Abstract
The human umbilical cord blood (HUCB) mononuclear cell (MNC) fraction is a mixed population of cells that induces functional repair in rodent models of stroke when injected intravenously (i.v.). The transplanted cells are found in the infarcted hemisphere and the spleen. The goal of this project was to determine the nature of the interaction between the HUCB MNCs cells and splenic immune cells. Male Sprague Dawley rats underwent permanent middle cerebral artery occlusion (MCAO) and received i.v. injection of either vehicle (MCAO only), HUCB MNCs or MNCs depleted of CD14+ monocytes, CD133+ stem cells or CD19+ B cells 48 hours post-stroke. At 72 hours post-MCAO, the animals were euthanized and the spleens and blood MNCs harvested for flow cytometry and mitogen proliferation assays. All HUCB cell preparations decreased the percentage of T cells in the spleen and monocytes in the blood (p < 0.05). MNCs depleted of CD14+ and CD19+ decreased the percentage of macrophage (p < 0.001), while CD133 depleted MNCs increased the percentage of macrophage in spleen (p < 0.001); MNC did not alter the macrophage population from the level observed after MCAO. Only HUCB MNC significantly decreased Concanavalin A (ConA)-induced T cell stimulation (p < 0.05). These results suggest that the effects of HUCB MNC in the spleen are not due to a single HUCB population, but the interaction of all the subpopulations together.
Keywords: mononuclear cells, T cells, monocytes, macrophage, B cells, stroke
Introduction
HUCB cells induce both functional recovery and diminish infarct size when administered after middle cerebral artery occlusion (MCAO). The degree of recovery after HUCB administration is dependent on the number of cells administered [1]. However, the more important parameters of delivery are timing of cell delivery and route of administration. Two studies have now shown that the cells are effective when delivered prior to 72 hr after MCAO [2,3]. Further, when we delivered the cells at 48 hr after MCAO, the effective cell dose decreased from 107 to 106 cells [3]. When we compared intravenous (i.v.) delivery with direct intraparenchymal delivery into the striatum, we initially obtained similar degrees of functional recovery with both routes, but stable recovery was only induced with i.v. delivery [4]; those animals with intraparenchymal delivery lost all early behavioral improvements by 8 weeks post-MCAO, whereas i.v. treated animals did not. Further, Borlongan and associates [5] demonstrated that even 3 days post MCAO, there were few HUCB cells in the brain, but they were still neuroprotective. Together, these data suggest that the HUCB cells may be acting outside the brain.
If the cells are not producing long-lasting effects when transplanted directly into the brain, then it remains to be determined where they are acting. Indeed, when we examined the biodistribution of the i.v. injected cells, a few cells were found in the infarcted hemisphere while the majority of cells remaining were found in the spleen one month post-transplantation [1]. Subsequent studies have shown that with any cells injected by a vascular delivery route, the majority of them end up in the spleen, liver or kidney [6-9], but it is not clear what the cells are doing in these organs. We demonstrated that HUCB administration alters the ratio of CD4+ to CD8+ T cells in the spleen, decreases mitogen stimulated T cell proliferation and alters secretion of tumor necrosis factor (TNF), interferon gamma (IFNγ) and interleukin (IL)-10 from splenocytes [10]. The HUCB cells not only induced an anti-inflammatory profile in the spleen, but also in the infarcted brain [11,12]. This decreased pro-inflammatory profile seems to represent a common mechanism by which i.v. cell therapies elicit their effects since even neural stem cells administered i.v. in the collagenase model of intracerebral hemorrhage decreased cytokine expression in the spleen and splenectomy reversed all beneficial effects of the cell therapy [13].
The goal of this study was to determine the effect of HUCB administration on the immune cell populations of the spleen. Further, the HUCB mononuclear cell (MNC) preparation that we have used in past studies is composed of a mixture of immature immune cells, including T cells, B cells, monocytes and macrophage and multiple stem cell types. To date, there have been few studies that have examined the contribution of the different HUCB cell types to recovery after stroke. HUCB CD34+ cells were shown to induce neovascularization and neurogenesis after MCAO [14]. It was recently shown that both CD34+ cells enriched from HUCB or HUCB depleted of CD34+ cells improved neurologic deficits and reduced infarct volume [15,16], but neither were as effective as the whole MNC preparation [16]. Consistent with these results, the Finish research group demonstrated that CD34+ HUCB cells, induced behavioral recovery, but the cells were not neuroprotective in their hands [17]. However, the stem cell population of the MNC is very small, the majority of the cells being immune cells. There are no studies that have examined the effects of the other HUCB cell populations in a stroke model. The second objective of this study was to examine whether the HUCB MNC subpopulations all had similar effects on splenic immune populations.
Methods
Animals
This study was carried out under the auspices of the University of South Florida Institutional Animal Care and Use Committee in compliance with the National Institutes of Health Guidelines for the Care and Use of Animals. Adult male Sprague-Dawley rats (250-400 g, Harlan) were randomly assigned to one of the following groups: MCAO only, MNC, HUCB MNCs depleted of CD14+ monocytes (CD14−), HUCB MNCs depleted of CD133+ stem cells (CD133−) or HUCB MNCs depleted of CD19+ B cells (CD19−).
Middle Cerebral Artery Occlusion
The rats were anesthetized with lsofluorane (3% in oxygen). The right common, internal and external carotid arteries were isolated using blunt dissection techniques. A siliconized monofilament 40 mm in length (size 4.0) was inserted through the external carotid approximately 25 mm into the internal carotid and permanently tied in to block the origin of the right middle cerebral artery.
HUCB Cell Preparation and Transplantation
Cryopreserved HUCB MNCs were obtained from All Cells, LLC. Immediately prior to the experiment the HUCB cells were thawed in a 37 °C water bath for about one minute and then washed twice in 10 ml PBS with 100μl DNase (Sigma # D4527-40KU, 1 mg/ml). The number of viable cells was determined using the trypan blue exclusion method. Cell viability generally ranged from 80% to 90%. Using magnetic antibody cell sorting (MACS), some HUCB MNCs were depleted of either CD14+ monocytes, CD133+ stem cells or CD19+ B cells. To deplete these cells populations, the MNC were centrifuged at 1000 rpm × 10 minutes, the supernatant aspirated and the cells resuspended in 80 μl of buffer per 107 cells and incubated at 4°C for 15 min. Antibody conjugated paramagnetic microbeads (20μl/107 cells; CD133 labeled beads (cat#130-090-853, Miltenyi Biotech); CD14 labeled beads (cat# 130-050-201, Miltenyi Biotech); or CD19 labeled beads (cat# 130-050-301, Miltenyi Biotech)) were used to deplete stem cells, monocyte/macrophage or B cells, respectively. After incubation, the cells were washed with buffer and centrifuged at 1000 rpm for 10 min. The cells were resuspended in 500 μl of buffer, then passed through a magnetic column that trapped the paramagnetic beads and the cells they were attached to (AutoMacs Pro). The magnetic field was disengaged and the cells collected. Forty-eight hours after undergoing MCAO, the rats were injected i.v. with HUCB MNC (106 cells) or HUCB MNC depleted of CD133+ (9.9 × 105), CD14+ (8.5 × 105) or CD19+ (8.8 × 105) cells.
Splenocyte Preparation
Animals were euthanized 72 hours post-MCAO. Blood and spleen were harvested for analysis of cell populations. The spleens were placed in DMEM with 10% fetal bovine serum (FBS) and 1% antimitotic/antibiotic, dissected free of the surrounding tissue and weighed. They were then dissociated in the same media, collected and filtered through a 40 μm cell strainer. The cells and media were collected and then centrifuged at 250g for 7 min. The cells were resuspended in 1% PharmLysing buffer (BD Pharmingen) for 1 min before being centrifuged again. The cells were then transferred to RPMI 1640 with 10% FBS and 1% antimitotic/antibiotic. Viability was determined with the trypan blue dye exclusion method.
Mononuclear Cell Isolation
To obtain an enriched mononuclear fraction (MNCs), the blood samples were subjected to standard Ficoll separation. Briefly, 3 ml of Ficoll (Histopaque-1077, Sigma) was pipetted into the bottom of a 14 ml LeucoSep centrifuge tube containing a porous membrane frit (Greiner Bio-One) and then centrifuged 1000 × g for 30 s. The blood sample was diluted 1:1 (v/v) with PBS and then 6 ml of this diluted sample was added to each LeucoSep tube. The blood was centrifuged at 400× g for 40 minutes at room temperature. The mononuclear cells formed a layer above the frit at the interface between the plasma and the separation media. The mononuclear cells were transferred to a new centrifuge tube, washed with 10 ml of PBS and centrifuged twice before undergoing flow cytometry.
Flow Cytometry
Once the cells were isolated, they were incubated in blocking solution for 1 h. The cells were then incubated for 60 min at 4°C with monoclonal antibodies to CD4 (T cells or macrophage) or CD45Ra+ (B cells) conjugated to fluorescent reporters (BD Biosciences/PharMingen). The cells were washed once and resuspended in 2% paraformaldehyde in PBS 10% FBS for flow cytometric analysis on the BD Immunocytometry Systems LSR II using the forward and side scatter physical parameters. Acquisition time was 45 s. Dead cells were excluded using propidium iodide staining. Isotype controls were used in parallel. CD4+ T cells and macrophage were distinguished by gating.
Mitogen Stimulation of Splenocytes
Splenic T and B cell activation were quantified using an in vitro proliferation assay. Isolated splenocytes were washed and resuspended in RPMI 1640 medium with 10% FBS, 1% antimitotic/antibiotic and then plated 5 × 104 cells/cm2 in a 96 well plate. After 72 hours, T cell proliferation in the spleen was tested using concanavalin A (Con A, 2.0 μg/ml) mitogen. B cell proliferation was stimulated with exposure to lipopolysaccharide (LPS, 1.0 μg/ml). Mitogen was added to the culture for 24 hours which was followed by CellTiter 96® AQueous Non-Radioactive Cell Proliferation Assay (Promega) performed according to the manufacturer's specifications. The plate was then read on a plate reader with absorbance at 490 nm. The amount of reaction product (formazan) was directly proportional to the number of living cells in the culture.
Histology
The brains were harvested and preserved at 4°C in 4% paraformaldehyde for 24 hours and then cryoprotected in 20% sucrose at 4°C. Brains were cut into 30 μm coronal sections on a Micro cryostat (Richard-Allan Scientific) and thaw mounted. The slides were Nissl stained as previously described [3] to visualize neurons. Slides were rehydrated and then incubated in the Nissl Thionin prepared in a glacial acetic acid/ 1N NaOH solution for 1.5 min. The slides were rinsed in distilled water for 2 min, dehydrated through a series of alcohols, cleared in xylene and coverslipped with Permount. At 6 predetermined levels at 1 mm intervals from 1.7 mm anterior to bregma to 3.3 mm posterior to bregma, we measured the area (mm2) of intact tissue in the normal hemisphere and the damaged area of the infarcted hemisphere using Image Pro Plus. The relative infarct size was calculated as a percent of intact hemisphere.
Results
HUCB Cells Alter Blood and Spleen Cell Populations
At 72 hours, post MCAO, the animals were weighed, euthanized and blood, spleen and brains harvested. The size of the spleens was not significantly different between groups (see Table 1). We then used flow cytometry to examine the T cell, B cell and monocyte/macrophage composition of the spleen and the mononuclear fraction of blood from rats that had undergone permanent MCAO followed 48 hrs later by i.v. injection of HUCB cells. We also collected blood and spleen samples from naïve rats (n=2) to provide reference values for cellular composition of blood and spleen. The data are presented as mean percentage ± SEM of the total living cells within the sample and were analyzed with Analysis of Variance followed by Neuman-Keuls post-hoc analysis.
Table 1. Spleen Weight.
| Group | Raw Wt (mg) | Spleen Weight/ Body Weight (mg/g) |
|---|---|---|
| MCAO only | 681 ± 80 | 216 ± 21 |
| HUCB MNC | 739 ± 87 | 246 ± 29 |
| CD14− | 596 ± 44 | 191 ± 25 |
| CD133− | 775 ± 73 | 239 ± 19 |
| CD19− | 761 ± 51 | 254 ± 15 |
In normal rats the percentage of CD4+ T cells was 19.7 ± 4.0 % in the blood, while in those animals that underwent MCAO the percentage was 53.3 ± 7.8%. The CD4+ T cell population within the blood remained fairly stable after i.v. administration of the HUCB cell preparations. While there was a small decrease in the percentage of T cells in the blood with cell transfusion, the decrease was similar across all cell groups and did not reach statistical significance (Fig 1A). In contrast, there were significant decreases in the percentage of T cells present in the spleen after MNC (p < 0.05), CD14 depleted (p< 0.001), CD133 depleted (p < 0.001) and CD19 depleted HUCB preparations (p < 0.001) (Fig 1B). Splenic T cells were significantly elevated in MCAO rats compared to naïve rats (p < 0.01).
Fig. 1. Flow Cytometric Analysis of Blood (A, C, E) and Splenic (B, D, F) Cell Populations 24 Hours after HUCB treatment, 72 hours after MCAO.
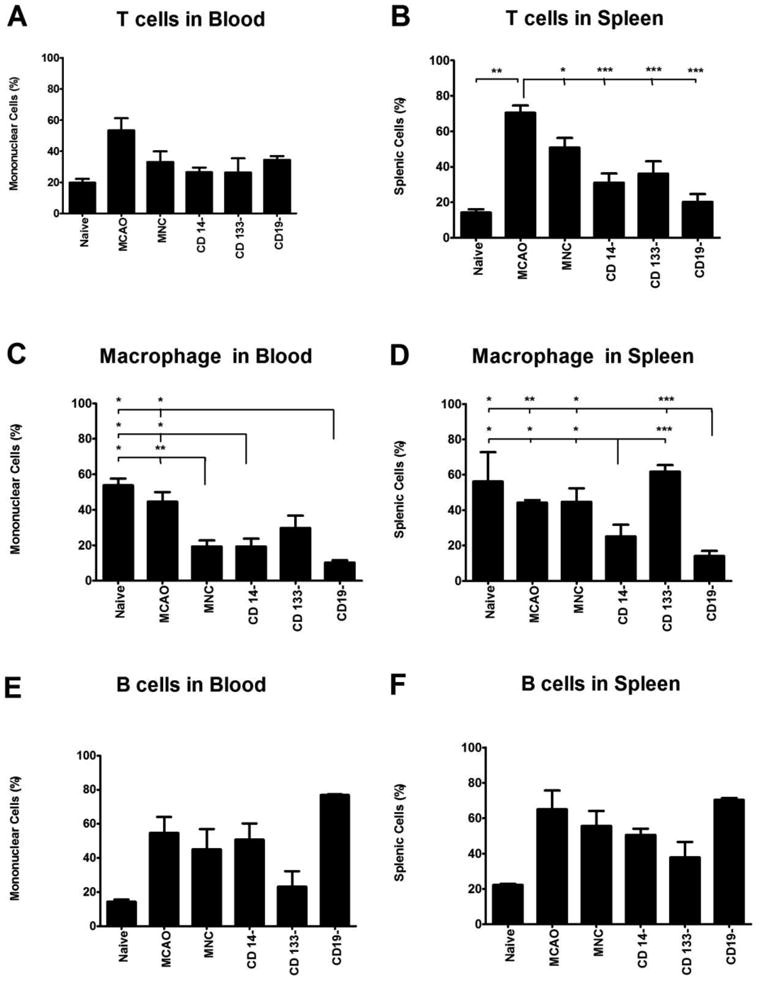
A)The profile of T cells circulating in the blood did not significantly change regardless of the HUCB cells that were administered. B) All HUCB cells significantly decreased the number of T cells present in the spleen compared to the MCAO only treatment. The brackets indicate the comparisons between groups. Every group encompassed by the bracket was compared to the group indicated by the long tail. The level of significance for the specific comparisons is indicated by the asterisks directly above the tickmark. For example, the degree of significance when percentage of T cells in the MNC treated group was compared to the MCAO only group was p <0.05. (C) Administering HUCB MNC, or MNC depleted of either CD14 or CD19 cells significantly decreased circulating macrophage. D) Removal of the CD14+ and CD19+ cells from the MNC significantly decreased the number of macrophage in the spleen compared to the MCAO only group, whereas removal the CD133+ cells significantly increased splenic macrophage. E) There were no significant differences between the treatment groups in blood or F) spleen B cell populations. * p < 0.05, ** p < 0.01, *** p < 0.001
The mean percentage of monocytes/macrophages in the blood from normal rats was 53.8 ± 3.8%. The percentage of the blood mononuclear fraction composed of monocytes/macrophage in rats that underwent MCAO was 44.6 ± 5.3%. When we compared the percentage of monocytes/macrophage in the blood to the percentage observed in naïve and MCAO only rats, HUCB MNC decreased circulating monocytes/macrophage significantly (Fig 1C; p < 0.05 and p < 0.01, respectively). CD14− and CD19− HUCB preparations also had significantly fewer macrophage than did the naïve and MCAO only group (p < 0.05). All cell preparations decreased the percentage of macrophage in the blood to a similar extent. The macrophage profile in the spleen, however, was much different (Fig 1D). In naive rats, the percentage of splenocytes that were monocytes/macrophage was 56.2 ± 16.7%. After MCAO, the percentage of macrophage splenocytes was 44.2 ± 1.5%. The HUCB MNC fraction did not alter the percentage of macrophage present in the spleen. However, with removal of the CD14 cell fractions, the percent of splenic monocytes/macrophage were significantly fewer than observed in the naïve (p < 0.05), MCAO (p < 0.05), MNC (p < 0.05) and CD133− (p <0.001). The effect of removal of the CD19− HUCB cells was similar to that observed with CD14 removal (naïve, p < 0.05; MCAO, p < 0.05; MNC, p < 0.05; CD133−, p <0.001).
The B cell profile as determined with an antibody to the CD45Ra receptor in the blood of the normal rats was 14.4 ± 2.0 %. After MCAO the percentage was 54.8 ± 9.2 % (Fig 1E). There were no significant differences between groups, although there was a tendency for circulating B cells to increase after MCAO. A similar profile existed in the spleen (Fig 1F), with no significant differences between groups observed.
HUCB Cells Alter the Activity of Splenic T cells?
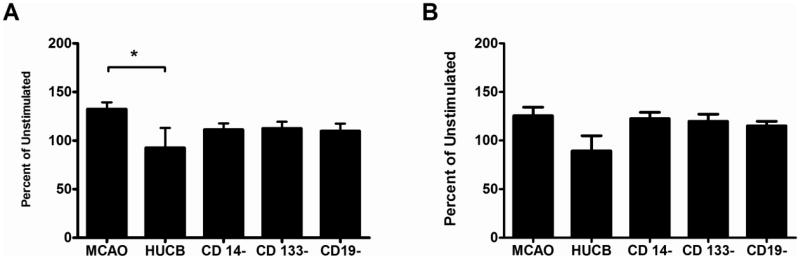
We also examined whether splenocytes harvested after permanent MCAO and HUCB administration were differentially responsive to mitogen stimulation. Cultures were stimulated with either Con A (T cell mitogen) or LPS (B cells and macrophage mitogen) and cell proliferation quantified and expressed as mean percentage of unstimulated cell proliferation ± SEM.
HUCB MNC significantly reduced the T cell proliferative response to Con A stimulation after MCAO (Fig 2A). After MCAO, Con A induced proliferation was 132.4 ± 7.7% of unstimulated cells from the same animal. In animals transplanted with HUCB MNC, proliferation significantly decreased to 92.5 ± 25.0 (p < 0.05). While there was a tendency for proliferation to be decreased in all the other transplant conditions, these changes were not significant.
Fig. 2. Ex vivo Mitogen Stimulation of Splenocytes.
Splenocytes were harvested 72 hr after MCAO and then cultured for 72 hr. A) Con A was added to the cultured splenocytes to stimulate T cell proliferation. Only the HUCB MNCs inhibited T cell proliferation. B) When LPS was added to the cultured splenocytes to stimulate B cell proliferation, there were no significant differences between the groups. * p < 0.05
Proliferation of splenocytes harvested from MCAO-treated rats in response to LPS was 125.6 ± 9.5%. None of the HUCB cell preparations significantly altered LPS-induced proliferation (Fig 2B).
Do Depleted HUCB Cell Fractions Decrease Infarct Size?
Brains were harvested, stained with Nissl thionin and sections at 1 mm intervals from 1.7 mm anterior to bregma to 3.3 mm posterior to bregma were chosen to measure infarct size. The overall analysis of variance was significant (p < 0.01). Those animals in the MCAO only group had large lesions ipsilateral to the occluded vessel (Fig 3). There was a tendency for infarct size to decrease after injection of HUCB MNC, but this difference was not significant. Only removal of CD133 cells significantly decreased infarct size (p < 0.05).
Fig. 3. Removal of the CD14+ monocytes from the mononuclear fraction resulted in a loss of HUCB protective effects.
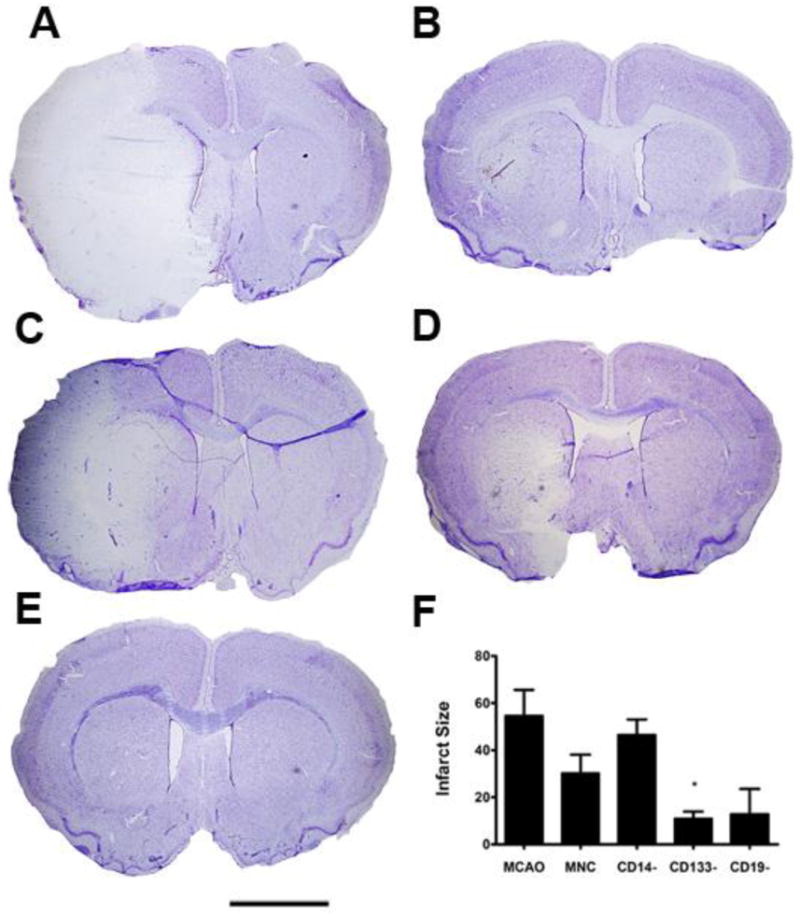
Nissl Thionin staining was used to label neurons. A) At 72 hr post-MCAO, the ipsilateral hemisphere had few thionin labeled neurons surviving, while the contral lateral hemisphere was intact. B) In the HUCB MNC treated rats, the size of the infarct was much smaller. C) When CD14+ monoyctes were removed from the MNC, there was a large infarct in the lateral striatum and overlying cortex. D) Removing CD133+ and E) CD19+ HUCB cells also appeared to decrease infarct size compared to the MCAO only controls. F) Quantification of infarct volume demonstrated that removal of CD14+ monocytes resulted in infarct size similar to the MCAO only animals while removal of the CD133+ stem cells significantly decreased infarct volume. * p < 0.05. Scale bar = 2.0 mm.
Discussion
We have previously demonstrated that HUCB cells improved functional outcome and decreased infarct size and these effects were dependent on route of administration [4], number of cells administered [1] and the timing of cell delivery [3]. One of the effects of HUCB administration after MCAO is a reduction in CD1 1b+ monocytes, macrophage or microglia and B220+ B cells in the stroke brain that is accompanied by a decrease in mRNA and protein concentration of pro-inflammatory cytokines [12]. The injected HUCB cells were also found in the spleen [10] altering T cell profile and cytokine production. The goal of this study was to further examine the effect of HUCB cell preparations on immune cell populations of the blood and spleen after MCAO through a flow cytometric analysis. We further wished to determine the role of specific subpopulations of the HUCB MNC fraction in these effects. Here we showed that in the spleen, the HUCB cells significantly alter T cell and monocyte/macrophage populations, but have very little effect on B cells. The main effect of HUCB administration on circulating immune cells is on the monocytes or macrophage populations.
There have been multiple reports that there is an activation of the peripheral immune response early after MCAO in rodents. Offner et al [18] reported that there is an increase in pro-inflammatory cytokines TNF-α, IFN-γ, IL-6, MCP-1, and IL-2 in blood & spleen as early as 6 hr after MCAO, but that is still maintained at 22 hr. At 48 hr post-MCAO in the rat, however, mRNA for TNF-α tended to decrease, while mRNA for IFN-γ and IL-10, tended to increase [10] in the spleen. When splenocytes from sham or MCAO animals were stimulated with ConA, MCAO did not increase IFNγ concentration above that secreted by splenocytes from sham animals, but it did decrease IL-10 concentration, shifting the balance to a pro-inflammatory state. Intravenous injection of HUCB cells switched the balance toward an anti-inflammatory state, significantly decreasing IFNy and increasing IL-10. These changes in cytokine production were associated with a change in the balance between CD4 and CD8 T cells after MCAO which was restored by MNC administration. By 96 hrs, Offner et al [19] reported CD3+, CD4+ and CD8+ T cells in the spleen, but not CD4+ FoxP3+ T cells, were undergoing apoptosis and as a consequence, there was a profound immune suppression that may be analogous to the post-stroke immune suppression seen in patients. The apoptosis of T cells was suggested to account for the decrease in size of the spleen that has been observed in a number of studies post-MCAO [10,19]. In contrast to the effect of splenic weight observed in the study by Vendrame et al [10], splenic weight in this study was similar across MCAO and treated groups. It may be that the decrease in splenic size observed in the earlier study after MCAO and the restoration by HUCB MNC is a transient effect. A careful time course study will be required to determine if this is the case.
In our current study, we transplanted the HUCB cells 48 hr after stroke and examined the splenic and blood profiles at 72 hr. Previous studies have shown that the optimal therapeutic benefits are obtained with administration at this time [3]. Using this protocol, there was a generalized decrease in T cells in the spleen, regardless of the type of HUCB preparation used. It should be noted that MCAO significantly increased splenic T cells from the percentage observed in naïve rats. The decreases in splenic T cells observed with HUCB cell administrations acted to restore the normal percentage of T cells in the spleen. One explanation for the apparent loss of T cells from the spleen is through a catecholamine-induced release of T cells into circulation. Spleen decreases in size after MCAO [10,19] and administration of the adrenergic antagonist carvedilol blocks this decrease [20]. However, there was a tendency for the T cell population in the blood of HUCB treated animals to also decrease from MCAO only levels. We know that there is an increase in T cell infiltration in the brain after MCAO, but HUCB MNCs do not alter the T cell concentration in the brain [12], making tissue redistribution of T cells unlikely as the main explanation for this decrease and an apoptotic mechanism more likely [19]. Alternatively, IL-10 produced by HUCB-derived T cells may directly inhibit proliferation of splenic T cells [21].
The role of T helper cells is to recognize antigen and through production of cytokines such as IFNγ and chemokines, induce infiltration of monoyctes and macrophage to the site of infection or injury followed by their activation leading to the release of other cytokines such as TNFα and IL-1. It could be argued that with the generalized suppression in T cell number after HUCB administration reported here, the circulating monocyte population simply followed suit. The situation in the spleen, however, is more complicated since there were differential effects of HUCB preparations. While MNC did not alter the splenic macrophage population from that observed after MCAO, removal of CD14+ or CD19+ cells from the HUCB MNC significantly decreased the population of macrophage in the spleen. The CD14+ HUCB cells may have contributed to the macrophage pool and with their removal from the MNC, the pool size decreased. This does not explain why there were significantly fewer macrophage when CD19+ B cells were removed from the MNC, however. Monocytes support normal B cell function [22] but the influence of B cells on monocytes and macrophage is less clear. However, B cells do secrete granulocyte and macrophage colony stimulating factor (GM-CSF) [23], a potent mitogen for macrophage. With removal of the HUCB CD19+ cells, this signal may have decreased. Regardless of the likely cause of the profound decreases in the circulating and splenic monocytes and macrophage, these changes are likely a contributing factor to the anti-inflammatory effects of HUCB cells, explaining why MNC significantly reduce the number of CD1 1b+ cells in the brain after MCAO and the reduced concentrations of pro-inflammatory cytokines [12]. This observation also raises a number of questions among which the most important to address from a clinical perspective are how long these cell counts are suppressed, do they return to normal, and are these rats susceptible to opportunistic infections because of a suppressed innate immune system.
In contrast to the effect of removing CD14+ and CD19+ cells from the MNC, removal of CD133+ cells significantly increased the percentage of macrophage in the spleen above the levels seen in the MCAO only animals. The CD133 antigen is not enough to distinguish between hematopoietic stem cells, mesenchymal cells (MSC) or endothelial precursors, all of which are present in the HUCB MNC [24-26]. MSCs shift the balance from a pro-inflammatory state to an anti-inflammatory state [27]. They can decrease TNFα and increase IL-10 secretion from dendritic cells, decrease IFNγ secretion from Th 1 and natural killer cells and increase IL-4 from Th 2 cells. The net effect of removing a MSC-like population would be to enhance pro-inflammatory signals. If this were the case, then we might have expected that removal of the CD133+ cells would increase infarct size, but this did not happen; infarct volume decreased when the CD133+ cells were removed suggesting that the presence of the cells actually contributed to secondary neurodegenerative damage in the brain. Analogous to the distinction between the Th1 and Th2 T cell immune states, there appear to be subsets of monocytes and macrophage in the innate immune system that have pro-inflammatory or anti-inflammatory functions [28]. Conceivably, while there are more macrophage in the spleen when CD133+ cells are removed, the remaining HUCB cells may have stimulated the production of anti-inflammatory macrophages which can then infiltrate the infarcted brain and provided a neuroprotective enrivonment. An alternative explanation for this apparent paradox is that CD133+ cells produce cytokines or growth factors that mobilize macrophage from the spleen, which then can respond to cytokine and chemokine signals to infiltrate the brain. When CD133+ cells are removed from the HUCB MNC fraction, macrophage remain sequestered in the spleen, decreasing the migration of pro-inflammatory macrophage into the brain.
The decrease in infarct volume when the CD133+ cells were removed appears to contradict earlier studies in which HUCB stem cells were administered and recovery was reported. Most of the HUCB studies have used the MNC fraction. Those studies that have used HUCB stem cells have used the CD34+ hematopoietic stem cells. CD133 is a more primitive stem cell antigen than CD34 [29] and expression precedes expression of CD34 on hematopoietic stem cells. CD133 is also expressed on MSCs and endothelial progenitors. Theoretically, by choosing to remove CD133+ cells in this study, we are removing the source of most of the stem cells present in the HUCB MNC. The CD34+ HUCB cells were shown to induce angiogenesis and neurogenesis after stroke [14]. In those studies that have injected CD34+ HUCB cells, they routinely observe that both CD34+ and CD34− HUCB preparations induce behavioral repair [15]. However, more recent studies have suggested that the MNC fraction is superior to either CD34+ or CD34− HUCB preparations in inducing functional recovery [16]. The effect of CD133+ cells specifically on functional recovery and long-term brain repair remains to be determined.
One caveat that must be considered with any study that injects human cells across species into rodents is that the xenograft is not immunologically neutral. Xenografts are often very quickly rejected by circulating antibodies and complement and those that survive immune attack may not survive long because the needed trophic support is not present [30]. Rejection is accompanied by increased pro-inflammatory cytokines [31]. But most studies that have examined HUCB or human bone marrow cells, routinely report that they have an anti-inflammatory effect even with cross species transplantation [10,12,32,33]. This issue will need to be examined in greater detail in an allograft model before this treatment is used clinically to treat stroke.
In summary, the HUCB cells altered T cell and monocyte/macrophage populations in the spleens of animals that had undergone MCAO. While all the HUCB cell populations tested had similar effects on T cells in the spleen, none of the depleted MNC fractions elicited the same effect on splenic macrophage as the entire MNC fraction. The importance of this observation for long-term functional outcome after stroke remains to be determined.
Acknowledgments
This research was supported in part by the Florida Department of Health James and Esther King Biomedical Research Program (07KB-07) and the National Institute of Neurological Diseases and Stroke (RO1NS52839 to AEW).
Bibliography & Literature Cited
- 1.Vendrame M, Cassady CJ, Newcomb J, Butler T, Pennypacker KR, Zigova T, et al. Infusion of human umbilical cord blood cells in a rat model of stroke dose-dependently rescues behavioral deficits and reduces infarct volume. Stroke. 2004;35:2390–5. doi: 10.1161/01.STR.0000141681.06735.9b. [DOI] [PubMed] [Google Scholar]
- 2.Boltze J, Schmidt UR, Reich DM, Kranz A, Reymann KG, Strassburger M, et al. Determination of the therapeutic time window for human umbilical cord blood mononuclear cell transplantation following experimental stroke in rats. Cell Transplant. 2011 Dec 13; doi: 10.3727/096368911X589609. [DOI] [PubMed] [Google Scholar]
- 3.Newcomb JD, Ajmo CT, Davis Sanberg C, Sanberg PR, Pennypacker KR, Willing AE. Timing of cord blood treatment after experimental stroke determines therapeutic efficacy. Cell Transplant. 2006;15(3):213–23. doi: 10.3727/000000006783982043. [DOI] [PubMed] [Google Scholar]
- 4.Willing AE, Lixian J, Milliken M, Poulos S, Zigova T, Song S, et al. Intravenous versus intrastriatal cord blood administration in a rodent model of stroke. J Neurosci Res. 2003;73(3):296–307. doi: 10.1002/jnr.10659. [DOI] [PubMed] [Google Scholar]
- 5.Borlongan CV, Hadman M, Davis Sanberg C, Sanberg PR. CNS entry of peripherally injected umbilical cord blood cells is not required for neuroprotection in stroke. Stroke. 2004;35(10):2385–9. doi: 10.1161/01.STR.0000141680.49960.d7. [DOI] [PubMed] [Google Scholar]
- 6.Keimpema E, Fokkens MR, Nagy Z, Agoston V, Luiten PG, Nyakas C, et al. Early transient presence of implanted bone marrow stem cells reduces lesion size after cerebral ischaemia in adult rats. Neuropathol Appl Neurobiol. 2009 Feb;35(1):89–102. doi: 10.1111/j.1365-2990.2008.00961.x. [DOI] [PubMed] [Google Scholar]
- 7.Fischer UM, Harting MT, Jimenez F, Monzon-Posadas WO, Xue H, Savitz SI, et al. Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first-pass effect. Stem Cells Dev. 2009 Jun;18(5):683–92. doi: 10.1089/scd.2008.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lappalainen RS, Narkilahti S, Huhtala T, Liimatainen T, Suuronen T, Narvanen A, et al. The SPECT imaging shows the accumulation of neural progenitor cells into internal organs after systemic administration in middle cerebral artery occlusion rats. Neurosci Lett. 2008 Aug 8;440(3):246–50. doi: 10.1016/j.neulet.2008.05.090. [DOI] [PubMed] [Google Scholar]
- 9.Battistella V, de Freitas GR, da Fonseca LM, Mercante D, Gutfilen B, Goldenberg RC, et al. Safety of autologous bone marrow mononuclear cell transplantation in patients with nonacute ischemic stroke. Regen Med. 2011 Jan;6(1):45–52. doi: 10.2217/rme.10.97. [DOI] [PubMed] [Google Scholar]
- 10.Vendrame M, Gemma C, Pennypacker KR, Bickford PC, Davis Sanberg C, Sanberg PR, et al. Cord Blood Rescues Stroke-Induced Changes In Splenocyte Phenotype and Function. Exp Neurol. 2006;199:191–200. doi: 10.1016/j.expneurol.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 11.Leonardo C, Hall AA, Collier LA, Ajmo CT, Jr, Willing AE, Pennypacker KR. HUCB cell therapy blocks the morphological change and recruitment of CD11b-expressing, isolectin-binding proinflammatory cells after MCAO. J Neurosci Res. 2010;88:1213–22. doi: 10.1002/jnr.22306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vendrame M, Gemma C, de Mesquita D, Collier L, Bickford PC, Davis Sanberg C, et al. Anti-inflammatory Effects Of Human Cord Blood Cells In A Rat Model Of Stroke. Stem Cells Dev. 2005;14:595–604. doi: 10.1089/scd.2005.14.595. [DOI] [PubMed] [Google Scholar]
- 13.Lee ST, Chu K, Jung KH, Kim SJ, Kim DH, Kang KM, et al. Anti-inflammatory mechanism of intravascular neural stem cell transplantation in haemorrhagic stroke. Brain : a journal of neurology. 2008 Mar;131(Pt 3):616–29. doi: 10.1093/brain/awm306. [DOI] [PubMed] [Google Scholar]
- 14.Taguchi A, Soma T, Tanaka H, Kanda T, Nishimura H, Yoshikawa H, et al. Administration of CD34+ cells after stroke enhances neurogenesis via angiogenesis in a mouse model. J Clin Invest. 2004;114(3):330–8. doi: 10.1172/JCI20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boltze J, Kowalski I, Geiger K, Reich D, Gunther A, Buhrle C, et al. Experimental treatment of stroke in spontaneously hypertensive rats by CD34+ and CD34− cord blood cells. Ger Med Sci. 2005;3:Doc09. [PMC free article] [PubMed] [Google Scholar]
- 16.Boltze J, Reich DM, Hau S, Reymann KG, Strassburger M, Lobsein D, et al. Assessment of neuroprotective effects of human umbilical cord blood mononuclear cell subpopulations in vitro and in vivo. Cell Transplant. 2012;21:723–37. doi: 10.3727/096368911X586783. [DOI] [PubMed] [Google Scholar]
- 17.Nystedt J, Makinen S, Laine J, Jolkkonen J. Human cord blood CD34+ cells and behavioral recovery following focal cerebral ischemia in rats. Acta Neurobiologie Experimentalis. 2006;66:293–300. doi: 10.55782/ane-2006-1618. [DOI] [PubMed] [Google Scholar]
- 18.Offner H, Subramanian S, Parker SM, Afentoulis ME, Vandenbark AA, Hurn PD. Experimental stroke induces massive, rapid activation of the peripheral immune system. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2006 May;26(5):654–65. doi: 10.1038/sj.jcbfm.9600217. [DOI] [PubMed] [Google Scholar]
- 19.Offner H, Subramanian S, Parker SM, Wang C, Afentoulis ME, Lewis A, et al. Splenic Atrophy in Experimental Stroke Is Accompanied by Increased Regulatory T Cells and Circulating Macrophages. J Immunol. 2006;176:6523–31. doi: 10.4049/jimmunol.176.11.6523. [DOI] [PubMed] [Google Scholar]
- 20.Ajmo J CT, Collier LA, Hall AA, Cuevas J, Green SM, et al. Blockade of adrenoreceptors inhibits the splenic response to stroke. Exp Neurol. 2009;218:47–55. doi: 10.1016/j.expneurol.2009.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rainsford E, Reen DJ. Interleukin 10, produced in abundance by human newborn T cells, may be the regulator of increased tolerance associated with cord blood stem cell transplantation. Br J Haematol. 2002;116(3):702–9. doi: 10.1046/j.0007-1048.2001.03321.x. [DOI] [PubMed] [Google Scholar]
- 22.Mueller CG, Boix C, Kwan W-H, Daussy C, Fournier E, Fridman WH, et al. Critical role of monocytes to support normal B cell and diffuse large B cell lymphoma survival and proliferation. Journal of Leukocyte Biology. 2007;82(3):567–75. doi: 10.1189/jlb.0706481. [DOI] [PubMed] [Google Scholar]
- 23.Jagannathan M, McDonnell M, Liang Y, Hasturk H, Hetzel J, Rubin D, et al. Toll-like receptors regulate B cell cytokine production in patients with diabetes. Diabetol. 2010 Jul;53(7):1461–71. doi: 10.1007/s00125-010-1730-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vanneaux V, El-Ayoubi F, Delmau C, Driancourt C, Lecourt S, Grelier A, et al. In vitro and in vivo analysis of endothelial progenitor cells from cryopreserved umbilical cord blood: are we ready for clinical application? Cell Transplant. 2010;19(9):1143–55. doi: 10.3727/096368910X504487. [DOI] [PubMed] [Google Scholar]
- 25.Chang JW, Hung SP, Wu HH, Wu WM, Yang AH, Tsai HL, et al. Therapeutic effects of umbilical cord blood-derived mesenchymal stem cell transplantation in experimental lupus nephritis. Cell Transplant. 2011;20(2):245–57. doi: 10.3727/096368910X520056. [DOI] [PubMed] [Google Scholar]
- 26.Ivanovic Z, Duchez P, Chevaleyre J, Vlaski M, Lafarge X, Dazey B, et al. Clinical-scale cultures of cord blood CD34(+) cells to amplify committed progenitors and maintain stem cell activity. Cell Transplant. 2011;20(9):1453–63. doi: 10.3727/096368910X552853. [DOI] [PubMed] [Google Scholar]
- 27.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005 Feb 15;105(4):1815–22. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 28.Geissmann F, Auffray C, Palframan R, Wirrig C, Ciocca A, Campisi L, et al. Blood monocytes: distinct subsets, how they relate to dendritic cells, and their possible roles in the regulation of T-cell responses. Immunology and Cell Biology. 2008;86:398–408. doi: 10.1038/icb.2008.19. [DOI] [PubMed] [Google Scholar]
- 29.McGuckin CP, Pearce D, Forraz N, Tooze JA, Watt SM, Pettengell R. Multiparametric analysis of immature cell populations in umbilical cord blood and bone marrow. European Journal of Haematology. 2003;71(5):341–50. doi: 10.1034/j.1600-0609.2003.00153.x. [DOI] [PubMed] [Google Scholar]
- 30.Saadi S, Platt JL. Immunology of xenotransplantation. Life Sci. 1998;62(5):365–87. doi: 10.1016/s0024-3205(97)00964-8. [DOI] [PubMed] [Google Scholar]
- 31.Saadi S, Holzknecht RA, Patte CP, Stern DM, Platt JL. Complement-mediated regulation of tissue factor activity in endothelium. J Exp Med. 1995 Dec 1;182(6):1807–14. doi: 10.1084/jem.182.6.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mezey E, Mayer B, Nemeth K. Unexpected roles for bone marrow stromal cells (or MSCs): a real promise for cellular, but not replacement, therapy. Oral Dis. 2010 Mar;16(2):129–35. doi: 10.1111/j.1601-0825.2009.01605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nemeth K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009 Jan;15(1):42–9. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]