Introduction
Although the death rates from cardiovascular diseases (CVD) have declined over the last decades, the burden of disease remains high and CVD remains one of the leading causes of morbidity and mortality in Western developed countries [1, 2] including the Netherlands. [3–6] Tremendous efforts worldwide are put into research and development of novel treatment modalities for CVD to further halt the disease burden, and frequent updates of primary and secondary prevention guidelines should result in the most optimal treatment of each patient with CVD. Besides major improvements in pharmacological treatment with for instance statins, beta-blockers, angiotensin-converting-enzyme inhibitors and antiplatelet agents, interventional treatment modalities such as percutaneous coronary intervention (PCI) have markedly improved the outcome of patients with coronary artery disease.
An important complication of percutaneous coronary intervention (PCI) that is still present is coronary restenosis.[7] Coronary restenosis - the renarrowing of the treated obstruction - results in high morbidity [8] and is even reported to be associated with an increased risk of mortality.[9, 10] Data on long-term follow-up of patients with restenosis are, however, scarce. To explore the relation of restenosis development with long-term mortality, we analysed the 10-year survival of the patients included in the GENetic DEterminants of Restenosis (GENDER) study.[11] The second objective of this report was to investigate whether the treatment strategies after PCI, applied in daily clinical practice over the past 10 years, resulted in a shift in mortality rates and causes of death of these confirmed coronary artery disease (CAD) patients compared with the general population.
Methods
The GENDER project was designed to study the genetic background of coronary restenosis development after successful PCI. It is a multicentre prospective follow-up study with clinical restenosis within 9 months as its primary endpoint. The first patient was included in March 1999 and inclusion lasted up to June 2001. In total 3104 consecutive patients were treated successfully by PCI for stable angina, non-ST-elevation acute coronary syndromes or silent ischaemia in four referral centres for interventional cardiology in the Netherlands: Academic Medical Center in Amsterdam, University Medical Center Groningen, Leiden University Medical Center and University Hospital Maastricht. Only patients treated for acute ST-elevation myocardial infarction (MI) were excluded. The study was primarily supported by the Interuniversity Cardiology Institute of the Netherlands.
The study protocol conforms to the Declaration of Helsinki and was approved by the ethics committees of each participating institution. After having obtained written informed consent, blood was sampled for DNA isolation and future analysis. Clinical and procedural data were gathered prospectively. Clinical restenosis was defined as the composite of death, myocardial infarction and target vessel revascularisation. An independent endpoint committee evaluated all potential endpoints. During the in-study follow-up period, 346 of the patients developed clinical restenosis. After the follow-up period patients returned to usual guideline-based clinical care, either with the general practitioner or if deemed necessary with the cardiologist. Currently, approximately 10 years after completion of the study, we have obtained long-term mortality data, including specific causes of death, for the complete GENDER population from the Dutch Central Bureau of Statistics (CBS). Data were available up to 31 December 2011 and the primary cause of death was described by ICD-10 codes.
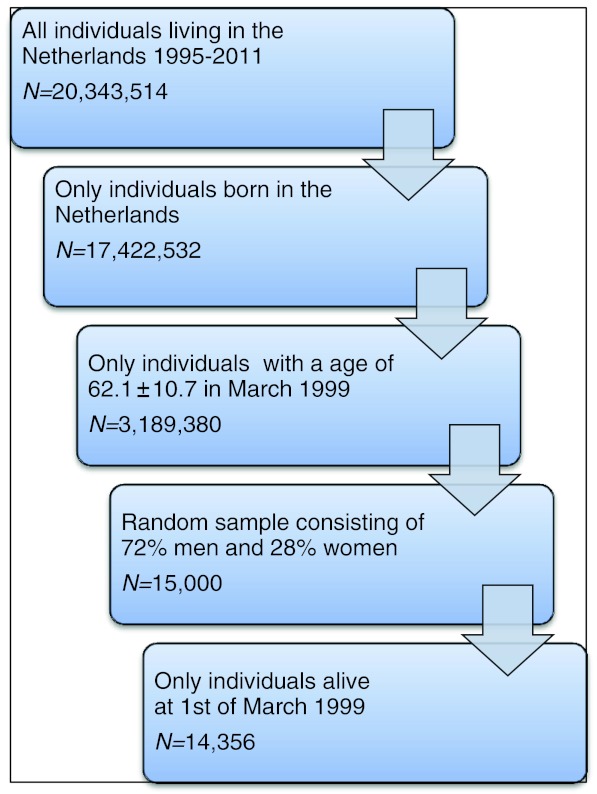
As a control population for the GENDER study we extracted a random sample of 15,000 individuals, matched for age and gender, from the general population of individuals born in the Netherlands and living in the Netherlands in the timeframe 1995–2011. Individuals who died before March 1999 were excluded from further analyses (N = 644) (Fig. 1).
Fig. 1.
Random sample of general Dutch population, matched for age and sex
Results and discussion
Restenosis and mortality
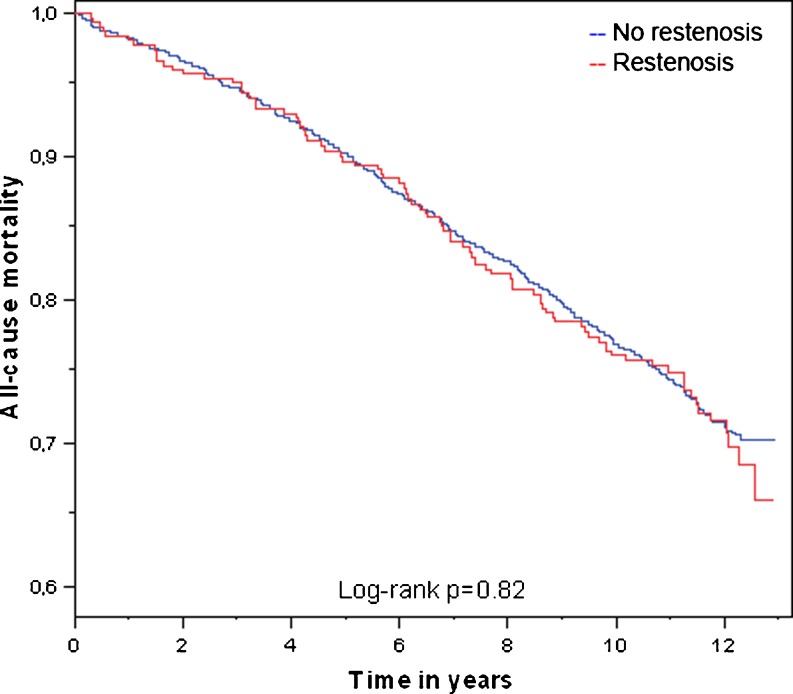
Baseline characteristics of the GENDER study population are shown in Table 1. Twenty patients (0.6 %) of the total GENDER population could not be identified in the CBS database and were considered lost to follow-up. A total of 865 patients died between inclusion in the GENDER study and 1 January 2012. When comparing the patients suffering from coronary restenosis after PCI with those who did not develop restenosis, no differences in mortality rates were observed (Fig. 2). Of the 300 patients with restenosis, 86 (28.7 %) died, which was similar to the 770 (27.7 %) of the patients without restenosis (N = 2784), hazard ratio (HR) 1.13, 95 % confidence interval (CI) 0.90–1.41, p = 0.28, with adjustment for age and sex. Also no difference was observed for death due specifically to CVD (12.3 % versus 11.4 % in the group with restenosis and the group without respectively, p = 0.64).
Table 1.
Baseline characteristics of the GENDER study population
| Parameter | GENDER study population (N = 3104) |
|---|---|
| Age | 62.1 ± 10.7 |
| Sex (male) | 2219 (71.5 %) |
| Diabetes | 453 (14.6 %) |
| Hypertension | 1259 (40.6 %) |
| Hypercholesterolaemia | 1890 (60.9 %) |
| Current smoker | 762 (24.5 %) |
| Multi-vessel disease | 1462 (46.1 %) |
| Total occlusion | 428 (13.8 %) |
| Stenting | 2309 (74.4 %) |
| Residual stenosis >20 % | 350 (11.3 %) |
Fig. 2.
Kaplan-Meier curve comparing mortality of patients with and without coronary restenosis of the GENDER study population
All-cause mortality in GENDER compared with the general population
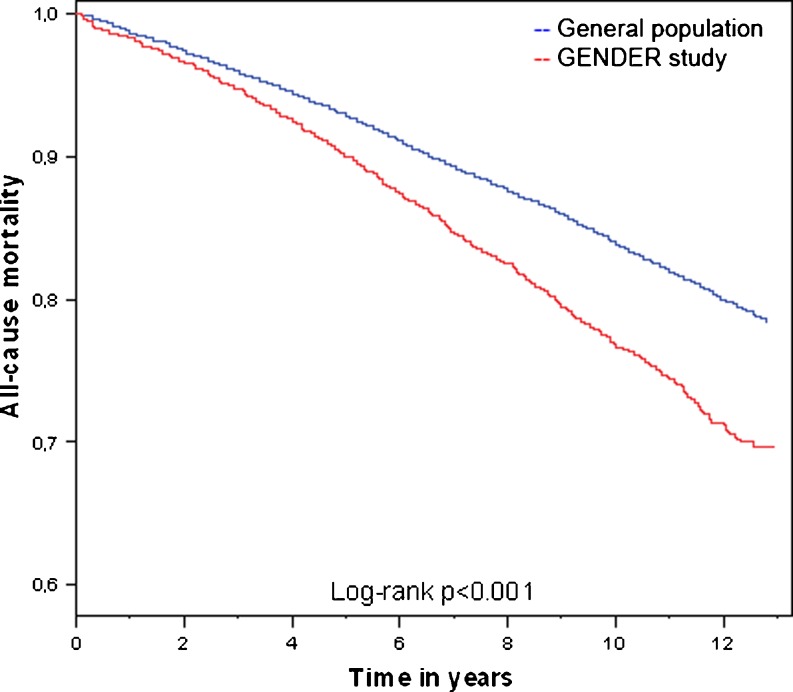
When comparing the total GENDER population with a random sample from the general population, we observed a higher mortality rate in the GENDER study population than in the control cohort, 27.8 % versus 21.6 %, P < 0.001. This difference is visualised in Fig. 3. However, when adjusting for age and sex, this increased 10-year mortality risk was no longer statistically significant, HR of 1.05, 95 % CI 0.97–1.14, p = 0.24. This was mainly caused by the influence of sex. The male subjects of GENDER did not have an increased all-cause mortality risk compared with the general population (HR 0.97, 95 % CI 0.87–1.05). In contrast, female patients with known coronary artery disease had an 1.39-fold (95 % CI 1.18–1.63, p < 0.001) increased risk to die during long-term follow-up, compared with the female individuals in the general population.
Fig. 3.
Kaplan-Meier curves comparing mortality of the GENDER study population (n = 3084) with a random sample from the Dutch general population (n = 14,356)
Primary cause of death
In Table 2, the primary causes of death are shown. The majority of the patients died of a cardiovascular cause (N = 355, 11.5 % of the total population and 41.5 % of all-cause mortality). Compared with the random sample from the general Dutch population, cardiovascular causes of death were more frequent in the GENDER study, 11.5 % (N = 355) versus 6.7 % (N = 957), p < 0.001. Patients in the GENDER cohort had a 1.29-fold increased risk (95 % CI 1.13–1.48, p < 0.001) for CVD death compared with the control cohort, adjusted for age and sex. Most of these CVD deaths were caused by acute myocardial infarction in both cohorts, 91 (3.0 % of the total population) in GENDER and 244 (1.6 % of the total population) in the control group respectively. Death due to stroke accounted for a similar percentage of all deaths in both populations, N = 47 (1.5 %) in GENDER versus N = 199 (1.3 %) in the control cohort.
Table 2.
Causes of death of the GENDER study population and of the Dutch general population from March 1999 up to December 2011
| Cause | ICD-10 code | GENDER (N = 3084) | General population (N = 14,356) | P-value | ||
|---|---|---|---|---|---|---|
| N (%) | %a | N (%) | %a | |||
| Cardiovascular disease | I000-I999 | 355 (11.5 %) | 41.5 % | 957 (6.7 %) | 30.8 % | <0.001 |
| Cancer | C000-D489 | 245 (7.9 %) | 28.6 % | 1283 (8.9 %) | 41.3 % | 0.08 |
| Infectious | A000-B999, J120-J180 | 34 (1.1 %) | 4.0 % | 140 (1.0 %) | 4.5 % | 0.39 |
| Metabolic disease | E000-E909 | 36 (1.2 %) | 4.2 % | 80 (0.6 %) | 2.6 % | <0.001 |
| Psychic disorders | F000-F999 | 17 (0.6 %) | 2.0 % | 56 (0.4 %) | 1.8 % | 0.21 |
| Respiratory systemb | J000-J999 (excl. J120-J180) | 41 (1.3 %) | 4.8 % | 192 (1.3 %) | 6.2 % | 0.97 |
| Gastro-intestinal system | K000-K939 | 29 (0.9 %) | 3.4 % | 101 (0.7 %) | 3.2 % | 0.17 |
| Urinary tract and reproductive organs | N000-N999 | 23 (0.7 %) | 2.7 % | 43 (0.3 %) | 1.4 % | <0.001 |
| Non-natural cause of death | V010-Y899 | 20 (0.6 %) | 2.3 % | 68 (0.5 %) | 2.2 % | 0.21 |
| Others | 21 (0.7 %) | 2.5 % | 79 (0.6 %) | 2.5 % | 0.41 | |
| Unknown | R000-R999 | 35 (1.1 %) | 4.1 % | 109 (0.8 %) | 3.5 % | 0.04 |
| Total | 856 (27.8 %) | 100.0 % | 3108 (21.6 %) | 100 % | <0.001 | |
aPercentage of all-cause mortality. bExcluding pneumonia
The second major cause of death in GENDER was cancer-related. A total of 2045 patients (7.9 % of the population and 28.6 % of all-cause mortality) died of cancer, which was slightly less frequent than in the general population (8.6 % of the population and 41.3 % of all-cause mortality). However, this difference was not statistically significant (p = 0.08). The patients included in GENDER did have a higher risk of dying from a metabolic disease, mostly diabetes, a well-known risk factor for CVD. Moreover, also death caused by urological disorders or diseases of the reproductive organs was higher in the GENDER population than in the general population. No differences were observed between other causes of death. Approximately 4 % of deaths were of infectious nature, 2 % due psychiatric disorders, 3 % due pulmonary disease and 2 % due to non-natural causes.
Cardiovascular death in GENDER
When looking more specifically into the cardiovascular causes of death in the GENDER population, ischaemic heart disease was the most frequent cause of death, responsible for almost half (47 %) of all CVD deaths (Table 3). Of these 167 patients dying from ischaemic heart disease, 91 died as the consequence of an acute myocardial infarction. Heart failure was the next most frequent occurring CVD death (15.4 %), followed by cerebrovascular disease (13.1 %) and valvular disease (7.9 %). Rhythm and conductions disorders and aneurysmal disease each accounted for approximately 4 % of the CVD related deaths.
Table 3.
Specific cardiovascular causes of death of the GENDER population
| Cause of death | ICD-10 code | GENDER | |
|---|---|---|---|
| N | %a | ||
| All cardiovascular disease | I000-I999 | 355 | 100 % |
| Ischaemic heart disease | I200-I259 | 167 | 47.0 % |
| Acute myocardial infarction | I210-I219 | 91 | 25.6 % |
| Valve disease | I340-I389 | 28 | 7.9 % |
| Rhythm and conduction disorders | I440-I499 | 16 | 4.5 % |
| Heart failure and cardiomyopathies | I420-I439, I500-I509 | 54 | 15.2 % |
| Cerebrovascular disease | I600-I699 | 47 | 13.2 % |
| Aneurysmal disease | I710-I729 | 15 | 4.2 % |
| Other cardiovascular disease | 28 | 7.8 % | |
Other cardiovascular disease includes hypertensive heart disease, rheumatic heart disease, pulmonary heart disease, peripheral vascular disease and unspecified heart disease
aPercentage of all cardiovascular death
Limitations
One study limitation should be mentioned. For this study we analysed the primary cause of death recorded by the Dutch CBS. Although this is the most specific mortality data available a small margin of error should be taken into account. For instance, the coronary artery disease patients in GENDER who are now classified with diabetes as their primary cause of death, likely died from a cardiovascular cause of death instead. Autopsy would result in the most specific cause of death, but this is not performed in most individuals.
Conclusions
The data presented above allow two conclusions. First, although previous studies have shown that patients developing coronary restenosis after PCI have a higher (CVD) morbidity and mortality[9, 10], the current study demonstrates that, in the long-term, no differences in mortality rates are present between patients who develop restenosis and those who do not develop restenosis. This indicates that the current treatment of coronary restenosis, by repeated intervention, is good enough to prevent an increased mortality risk in the long-term. However, this patient population with confirmed coronary artery disease, despite guideline-based treatment, does have an increased mortality risk compared with the general population, especially pronounced in female patients. Since this increased risk is still attributable to CVD mortality, and not to other causes of death, there still seems room to improve current treatment strategies for CVD. Although speculative, likely the most room for improvement is in primary prevention and not secondary prevention as in the current study population. Future studies in both primary and secondary prevention cohorts will show us whether we are still heading in the right direction for optimal treatment of our patients, ultimately shifting their mortality risk all the way back to that of the general population.
References
- 1.Murray CJ, Lopez AD. Mortality by cause for eight regions of the world: global burden of disease study. Lancet. 1997;349(9061):1269–1276. doi: 10.1016/S0140-6736(96)07493-4. [DOI] [PubMed] [Google Scholar]
- 2.Roger VL, Go AS, Lloyd-Jones DM, et al. Executive summary: heart disease and stroke statistics–2012 update: a report from the American Heart Association. Circulation. 2012;125(1):188–197. doi: 10.1161/CIR.0b013e3182456d46. [DOI] [PubMed] [Google Scholar]
- 3.Vaartjes I, O’Flaherty M, Grobbee DE, et al. Coronary heart disease mortality trends in the Netherlands 1972–2007. Heart. 2011;97(7):569–573. doi: 10.1136/hrt.2010.206565. [DOI] [PubMed] [Google Scholar]
- 4.van Nooten F, Davies GM, Jukema JW, et al. Economic evaluation of ezetimibe combined with simvastatin for the treatment of primary hypercholesterolaemia. Neth Heart J. 2011;19:61–67. doi: 10.1007/s12471-010-0061-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liew D, Webb K, Meerding WJ, et al. Potential cardiovascular consequences of switching from atorvastatin to generic simvastatin in the Netherlands. Neth Heart J. 2012;20:197–201. doi: 10.1007/s12471-012-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dharma S, Juzar DA, Firdaus I, et al. Acute myocardial infarction system of care in the third world. Neth Heart J. 2012;20:254–259. doi: 10.1007/s12471-012-0259-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jukema JW, Verschuren JJ, Ahmed TA, et al. Restenosis after PCI. Part 1: pathophysiology and risk factors. Nat Rev Cardiol. 2012;9(1):53–62. doi: 10.1038/nrcardio.2011.132. [DOI] [PubMed] [Google Scholar]
- 8.Weintraub WS, Ghazzal ZM, Douglas JS, Jr, et al. Long-term clinical follow-up in patients with angiographic restudy after successful angioplasty. Circulation. 1993;87(3):831–840. doi: 10.1161/01.CIR.87.3.831. [DOI] [PubMed] [Google Scholar]
- 9.Espinola-Klein C, Rupprecht HJ, Erbel R, et al. Impact of restenosis 10 years after coronary angioplasty. Eur Heart J. 1998;19(7):1047–1053. doi: 10.1053/euhj.1997.0863. [DOI] [PubMed] [Google Scholar]
- 10.Schuhlen H, Kastrati A, Mehilli J, et al. Restenosis detected by routine angiographic follow-up and late mortality after coronary stent placement. Am Heart J. 2004;147(2):317–322. doi: 10.1016/j.ahj.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 11.Agema WR, Monraats PS, Zwinderman AH, et al. Current PTCA practice and clinical outcomes in the Netherlands: the real world in the pre-drug-eluting stent era. Eur Heart J. 2004;25(13):1163–1170. doi: 10.1016/j.ehj.2004.05.006. [DOI] [PubMed] [Google Scholar]