4:375–391. doi: 10.1162/jocn.1992.4.4.375. [DOI] [PubMed] [Google Scholar]
- 17.Paller K A, Kutas M, McIsaac H K. Psychol Sci. 1995;6:107–111. [Google Scholar]
- 18.Rugg M D, Mark R E, Walla P, Schloerscheidt A M, Birch C S, Allan K. Nature (London) 1998;392:595–598. doi: 10.1038/33396. [DOI] [PubMed] [Google Scholar]
- 19.Düzel E, Yonelinas A P, Mangun G R, Heinze H J, Tulving E. Proc Natl Acad Sci USA. 1997;94:5973–5978. doi: 10.1073/pnas.94.11.5973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Curran T. Neuropsychologia. 1999;37:771–785. doi: 10.1016/s0028-3932(98)00133-x. [DOI] [PubMed] [Google Scholar]
- 21.Curran T. Mem Cognit. 2000;28:923–938. doi: 10.3758/bf03209340. [DOI] [PubMed] [Google Scholar]
- 22.Paller K A, Kutas M, McIsaac H K. Conscious Cogn. 1998;7:54–66. doi: 10.1006/ccog.1998.0329. [DOI] [PubMed] [Google Scholar]
- 23.Wilding E L, Rugg M D. Brain. 1996;119:889–905. doi: 10.1093/brain/119.3.889. [DOI] [PubMed] [Google Scholar]
- 24.Wilding E. Int J Psychophysiol. 2000;35:81–87. doi: 10.1016/s0167-8760(99)00095-1. [DOI] [PubMed] [Google Scholar]
- 25.Yonelinas A P, Jacoby L L. Conscious Cognit. 1996;5:131–141. [PubMed] [Google Scholar]
- 26.Nessler D, Mecklinger A, Penney T B. Brain Res Cogn Brain Res. 2001;10:283–301. doi: 10.1016/s0926-6410(00)00049-5. [DOI] [PubMed] [Google Scholar]
- 27.Kutas M, Van Petten C K. In: Handbook of Psycholinguistics. Gernsbacher M A, editor. San Diego: Academic; 1994. pp. 83–143. [Google Scholar]
- 28.Olichney J M, Van Petten C, Paller K A, Salmon D P, Iragui V J, Kutas M. Brain. 2000;123:1948–1963. doi: 10.1093/brain/123.9.1948. [DOI] [PubMed] [Google Scholar]
- 29.Baayen R L, Piepenbrock R, von Rijn H. The CELEX Lexical Database. Univ. of Pennsylvania, Philadelphia: Linguistic Database Consortium; 1993. [Google Scholar]
- 30.Düzel E, Cabeza R, Picton T W, Yonelinas A P, Scheich H, Heinze H J, Tulving E. Proc Natl Acad Sci USA. 1999;96:1794–1799. doi: 10.1073/pnas.96.4.1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Winer B J. Statistical Principles in Experimental Design. New York: McGraw-Hill; 1971. [Google Scholar]
- 32.McCarthy G, Wood C C. Electroencephalogr Clin Neurophysiol. 1985;62:203–208. doi: 10.1016/0168-5597(85)90015-2. [DOI] [PubMed] [Google Scholar]
- 33.Rugg M D. Mem Cognit. 1990;18:367–379. doi: 10.3758/bf03197126. [DOI] [PubMed] [Google Scholar]
- 34.Münte T F, Urbach P U, Düzel E, Kutas M. In: Handbook of Neuropsychology. Kutas M, editor. Amsterdam: Elsevier; 2001. , in press. [Google Scholar]
- 35.Suzuki W A. Neuron. 1999;24:295–298. doi: 10.1016/s0896-6273(00)80844-2. [DOI] [PubMed] [Google Scholar]
- 36.Stark C E, Squire L R. J Neurosci. 2000;20:7776–7781. doi: 10.1523/JNEUROSCI.20-20-07776.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eldridge L L, Knowlton B J, Furmanski C S, Bookheimer S Y, Engel S A. Nat Neurosci. 2000;3:1149–1152. doi: 10.1038/80671. [DOI] [PubMed] [Google Scholar]
- 38.Wheeler M A, Stuss D T, Tulving E. Psychol Bull. 1997;121:331–354. doi: 10.1037/0033-2909.121.3.331. [DOI] [PubMed] [Google Scholar]
- 39.Murray E A, Bussey T J. Trends Cogn Sci. 1999;3:142–151. doi: 10.1016/s1364-6613(99)01303-0. [DOI] [PubMed] [Google Scholar]