Abstract
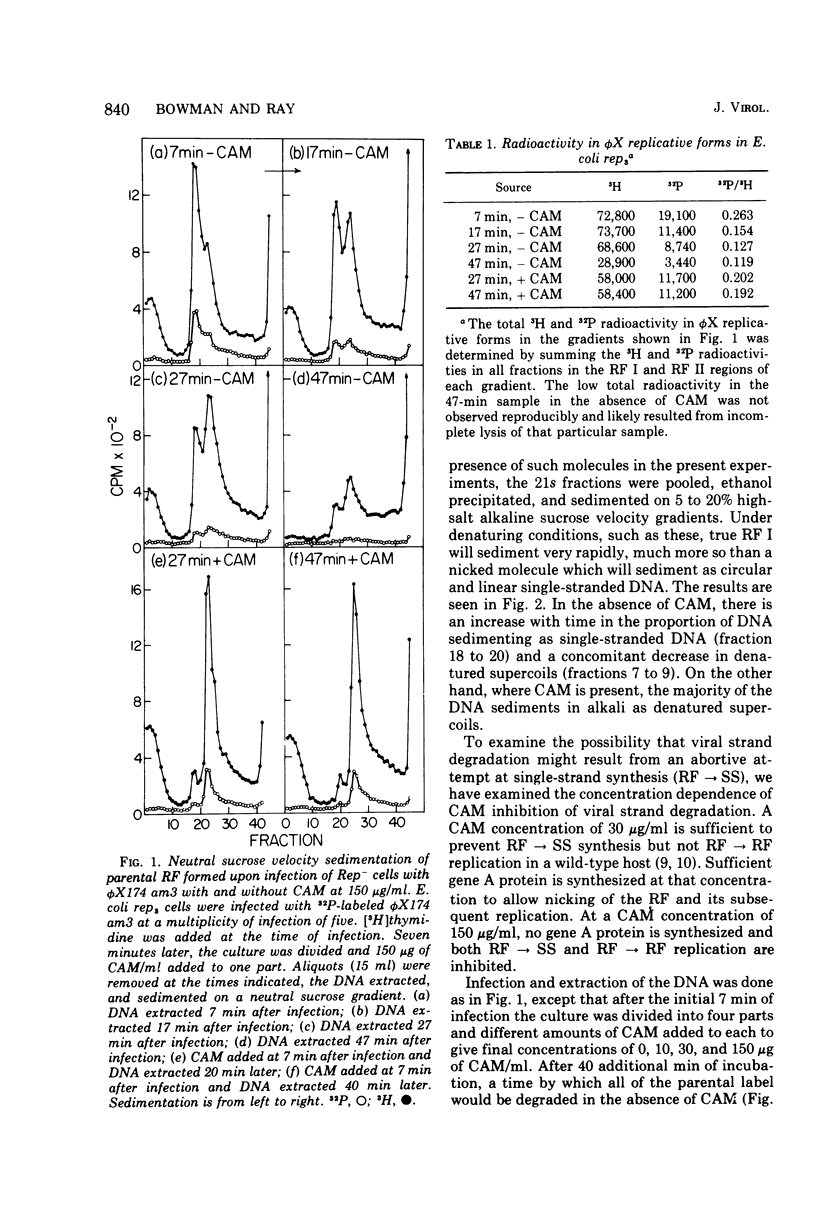
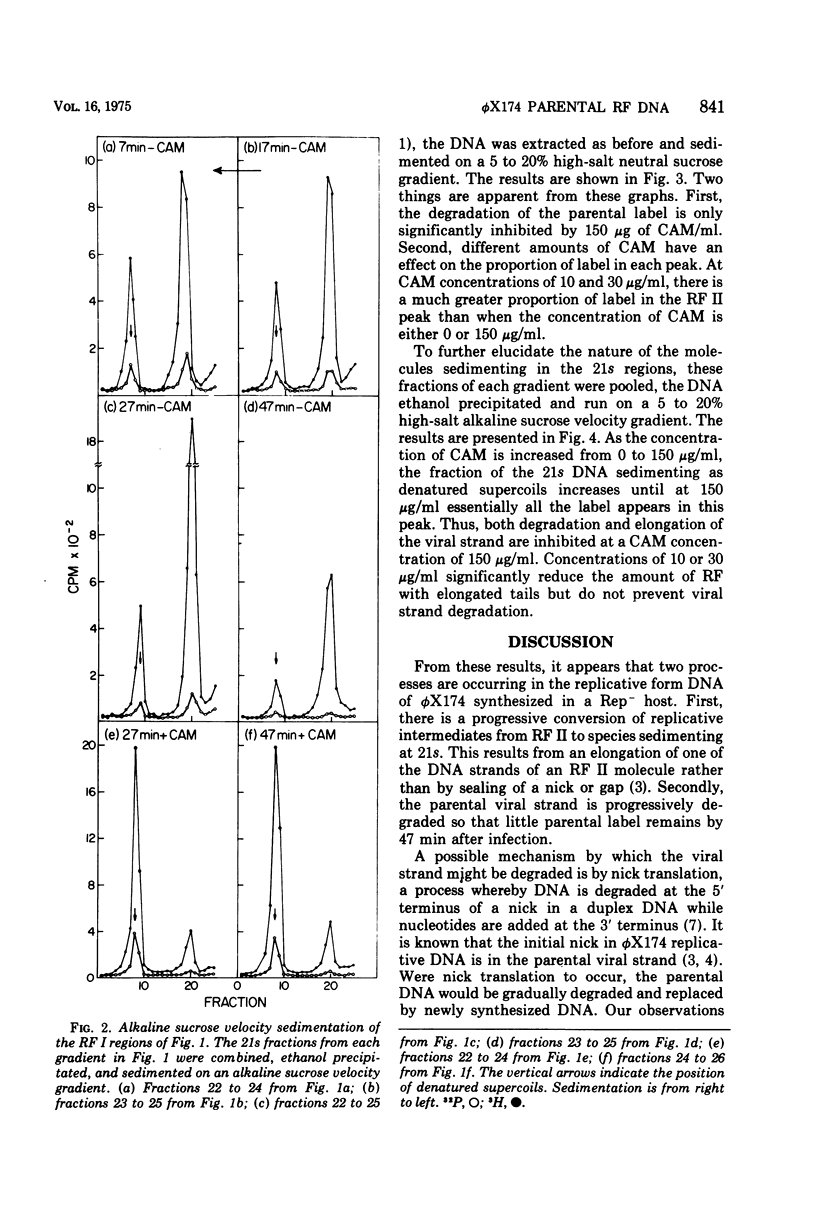
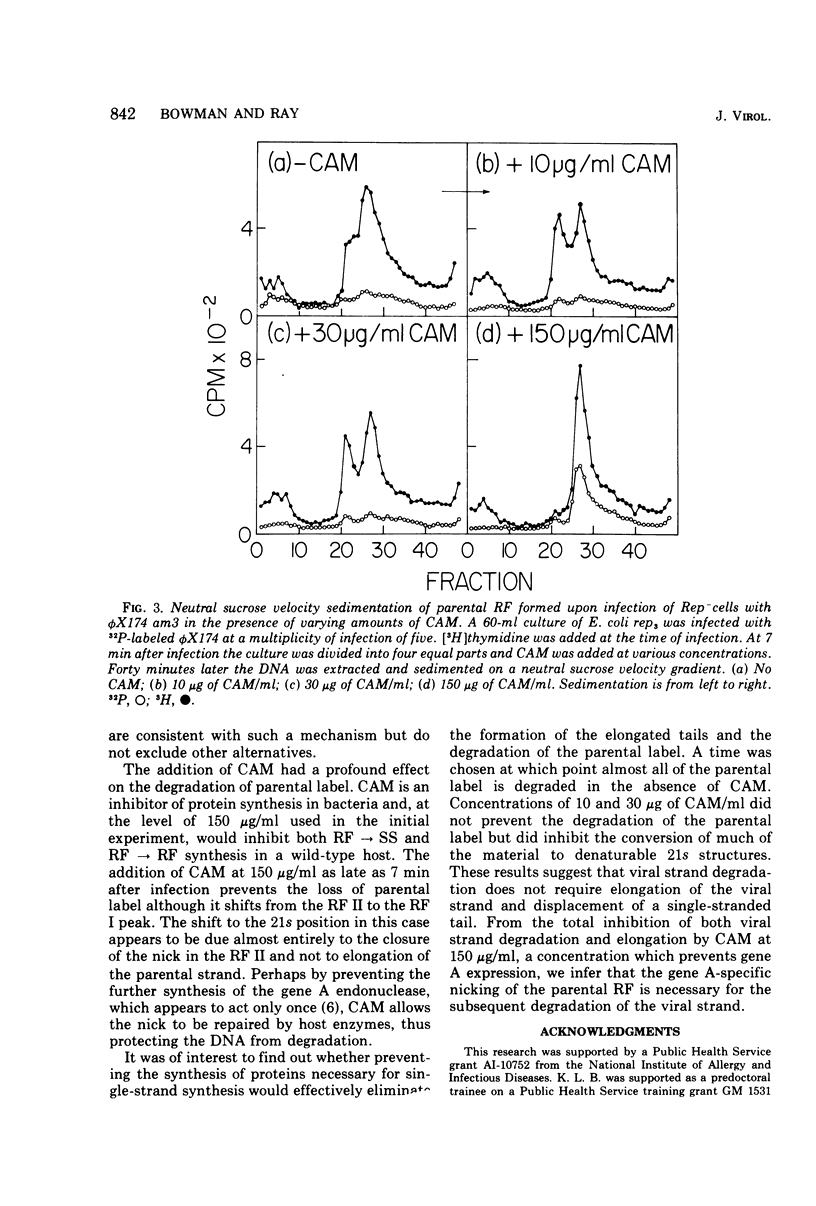
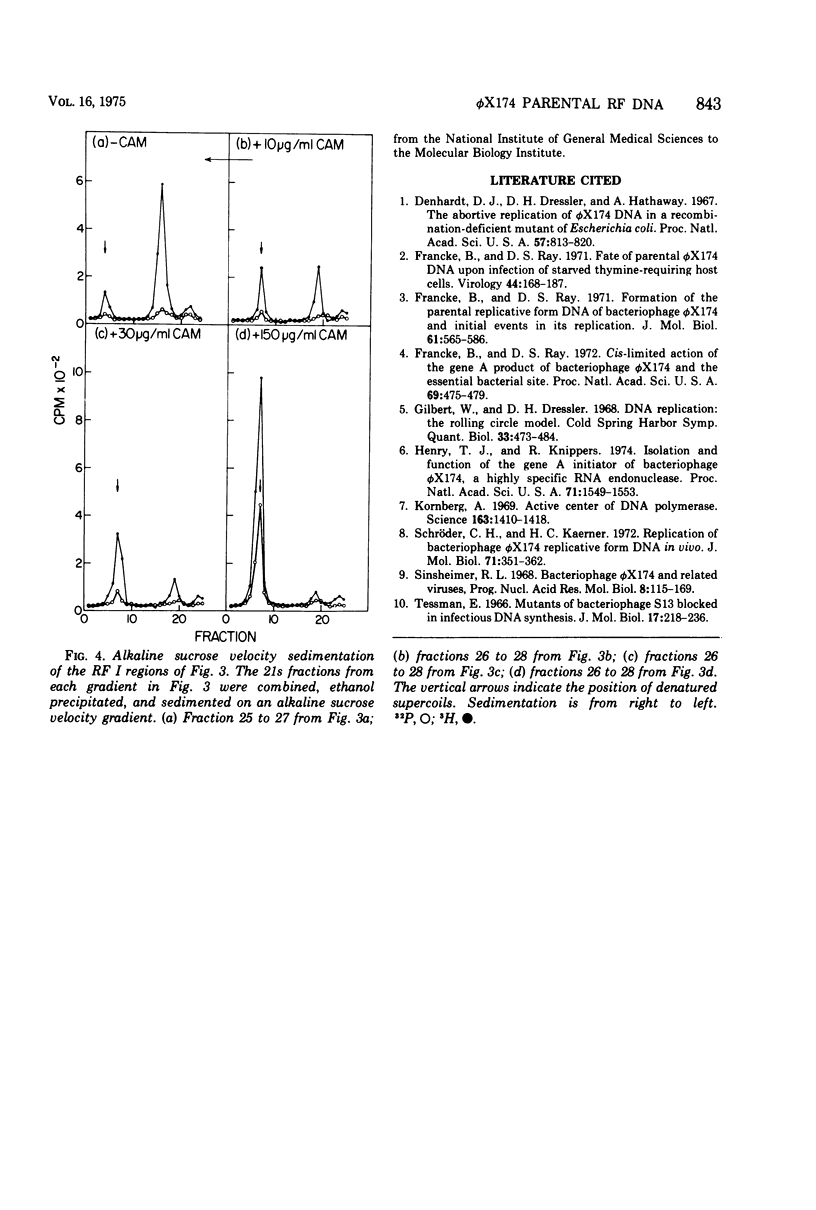
A progressive degradation of the parental viral strand label is observed upon infection of a Rep- mutant of Escherichia coli by 32P-labeled phiX174. Very little parental label remains in the RF (replicative form) by 47 min after infection. Concomitant with this degradation, replicative intermediates are formed which sediment at 21s, the rate of RF I (supercoiled-closed circular DNA), in a neutral sucrose gradient but which denature and sediment in alkaline gradients as single strands of unit size and larger. These denaturable 21s replicative intermediates have been shown previously to be RF molecules containing an elongated viral strand. Addition of chloramphenicol at 7 min after infection at 30 mug/ml, a concentration sufficient to block RF leads to SS (single strand) synthesis but not RF leads to RF synthesis in a wild-type host cell, reduced the amount of viral strand elongation but did not prevent viral strand degradation. The addition of chloramphenicol at 150 mug/ml at 7 min after infection totally prevents both the degradation of the parental label and the formation of the replicative intermediates with elongated tails. We infer that degradation of the viral strand requires the gene A-mediated nicking of the viral strand but not the concomitant elongation of the viral strand.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Denhardt D. T., Dressler D. H., Hathaway A. THE ABORTIVE REPLICATION OF PhiX174 DNA IN A RECOMBINATION-DEFICIENT MUTANT OF Escherichia coli. Proc Natl Acad Sci U S A. 1967 Mar;57(3):813–820. doi: 10.1073/pnas.57.3.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francke B., Ray D. S. Cis-limited action of the gene-A product of bacteriophage phiX174 and the essential bacterial site (E. coli-electron microscopy-cis-acting protein-specifically-nicked RF). Proc Natl Acad Sci U S A. 1972 Feb;69(2):475–479. doi: 10.1073/pnas.69.2.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francke B., Ray D. S. Fate of parental phi X174-DNA upon infection of starved thymine-requiring host cells. Virology. 1971 Apr;44(1):168–187. doi: 10.1016/0042-6822(71)90163-2. [DOI] [PubMed] [Google Scholar]
- Francke B., Ray D. S. Formation of the parental replicative form DNA of bacteriophage phi-X174 and initial events in its replication. J Mol Biol. 1971 Nov 14;61(3):565–586. doi: 10.1016/0022-2836(71)90065-9. [DOI] [PubMed] [Google Scholar]
- Gilbert W., Dressler D. DNA replication: the rolling circle model. Cold Spring Harb Symp Quant Biol. 1968;33:473–484. doi: 10.1101/sqb.1968.033.01.055. [DOI] [PubMed] [Google Scholar]
- Henry T. J., Knippers R. Isolation and function of the gene A initiator of bacteriophage phi-chi 174, a highly specific DNA endonuclease. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1549–1553. doi: 10.1073/pnas.71.4.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg A. Active center of DNA polymerase. Science. 1969 Mar 28;163(3874):1410–1418. doi: 10.1126/science.163.3874.1410. [DOI] [PubMed] [Google Scholar]
- Schröder C. H., Kaerner H. C. Replication of bacteriophage phichi174 replicative form DNA in vivo. J Mol Biol. 1972 Nov 14;71(2):351–362. doi: 10.1016/0022-2836(72)90356-7. [DOI] [PubMed] [Google Scholar]
- Sinsheimer R. L. Bacteriophage phi-X174 and related viruses. Prog Nucleic Acid Res Mol Biol. 1968;8:115–169. [PubMed] [Google Scholar]
- Tessman E. S. Mutants of bacteriophage S13 blocked in infectious DNA synthesis. J Mol Biol. 1966 May;17(1):218–236. doi: 10.1016/s0022-2836(66)80104-3. [DOI] [PubMed] [Google Scholar]



