Summary
The purpose of this literature review is to disclose the relationship between the temporal profile of steno-occlusive changes in the cerebral arteries in moyamoya disease and the embryological evolution of the cerebral arteries. Steno-occlusive changes and progression occur in the sequence of embryological evolution of the primitive internal carotid artery in the early embryological stage. In other words, steno-occlusive changes in the cerebral arteries occur primarily near the bifurcation of the cranial and caudal divisions of the primitive internal carotid artery, evolve from the cranial division to the caudal one, and progress from the bifurcation centrifugally. Steno-occlusive changes do not occur essentially in the distal cortical branches of the primitive internal carotid artery, in any arteries in the external carotid system, which are derived from ventral pharyngeal system and primitive stapedial system, or in any cerebral arteries in the vertebrobasilar system, which are derived from the longitudinal neural arteries. These facts suggest that moyamoya disease is strongly related to the vasculogenesis of the primitive internal carotid artery and genetic factors play a major role in the clinical manifestations of moyamoya disease.
Key words: carotid-basilar anastomosis, embryology, moyamoya disease, primitive internal carotid artery, vasculogenesis
Introduction
Moyamoya disease (MD), known also as spontaneous occlusion of the circle of Willis, is a cerebrovascular occlusive disease with angiographic characteristics of stenosis or occlusion of the terminal portion of the internal carotid artery (ICA) and development of collaterals at the base of the brain, called “moyamoya vessels” because of their resemblance to a puff of cigarette smoke (moyamoya appearance in Japanese). These changes occur bilaterally and symmetrically11,21.
Typical symptoms of MD are brain ischaemia in children and brain haemorrhage in adults.
The aetiology of MD is still unknown since the first recognition of this disease some 40 years ago in Japan, but both congenital (gene-defined) and acquired (environmental) factors are believed to play roles in the clinical manifestation of MD.
With available data in the literature on the angiographic characteristics of MD, this paper attested the hypothesis that the temporal profile of steno-occlusive changes in MD follow the sequences of the embryological evolution of the primitive ICA occurring early in the embryological stage.
Steno-occlusive Changes at the Terminal Portion of the ICA (figure 1)
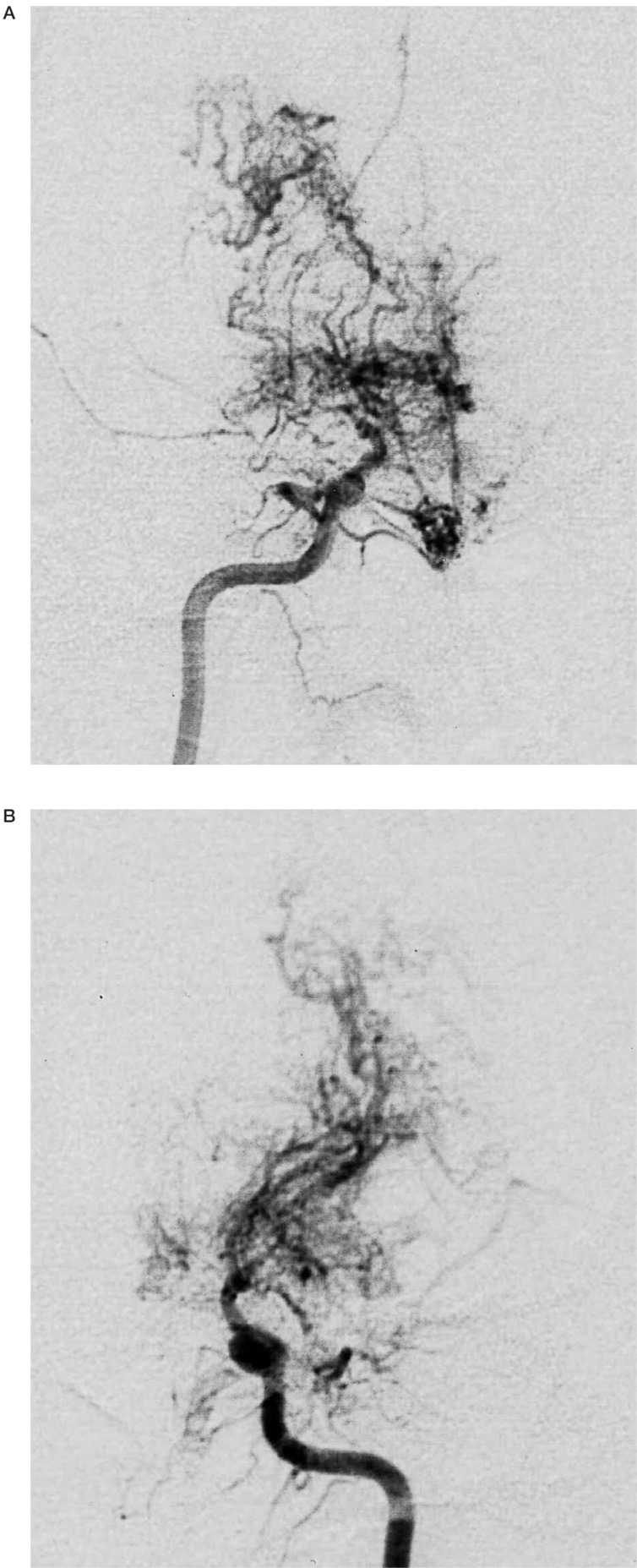
Figure 1.
A 24-year-old man with moyamoya disease who developed transient ischaemic attack. Right internal carotid artery injection (A, frontal view) shows occlusion of internal carotid artery at the terminal portion and well-developed moyamoya vessels. Left internal carotid artery injection (B, frontal view) shows the similar occlusive changes and moyamoya vessels to those on the right side.
In MD, stenosis or occlusion of the cerebral arteries is observed at the terminal portion of the bilateral ICAs and their vicinities2,11,20. These portions are embryologically near the bifurcation of the cranial and caudal divisions of the primitive ICA14. Bilateral and symmetrical involvements of the ICAs suggest structural defects of arterial walls, which are defined by congenital (gene-defined) rather than acquired (environmental) factors. The vertebrobasilar system proximal to the mid-portion between the anterior-superior cerebellar artery and anterior-inferior cerebellar artery (at the junctional point of the primitive trigeminal artery) is derived embryologically from the paired longitudinal neural arteries 14. This portion (proximal vertebrobasilar system) is not involved in MD19.
Angiographic Staging of the Anterior Circulation
Angiographic staging of MD is defined from stage 1 to stage 6 as follows17,21:
Stage 1: narrowing of the carotid bifurcation;
Stage 2: appearance of moyamoya vessels; dilatation of anterior and middle cerebral arteries;
Stage 3: intensification of moyamoya vessels; partial disappearance of anterior and middle cerebral arteries;
Stage 4: minimization of moyamoya vessels; advanced steno-occlusive changes of the ICA,
Stage 5: reduction of moyamoya vessels; absence of anterior and middle cerebral arteries;
Stage 6: disappearance of moyamoya vessels; only the collateral circulation from external carotid artery.
In this staging system, moyamoya vessels refer to the perforating arteries from the ICA system.
This angiographic staging system indicates that steno-occlusive changes in the anterior circulation start at the terminal portion of the ICA. The proximal portions of anterior and middle cerebral artery are progressively involved 1. The proximal ICA near the origin of the anterior choroidal artery, posterior communicating artery and ophthalmic artery are involved with advancement of the disease. This staging system suggests less involvement of the posterior circulation than the anterior one. Steno-occlusive changes in child onset MD evolve until adolescence, and stabilize by the age of 20 years1. It is still unclear whether adult onset MD passes through chronological changes identical to those in child onset MD.
Steno-occlusive Changes in the Posterior Circulation (figure 2)
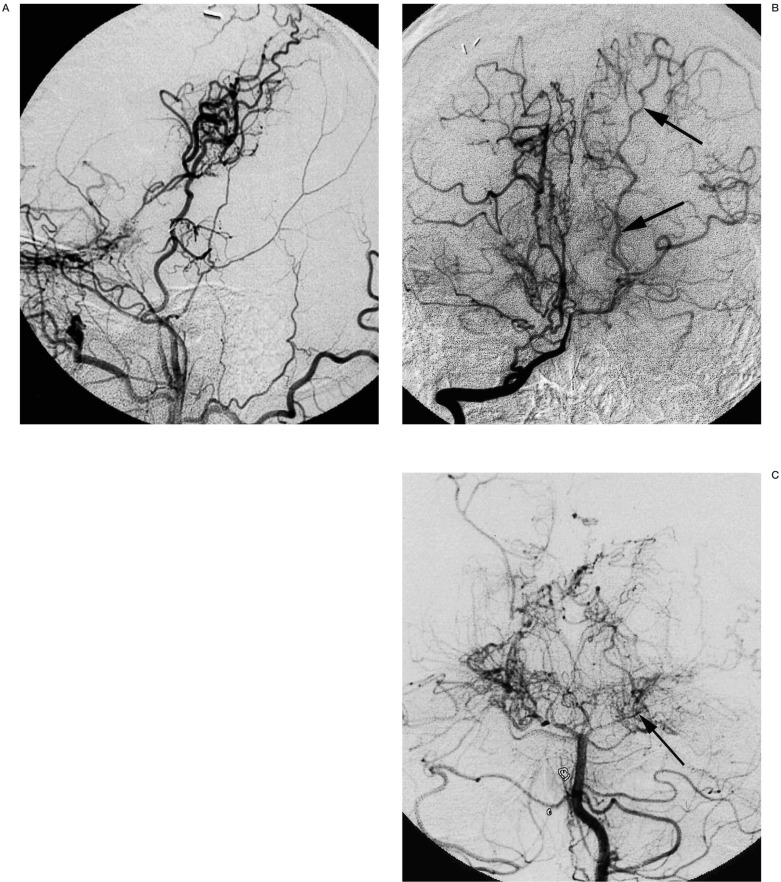
Figure 2.
A 23-year-old man with moyamoya disease whose initial ischaemic events occurred 13 years ago. Left common carotid artery injection (A, lateral view) shows occlusion of internal carotid artery below the origin of ophthalmic artery. Good collateral though the surgical anastomosis is observed. Right vertebral artery injection (B, frontal view) shows the patent left posterior cerebral artery (arrows). Five months after this angiogram, the patient developed right homonymous hemianopsia due to occlusion of the P2 portion of left posterior cerebral artery (C, frontal view, arrow).
Although steno-occlusive changes in the posterior circulation are not included in the diagnostic criteria of MD, these changes extend to the posterior circulation with an advancement of MD12,16,17,19,23. Steno-occlusive changes seem to be confined to the proximal portion of the posterior cerebral artery (PCA) usually P1 and P2 portions, and to the distal basilar artery. The posterior communicating artery is also commonly involved in MD and may disappear with an advancement of the disease. Satoh et Al 19 found steno-occlusive changes in the PCA in 18 cases among 34 paediatric moyamoya cases. They thought that the initial steno-occlusive changes start at the posterior communicating artery and extend to the proximal PCA, but distal PCA changes can also occur simultaneously.
Staging of the posterior circulation of MD has been proposed by Mugikura et Al as follows 17:
Stage 1: no occlusive changes in the PCA,
Stage 2: stenosis of the PCA with or without slightly developed PCA moyamoya,
Stage 3: severe stenosis or virtually complete occlusion of the PCA with well-developed PCA moyamoya,
Stage 4: occlusion of the PCA with decreased PCA moyamoya.
In this staging system, PCA moyamoya refers to moyamoya vessels from the PCA.
Embryologically, the posterior communicating artery and P1 portion of the PCA are the caudal divisions of the primitive ICA14. Later involvement of this caudal division makes an analogy of the later embryological event of distal annexation of cortical branches (P2-4 portions) of the primitive anterior choroidal artery.
Occlusion of the intracranial vertebral artery is extremely rare in MD (3/82 cases16). Although vertebral artery occlusion can be explained by progression of MD, the author believes that proximal basilar artery and vertebral artery are not involved in MD, and occlusion of the vertebral artery is the ultimate consequence of flow compromise in the vertebrobasilar system, which is similar to proximal ICA occlusion in the ultimate sequel of occlusive changes in the ICA system.
No steno-occlusive Changes in the Distal Cortical Arteries (figure 3)
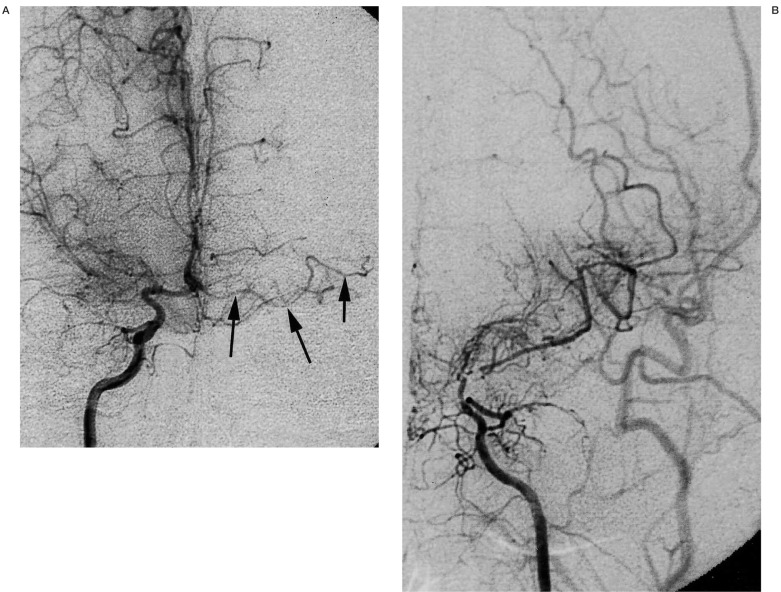
Figure 3.
A 30-year-old man with moyamoya disease who developed transient ischaemic attack 15 years ago. Right internal carotid injection (A, frontal view) shows occlusion of right middle cerebral artery and minimal development of moyamoya vessels. Arrows indicate left accessory middle cerebral artery without any stenotic changes. Left internal carotid injection (B, frontal view) shows marked stenosis at the terminal portion of internal carotid artery.
Stenosis or occlusion typically occur at the terminal portion of the ICA and not in the distal cortical branches. The accessory middle cerebral artery originates from the horizontal portion of the anterior cerebral artery, runs parallel to the proximal anterior and middle cerebral arteries laterally, and terminates in the anterior frontal lobe7. Embryologically, the accessory middle cerebral artery is a cortical branch variation of the middle cerebral artery(proximal annexation), which branches off from the primitive anterior cerebral artery7. Interestingly, this accessory middle cerebral artery does not show steno-occlusive change in MD even though it courses near the terminal portion of the ICA. This fact suggests topological preference of steno-occlusive changes exists in MD10. It is therefore postulated that the distal cortical branch is less involved than the proximal large cerebral arteries in MD.
Choroidal Arteries as a Collateral Circulation
Although the role of the anterior choroidal artery has not been emphasized, its role as a collateral in MD is obvious. It enlarges by means of angioectasia and is a major component of moyamoya vessels. Embryologically, this artery is an old branch of the cranial division of the primitive ICA 14. Later, cortical branches to the temporal, parietal and occipital lobes are transferred to the caudal division of the primitive ICA forming the P2-4 portions of the PCA (distal annexation). These distal portions function in MD as a collateral from the posterior circulation to the anterior one. In other words, the proximal portion of the primitive anterior choroidal artery remains as moyamoya vessels and may disappear with an advancement of MD, and the distal portion serves as a collateral from the posterior circulation to the anterior one. The posterior choroidal artery, which is derived form the caudal division of the primitive ICA, also forms moyamoya vessels functioning as a collateral to the anterior circulation in MD.
Lenticulostriate Arteries as a Collateral Circulation
In the progression of MD, the lenticulostriate arteries may become moyamoya vessels by means of angioectasia together with the choroidal arteries and thalamoperforating arteries 22. Lenticulostriate arteries may become a collateral pathway to the insular branches of the middle cerebral artery and/or to the cortical branches in the frontal and parietal lobes through the medullary arteries 22. Embryologically, the lenticulostriate artery is derived from the lateral striate artery (a branch of the primitive anterior cerebral artery) of the cranial division of the primitive ICA14.
Thalamoperforating Arteries as a Collateral Circulation
Moyamoya vessels consist of anterior and posterior choroidal arteries, lenticulostriate, thalamogeniculate, and thalamoperforating arteries. The anterior thalamoperforating artery originates from the posterior communicating artery, and the posterior thalamoperforating artery originates from the P1 portion of the PCA. The thalamogeniculate arteries originate from the P2 portion of the PCA. This means that thalamogeniculate and thalamoperforating arteries are embryologically derived from the caudal division of the primitive ICA.
No Steno-occlusive Changes in the External Carotid System
Increasing interest in the investigation of MD has focused on the changes in the external carotid system, especially in the superficial temporal artery and middle meningeal artery because surgical specimens of these vessels can be obtained during bypass surgery. Its angiographic changes3,9, histopathological changes15, and histochemical changes 4,20 have been extensively investigated expecting the changes occurring at the intracranial ICAs and their vicinities may also occur in the external carotid system. It is common to observe spontaneous transdural anastomoses in MD, that is, transdural collateral to the distal cortical arteries. Enlarged middle meningeal artery, superficial temporal artery, internal maxillary artery, and occipital artery may contribute to these dural anastomoses2,19. These anastomoses are due to angiogenesis, which is a remote response to the ischaemic triggers13. Although the high incidence of angiographic steno-occlusive changes in the external carotid system was reported3, Komiyama et Al did not find such changes9. The author still believes that arteriopathy in MD occurs solely in the ICA (primitive ICA system) and not in the external carotid system (ventral pharyngeal arterial system and stapedial arterial system).
The ophthalmic artery does not show stenosis or occlusion, but becomes hypertrophied when it gives rise to transdural collaterals 2. The ophthalmic artery occasionally originates from the middle meningeal artery instead of the ICA in MD 9. This means that the primitive ophthalmic arterial system is least involved in MDbecause this system is independent from the primitive ICA and is occasionally connected to the primitive stapedial system.
High Incidence of Embryological Carotid-Basilar Anastomoses (figure 4)
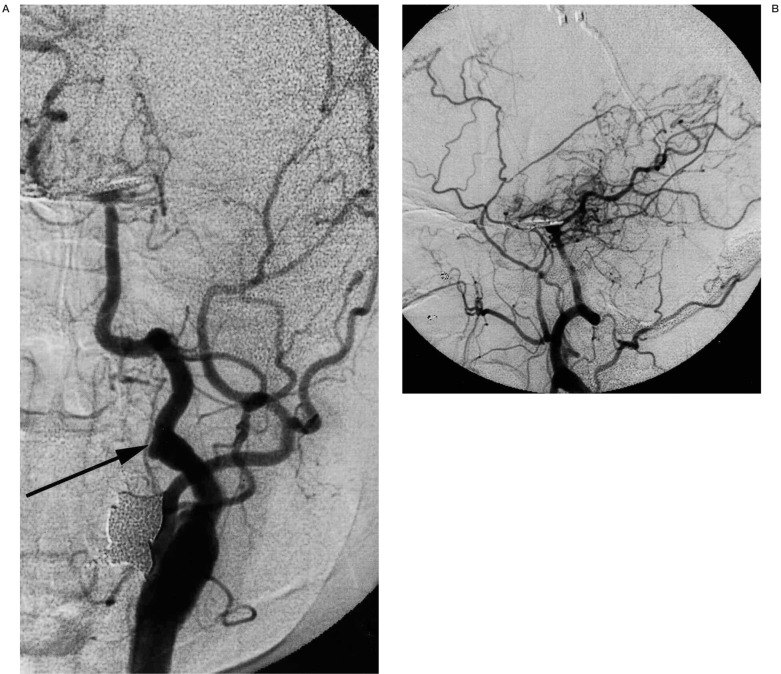
Figure 4.
A 54-year-old woman with moyamoya disease who initially developed ischaemia 10 years ago and subsequently developed subarachnoid haemorrhage 5 years ago due to ruptured basilar bifurcation aneurysm. Left common carotid injection (A, frontal view;B, lateral view) shows the previously patent, but recently occluded left internal carotid artery and persistent primitive hypoglossal artery (arrow) with development of moyamoya vessels. Basilar bifurcation aneurysm is surgically clipped.
Primitive carotid-basilar anastomoses, most of which are the primitive trigeminal artery and its variant, are frequently observed in MD2,6,8. Embryologically, these vessels serve as a shortcut from the ICA to the posterior circulation before development of the posterior communicating artery. The high incidence of persistence of carotid-basilar anastomoses in MD implies less development of the caudal division of the primitive ICA than these anastomoses, and their important role in the posterior circulation, which also sends collateral flow to the anterior circulation.
Metameric Disposition of the Steno-occlusive Arteries
It is hypothetically explained that the rostral mesoderm supplies the endothelium of the prosencephalon, the middle mesoderm supplies the endothelium of the diencephalon, and the caudal mesoderm supplies the endothelium and media of the mesencephalon and metencephalon 13.
The media of the prosencephalon and diencephalon are derived from the neural crest. Histopathological changes in the diseased arteries in MD are fibrous intimal thickening with laminated elastic fibers, without atherosclerotic or inflammatory changes 5,18. The metameric disposition of the intracranial vasculature suggests the endothelial cells of the prosencephalon and diencephalons, namely the brain supplied by the primitive ICA, are more prone to intimal thickening than those of the mesencephalon and metencephalon supplied by the ventral longitudinal neural arteries. It is, therefore, postulated that cerebral arteries in MD have unrecognized pre-existing (gene-defined) structural defects, which are clinically latent during vasculogenesis, but later represent phenotypic manifestations (fibrous intimal thickening) due to unknown triggering factors.
Conclusions
Steno-occlusive changes and their progression in the cerebral arteries in MD occur primarily near the bifurcation of the cranial and caudal divisions of the primitive ICA. The changes evolve from the cranial division to the caudal one, and progress from the bifurcation centrifugally; and steno-occlusive changes do not occur in the distal cortical portions of the primitive ICA, in any arteries of the external carotid system, or in any arteries of the vertebrobasilar system. Thus, it is strongly suggested that MD is a disease of the “primitive ICA” and genetic factors play a major role in its clinical manifestations.
References
- 1.Ezura M, Yoshimoto T, et al. Clinical and angiographic follow-up of childhood-onset moyamoya disease. Child’s Nerv Syst. 1995;11:591–594. doi: 10.1007/BF00300998. [DOI] [PubMed] [Google Scholar]
- 2.Handa J, Handa H. Progressive cerebral arterial occlusive disease: analysis of 27 cases. Neuroradiology. 1972;3:119–133. doi: 10.1007/BF00341494. [DOI] [PubMed] [Google Scholar]
- 3.Hoshimaru M, Kikuchi H. Involvement of the external carotid arteries in moyamoya disease: neuroradiological evaluation of 66 patients. Neurosurgery. 1992;31:398–400. doi: 10.1227/00006123-199209000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Hoshimaru M, Takahashi JA, et al. Possible roles of basic fibroblast growth factor in the pathogenesis of moyamoya disease: an immunohistochemical study. J Neurosurg. 1991;75:267–270. doi: 10.3171/jns.1991.75.2.0267. [DOI] [PubMed] [Google Scholar]
- 5.Hosoda Y. A pathological study of so-called “spontaneous occlusion of the circle of Willis” (“cerebrovascular moyamoya disease”) Folia Angiol. 1976;14:85–86. [Google Scholar]
- 6.Komiyama M, Kitano S, et al. An additional variant of the persistent primitive trigeminal artery: accessory meningeal artery - antero-superior cerebellar artery anastomosis associated with moyamoya disease. Acta Neurochir (Wien) 1998;140:1037–1042. doi: 10.1007/s007010050212. [DOI] [PubMed] [Google Scholar]
- 7.Komiyama M, Nakajima H, et al. Middle cerebral artery variations: duplicated and accessory arteries. Am J Neuroradiol. 1998;19:45–49. [PMC free article] [PubMed] [Google Scholar]
- 8.Komiyama M, Nakajima H, et al. High incidence of persistent primitive arteries in moyamoya and quasimoyamoya diseases. Neurol Med Chir (Tokyo) 1999;39:416–422. doi: 10.2176/nmc.39.416. [DOI] [PubMed] [Google Scholar]
- 9.Komiyama M, Nishikawa M, et al. Steno-occlusive changes in the external carotid system in moyamoya disease. Acta Neurochir (Wien) 2000;142:421–424. doi: 10.1007/s007010050452. [DOI] [PubMed] [Google Scholar]
- 10.Komiyama M, Yasui T. Accessory middle cerebral artery and moyamoya disease. J Neurol Neurosurg Psychiatr. 2001;71:129–130. doi: 10.1136/jnnp.71.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kudo T. Spontaneous occlusion of the circle of Willis: a disease apparently confined to Japanese. Neurology. 1968;18:485–496. doi: 10.1212/wnl.18.5.485. [DOI] [PubMed] [Google Scholar]
- 12.Kuroda S, Ishikawa T, et al. Clinical significance of posterior cerebral artery stenosis/occlusion in moyamoya disease. No Shinkei Geka. 2002;30:1295–1300. [PubMed] [Google Scholar]
- 13.Lasjaunias P, Berenstein A, et al. In: Surgical Neuroangiography I. Clinical vascular anatomy and variations. Second edition. Berlin: Springer-Verlag; 2001. Vascular anatomy and biological process; pp. 1–27. [Google Scholar]
- 14.Lasjaunias P, Berenstein A, et al. In: Surgical Neuroangiography I. Clinical vascular anatomy and variations. Second edition. Berlin: Springer-Verlag; 2001. Intradural arteries; pp. 479–629. [Google Scholar]
- 15.Li B, Wang CC, et al. A histological, ultrastructural and immunohistochemical study of superficial temporal arteries and middle meningeal arteries in moyamoya disease. Acta Pathol Jpn. 1991;41:521–530. doi: 10.1111/j.1440-1827.1991.tb02517.x. [DOI] [PubMed] [Google Scholar]
- 16.Miyamoto S, Kikuchi H, et al. Study of the posterior circulation in moyamoya disease. Clinical and neuroradiological evaluation. J Neurosurg. 1984;61:1032–1037. doi: 10.3171/jns.1984.61.6.1032. [DOI] [PubMed] [Google Scholar]
- 17.Mugikura S, Takahashi S, et al. The relationship between cerebral infarction and angiographic characteristics in childhood moyamoya disease. Am J Neuroradiol. 1999;20:336–343. [PMC free article] [PubMed] [Google Scholar]
- 18.Oka K, Yamashita M, et al. Cerebral haemorrhage in moyamoya disease at autopsy. Virchows Arch. 1981;392:247–261. doi: 10.1007/BF02155663. [DOI] [PubMed] [Google Scholar]
- 19.Satoh S, Shibuya H, et al. Analysis of the angiographic findings in cases of childhood moyamoya disease. Neuroradiology. 1988;30:111–119. doi: 10.1007/BF00395611. [DOI] [PubMed] [Google Scholar]
- 20.Suzui H, Hoshimaru M, et al. Immunochemical reactions for fibroblast growth factor receptor in arteries of patients with moyamoya disease. Neurosurgery. 1994;35:2025. doi: 10.1227/00006123-199407000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki J, Takaku A. Cerebrovascular “moyamoya” disease: disease showing abnormal net-like vessels in base of brain. Arch Neurol. 1969;20:288–299. doi: 10.1001/archneur.1969.00480090076012. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi M. Magnification angiography in moyamoya disease. New observation on collateral vessels. Radiology. 1980;136:379–386. doi: 10.1148/radiology.136.2.7403514. [DOI] [PubMed] [Google Scholar]
- 23.Yamada I, Himeno Y, et al. Posterior circulation in moyamoya disease: angiographic study. Radiology. 1995;197:239–246. doi: 10.1148/radiology.197.1.7568830. [DOI] [PubMed] [Google Scholar]