Abstract
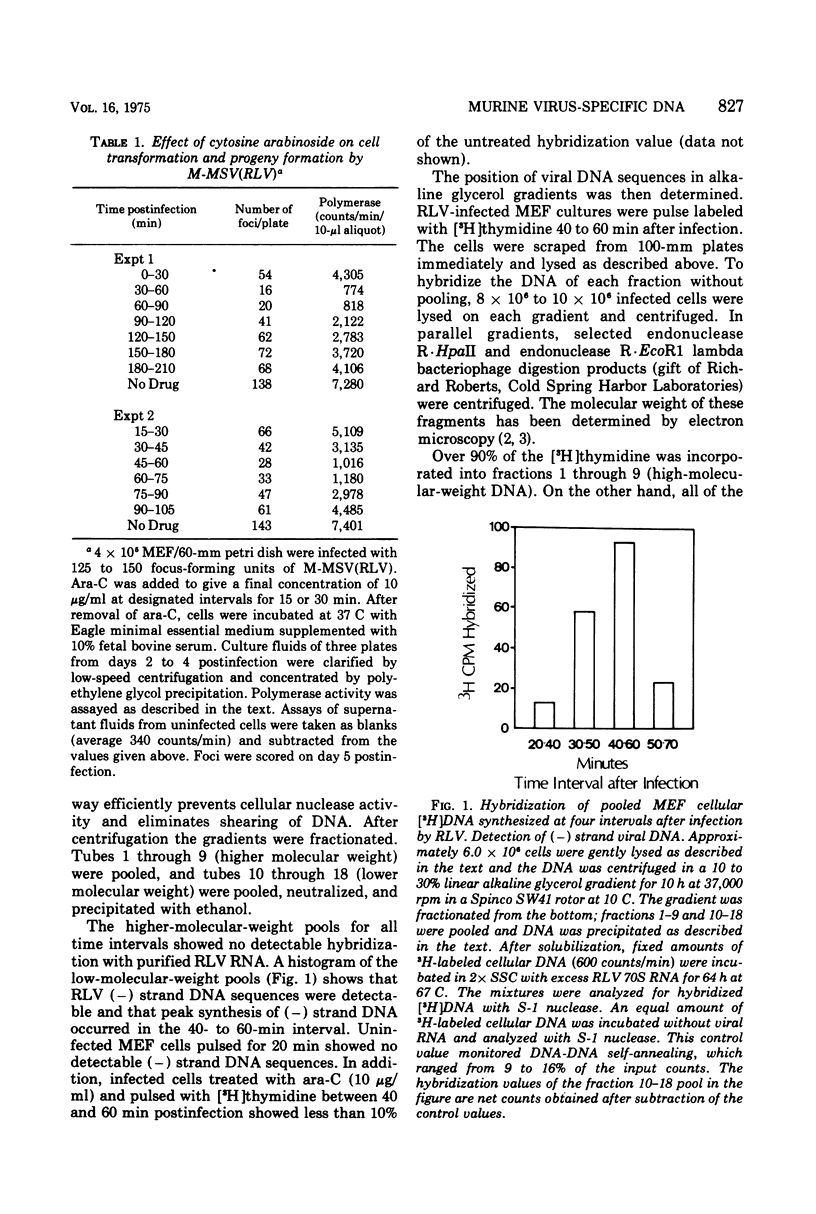
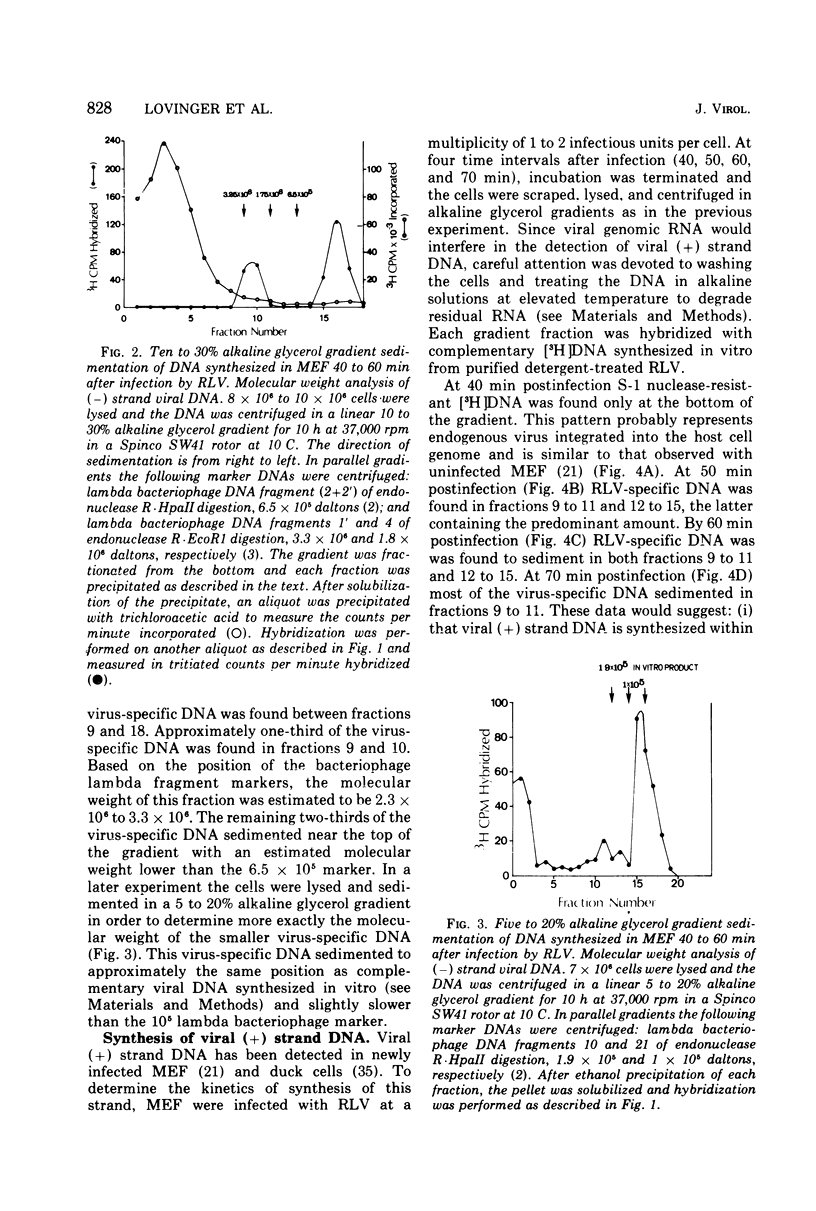
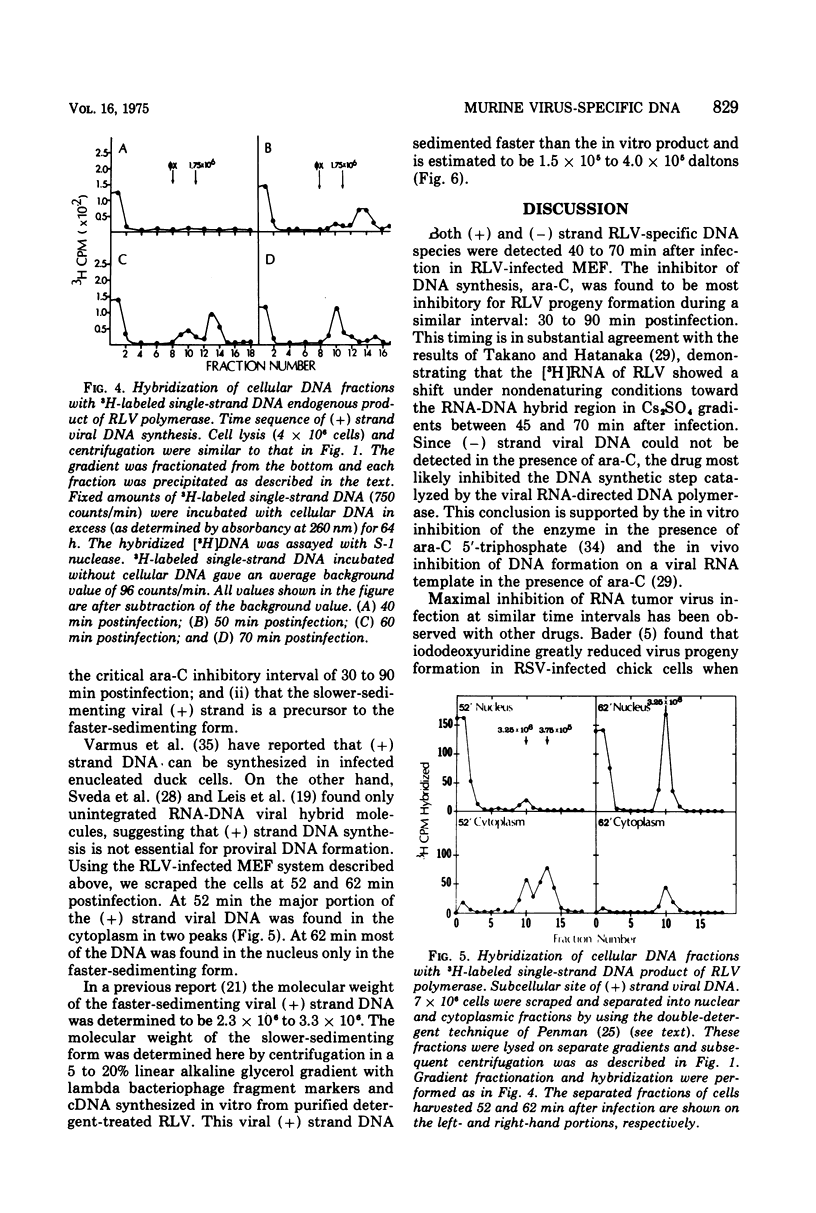
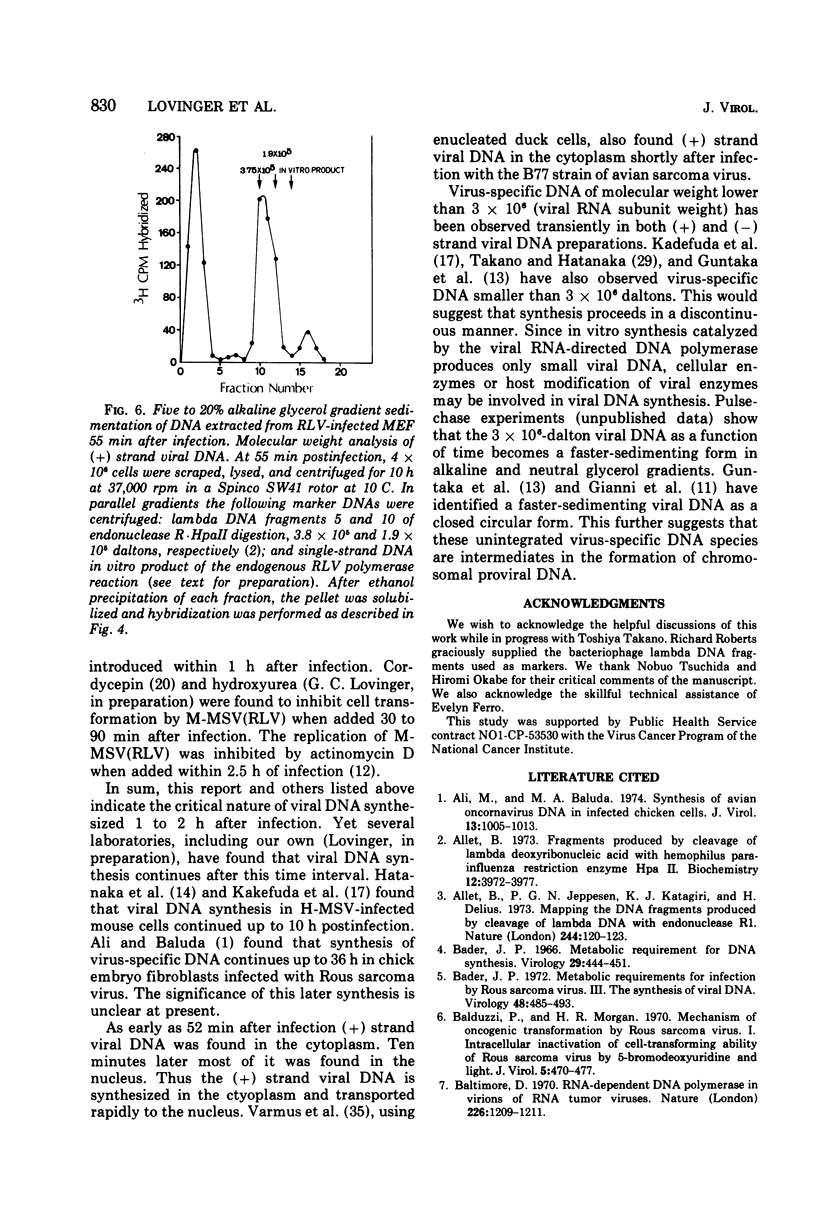
Replicating transforming functions of Rauscher leukemia virus (RLV) and the RLV pseudotype of Moloney sarcoma virus in mouse embryo fibroblasts were found to be most sensitive to inhibition by cytosine arabinoside (ara-C) 30 to 90 min after infection. The initiation of intracellular RLV DNA synthesis was detected by nucleic acid hybridization within this time interval. Treatment of infected cells with cytosine arabinoside abolished RLV DNA synthesis. Peak synthesis of the DNA complementary to the infecting RLV genome, the (-) strand, occurred 40 to 60 min after infection. During this interval two s two species of DNA were observed with estimated molecular weights of 0.5 X 10(5) to 1.0 X 10(5) and 3 X 10(6). Peak synthesis of the (+) strand viral DNA occurred 50 to 70 min after infection. The initial species detected had a molecular weight of 1.5 X 10(5) to 4.0 X 10(5) which shifted as a function of time to 3 X 10(6). Both (+) strand species were initially detected in the cytoplasm followed by a rapid (10-min interval) appearance of the faster-sedimenting species in the nucleus. The virus-specific (-) and (+) strand DNA species are presumably unintegrated intermediates in provirus formation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali M., Baluda M. A. Synthesis of avian oncornavirus DNA in infected chicken cells. J Virol. 1974 May;13(5):1005–1013. doi: 10.1128/jvi.13.5.1005-1013.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allet B. Fragments produced by cleavage of lambda deoxyribonucleic acid with the Hemophilus parainfluenzae restriction enzyme Hpa II. Biochemistry. 1973 Sep 25;12(20):3972–3977. doi: 10.1021/bi00744a029. [DOI] [PubMed] [Google Scholar]
- Allet B., Jeppesen P. G., Katagiri K. J., Delius H. Mapping the DNA fragments produced by cleavage by lambda DNA with endonuclease RI. Nature. 1973 Jan 12;241(5385):120–123. doi: 10.1038/241120a0. [DOI] [PubMed] [Google Scholar]
- Bader J. P. Metabolic requirements for infection by Rous sarcoma virus. 3. The synthesis on viral DNA. Virology. 1972 May;48(2):485–493. doi: 10.1016/0042-6822(72)90059-1. [DOI] [PubMed] [Google Scholar]
- Bader J. P. Metabolic requirements for infection by Rous sarcoma virus. I. The transient requirement for DNA synthesis. Virology. 1966 Jul;29(3):444–451. doi: 10.1016/0042-6822(66)90220-0. [DOI] [PubMed] [Google Scholar]
- Balduzzi P., Morgan H. R. Mechanism of oncogenic transformation by Rous sarcoma virus. I. Intracellular inactivation of cell-transforming ability of Rous sarcoma virus by 5-bromodeoxyuridine and light. J Virol. 1970 Apr;5(4):470–477. doi: 10.1128/jvi.5.4.470-477.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltimore D. RNA-dependent DNA polymerase in virions of RNA tumour viruses. Nature. 1970 Jun 27;226(5252):1209–1211. doi: 10.1038/2261209a0. [DOI] [PubMed] [Google Scholar]
- Boettiger D., Temin H. M. Light inactivation of focus formation by chicken embryo fibroblasts infected with avian sarcoma virus in the presence of 5-bromodeoxyuridine. Nature. 1970 Nov 14;228(5272):622–624. doi: 10.1038/228622a0. [DOI] [PubMed] [Google Scholar]
- Doering A., Keller J., Cohen S. S. Some effects of D-arabinosyl nucleosides on polymer syntheses in mouse fibroblasts. Cancer Res. 1966 Dec;26(12):2444–2450. [PubMed] [Google Scholar]
- Duesberg P. H., Vogt P. K. On the role of DNA synthesis in avian tumor virus infection. Proc Natl Acad Sci U S A. 1969 Nov;64(3):939–946. doi: 10.1073/pnas.64.3.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianni A. M., Smotkin D., Weinberg R. A. Murine leukemia virus: detection of unintegrated double-stranded DNA forms of the provirus. Proc Natl Acad Sci U S A. 1975 Feb;72(2):447–451. doi: 10.1073/pnas.72.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godard C., Robin J., Boiron M. Effects of actinomycin D on early steps of replication in vitro of murine sarcoma in virus (Moloney). J Gen Virol. 1974 Feb;22(2):293–296. doi: 10.1099/0022-1317-22-2-293. [DOI] [PubMed] [Google Scholar]
- Guntaka R. V., Mahy B. W., Bishop J. M., Varmus H. E. Ethidium bromide inhibits appearance of closed circular viral DNA and integration of virus-specific DNA in duck cells infected by avian sarcoma virus. Nature. 1975 Feb 13;253(5492):507–511. doi: 10.1038/253507a0. [DOI] [PubMed] [Google Scholar]
- Hatanaka M., Kakefuda T., Gilden R. V., Callan E. A. Cytoplasmic DNA synthesis induced by RNA tumor viruses. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1844–1847. doi: 10.1073/pnas.68.8.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauschka P. V. Analysis of nucleotide pools in animal cells. Methods Cell Biol. 1973;7:361–462. doi: 10.1016/s0091-679x(08)61787-2. [DOI] [PubMed] [Google Scholar]
- Hill M., Hillova J. Recovery of the temperature-sensitive mutant of Rous sarcoma virus from chicken cells exposed to DNA extracted from hamster cells transformed by the mutant. Virology. 1972 Jul;49(1):309–313. doi: 10.1016/s0042-6822(72)80034-5. [DOI] [PubMed] [Google Scholar]
- Kakefuda T., Dingman C. W., Bak T. M., Hatanaka M., Kitano Y. Reverse transcription of the viral genome associated with the plasma membrane after infection with RNA tumor viruses. Cancer Res. 1974 Apr;34(4):679–688. [PubMed] [Google Scholar]
- Leis J., Schincariol A., Ishizaki R., Hurwitz J. RNA-dependent DNA polymerase activity of RNA tumor viruses. V. Rous sarcoma virus single-stranded RNA-DNA covalent hybrids in infected chicken embryo fibroblast cells. J Virol. 1975 Mar;15(3):484–489. doi: 10.1128/jvi.15.3.484-489.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger G. G., Klein R. A., Gilden R. V., Hatanaka M. The effect of cordycepin on cell transformation by RNA tumor viruses. Virology. 1973 Oct;55(2):524–526. doi: 10.1016/0042-6822(73)90195-5. [DOI] [PubMed] [Google Scholar]
- Lovinger G. G., Ling H. P., Klein R. A., Gilden R., Hatanaka M. Unintegrated murine leukemia viral DNA in newly infected cells. Virology. 1974 Nov;62(1):280–283. doi: 10.1016/0042-6822(74)90323-7. [DOI] [PubMed] [Google Scholar]
- Moloney J. B. A virus-induced rhabdomyosarcoma of mice. Natl Cancer Inst Monogr. 1966 Sep;22:139–142. [PubMed] [Google Scholar]
- Nonoyama M., Pagano J. S. Separation of Epstein-Barr virus DNA from large chromosomal DNA in non-virus-producing cells. Nat New Biol. 1972 Aug 9;238(84):169–171. doi: 10.1038/newbio238169a0. [DOI] [PubMed] [Google Scholar]
- Okabe H., Gilden R. V., Hatanaka M. RD 114 virus-specific sequences in feline cellular RNA: detection and characterization. J Virol. 1973 Nov;12(5):984–994. doi: 10.1128/jvi.12.5.984-994.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J., Westphal H., Srinivasan P. R., Dulbecco R. The integrated state of viral DNA in SV40-transformed cells. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1288–1295. doi: 10.1073/pnas.60.4.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sveda M. M., Fields B. N., Soeiro R. Host restriction of friend leukemia virus; fate of input virion RNA. Cell. 1974 Aug;2(4):271–277. doi: 10.1016/0092-8674(74)90021-x. [DOI] [PubMed] [Google Scholar]
- TEMIN H. M. THE PARTICIPATION OF DNA IN ROUS SARCOMA VIRUS PRODUCTION. Virology. 1964 Aug;23:486–494. doi: 10.1016/0042-6822(64)90232-6. [DOI] [PubMed] [Google Scholar]
- Takano T., Hatanaka M. Fate of viral RNA of murine leukemia virus after infection. Proc Natl Acad Sci U S A. 1975 Jan;72(1):343–347. doi: 10.1073/pnas.72.1.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temin H. M. Mechanism of cell transformation by RNA tumor viruses. Annu Rev Microbiol. 1971;25:609–648. doi: 10.1146/annurev.mi.25.100171.003141. [DOI] [PubMed] [Google Scholar]
- Temin H. M., Mizutani S. RNA-dependent DNA polymerase in virions of Rous sarcoma virus. Nature. 1970 Jun 27;226(5252):1211–1213. doi: 10.1038/2261211a0. [DOI] [PubMed] [Google Scholar]
- Tuominen F. W., Kenney F. T. Inhibition of RNA-directed DNA polymerase from Rauscher leukemia virus by the 5'-triphosphate of cytosine arabinoside. Biochem Biophys Res Commun. 1972 Sep 26;48(6):1469–1475. doi: 10.1016/0006-291x(72)90879-0. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Vogt P. K., Bishop J. M. Integration of deoxyribonucleic acid specific for Rous sarcoma virus after infection of permissive and nonpermissive hosts. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3067–3071. doi: 10.1073/pnas.70.11.3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varmus H. E., Vogt P. K., Bishop J. M. Integration of deoxyribonucleic acid specific for Rous sarcoma virus after infection of permissive and nonpermissive hosts. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3067–3071. doi: 10.1073/pnas.70.11.3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright B. S., O'Brien P. A., Shibley G. P., Mayyasi S. A., Lasfargues J. C. Infection of an established mouse bone marrow cell line (JLS-V9) with Rauscher and Moloney murine leukemia viruses. Cancer Res. 1967 Sep;27(9):1672–1677. [PubMed] [Google Scholar]



