Abstract
In this study, the Vaccine Adverse Event Reporting System (VAERS) database, 1990–2010, was investigated; cases that specified either hospitalization or death were identified among 38,801 reports of infants. Based on the types of vaccines reported, the actual number of vaccine doses administered, from 1 to 8, was summed for each case. Linear regression analysis of hospitalization rates as a function of (a) the number of reported vaccine doses and (b) patient age yielded a linear relationship with r 2 = 0.91 and r 2 = 0.95, respectively. The hospitalization rate increased linearly from 11.0% (107 of 969) for 2 doses to 23.5% (661 of 2817) for 8 doses and decreased linearly from 20.1% (154 of 765) for children aged <0.1 year to 10.7% (86 of 801) for children aged 0.9 year. The rate ratio (RR) of the mortality rate for 5–8 vaccine doses to 1–4 vaccine doses is 1.5 (95% confidence interval (CI), 1.4–1.7), indicating a statistically significant increase from 3.6% (95% CI, 3.2–3.9%) deaths associated with 1–4 vaccine doses to 5.5% (95% CI, 5.2–5.7%) associated with 5–8 vaccine doses. The male-to-female mortality RR was 1.4 (95% CI, 1.3–1.5). Our findings show a positive correlation between the number of vaccine doses administered and the percentage of hospitalizations and deaths. Since vaccines are given to millions of infants annually, it is imperative that health authorities have scientific data from synergistic toxicity studies on all combinations of vaccines that infants might receive. Finding ways to increase vaccine safety should be the highest priority.
Keywords: VAERS, vaccine, childhood vaccines, immunization, epidemiology, infant mortality, SIDS, drug toxicology, human toxicology
Introduction
In 1986, Congress passed the National Childhood Vaccine Injury Act (PL-99-660) requiring health care providers to report suspected vaccine reactions to a centralized reporting system. As a result, the Vaccine Adverse Events Reporting System (VAERS), cosponsored by the Centers for Disease Control and Prevention (CDC) and the Food and Drug Administration (FDA), was established in 1990. VAERS is a postmarketing safety surveillance program that collects information about possible adverse reactions (side effects) that occur after the administration of vaccines licensed for use in the United States. Current and historic VAERS data are public access, available to health care providers, vaccine manufacturers, and the general public.
VAERS receives approximately 30,000 reports annually. Since 1990, VAERS has received over 350,000 reports, most of which describe mild side effects, such as fever and local reactions. About 13% of all reactions are classified as serious, involving life-threatening conditions, hospitalization, permanent disability, or death. By monitoring such events, VAERS helps to identify unusual patterns of reports and important safety concerns.
Several factors could contribute to whether an infant will have an adverse reaction to vaccines, including a genetic predisposition, illness (which may be a contraindication to vaccine administration), quality of vaccines (which can vary by manufacturing methods), and sensitivity to one or more vaccine components. Some infants might be more likely to experience an adverse reaction due to biochemical or synergistic toxicity associated with concurrent administration of multiple vaccines. Yet, studies have not been conducted to determine the safety (or efficacy) of administering multiple vaccine doses during a single physician visit based on the CDC’s recommended vaccine schedule.
To explore the correlation between the total number of vaccine doses administered and serious adverse events reported, a statistical analysis was performed. Cases that specified either hospitalization or death were identified among infants, defined as children aged <1 year, for whom reports were filed in the VAERS database from 1990 through the end of 2010. Based on the quantity and types of vaccines reported, the actual number of vaccine doses was summed for each case. Thus, relative trends in reported hospitalization and mortality rates as a function of the number of vaccine doses and age could be investigated.
Methodology
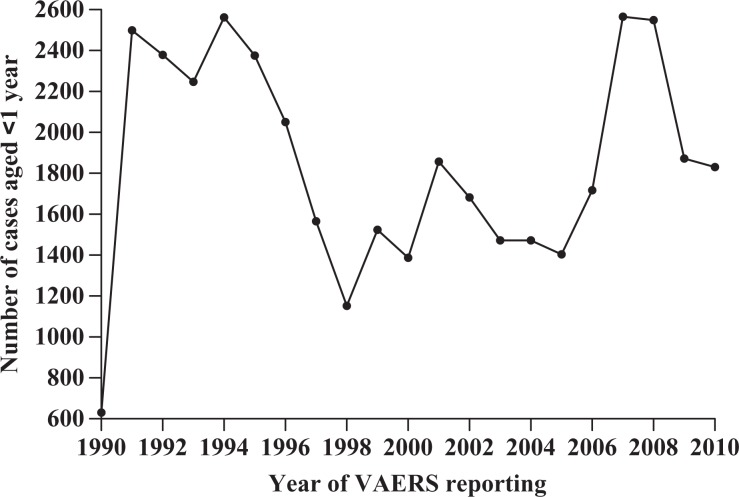
VAERS is a national passive reporting system managed by the CDC and FDA—agencies of the US Department of Health and Human Services. VAERS reports are filed by vaccine manufacturers (37%), health care providers (36%), state immunization programs (10%), vaccine recipients or their parent/guardians (7%), and other sources (10%). Each report includes information about the patients’ demographics, vaccine/vaccines administered, and symptoms related to their adverse event. The public access VAERS database, 1990 through 2010, was downloaded from the Internet (http://vaers.hhs.gov/data/data) on April 20, 2011, as comma-separated value (CSV) files (Figure 1). Records associated with 1990 were incomplete, containing only about 20% of the cases typically represented by each subsequent full year’s report. Foreign reports in the nondomestic VAERS database were not considered.
Figure 1.
Distribution of infant cases reported to Vaccine Adverse Event Reporting System (VAERS) by year, 1990–2010. Note: 1990 was a partial year of VAERS reporting.
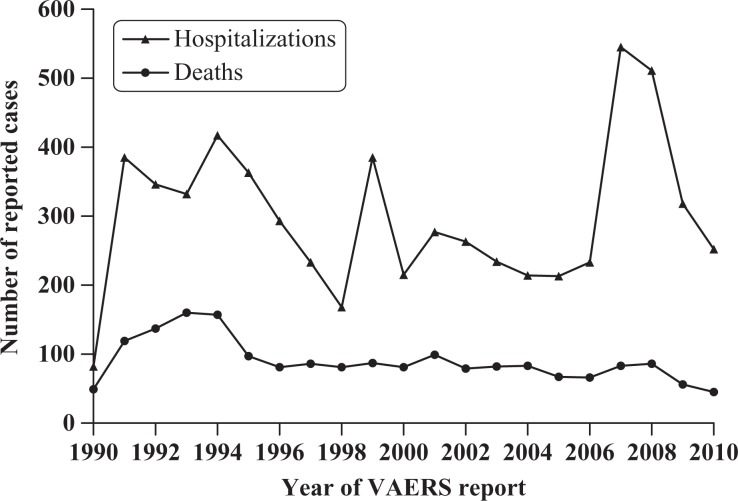
Of the total 327,331 cases contained in the downloaded VAERS files, 28,707 (8.7%) cases specified no age and another 10 cases (0.003%) specified an age of 100 years or older and thus were discarded. One VAERS case showed 0 vaccines, but this was corrected to indicate receipt of measles, mumps and rubella (MMR) vaccine (counted as 3 vaccine doses). Thus, 298,614 (91.2%) case reports of individuals aged <100 years were imported. Of these 298,614 total case reports, there were 39,082 (13.1%) infant cases. Of these, the cumulative 281 (0.7%) VAERS cases reporting 9 or more vaccine doses concurrently were insufficient to yield statistically significant results, especially when further stratified by age, and were therefore excluded from analysis. Thus, 38,801 (99.3%) infant cases receiving fewer than 9 vaccine doses concurrently were available for the various analyses performed with respect to hospitalization and mortality rates. The distributions by year of the total number of reported hospitalizations (6279) and deaths (1881) are shown in Figure 2.
Figure 2.
Distribution of infant cases reported as hospitalized or as a death to Vaccine Adverse Event Reporting System (VAERS) by year, 1990–2010. Note: 1990 was a partial year of VAERS reporting.
A Web-based (online) program (available at www.medicalveritas.com/vaers.php) was written (in HTML, Javascript, and PHP) to convert all specified vaccines for any given case to the equivalent number of doses (i.e., DTaP is administered with one injection but contains three separate vaccine doses for diphtheria, tetanus, and pertussis; Table 1). The data were then analyzed by inspecting the age of each case, summing the dose equivalents for each vaccine specified to obtain the total number of vaccine doses (1–8 doses) associated with each case. The hospitalization rate corresponding to each dose was computed by dividing the number of reported hospitalizations among infants for a given dose by the total number of VAERS reports received having that same given dose. The mortality rate was similarly computed, and both hospitalization and mortality rates were multiplied by a factor of 100 to yield a percentage figure.
Table 1.
Vaccine abbreviations as used in VAERS with the equivalent number of doses
| Vaccine | Doses |
|---|---|
| 6VAX-F | 3 |
| ADEN | 1 |
| ANTH | 1 |
| BCG | 1 |
| CEE | 1 |
| CHOL | 1 |
| DPIPV | 3 |
| DPP | 3 |
| DT | 2 |
| DTaP | 3 |
| DTAPH | 4 |
| DTAPHEPBIP | 5 |
| DTAPIPV | 4 |
| DTAPIPVHIB | 5 |
| DTIPV | 3 |
| DTOX | 1 |
| DTP | 3 |
| DTPHEP | 4 |
| DTPHIB | 4 |
| DTPIHI | 5 |
| DTPIPV | 4 |
| DTPPHIB | 5 |
| FLU | 1 |
| FLU(H1N1) | 1 |
| FLU(10-11) | 1 |
| FLUHD(10-11) | 1 |
| FLUN | 1 |
| FLUN(10-11) | 1 |
| FLUN(H1N1) | 1 |
| H5N1 | 1 |
| HBHEPB | 2 |
| HBPV | 1 |
| HEP | 1 |
| HEPA | 1 |
| HEPAB | 2 |
| HIBV | 1 |
| HPV | 1 |
| HPV2 | 1 |
| HPV4 | 1 |
| IPV | 1 |
| JEV | 1 |
| JEV1 | 1 |
| LYME | 1 |
| MEA | 1 |
| MEN | 1 |
| MER | 1 |
| MNC | 1 |
| MNQ | 1 |
| MM | 2 |
| MMR | 3 |
| MMRV | 4 |
| MU | 1 |
| MUR | 2 |
| OPV | 1 |
| PER | 1 |
| PLAGUE | 1 |
| PNC | 1 |
| PNC13 | 1 |
| PPV | 1 |
| RAB | 1 |
| ROT | 1 |
| ROTH1 | 1 |
| ROTHB5 | 1 |
| RUB | 1 |
| RV | 1 |
| SMALL | 1 |
| SSEV | 1 |
| TBE | 1 |
| TD | 2 |
| TDAP | 3 |
| TTOX | 1 |
| TYP | 1 |
| VARCEL | 1 |
| VARZOS | 1 |
| YF | 1 |
VAERS: Vaccine Adverse Event Reporting System.
The linear regression line for hospitalization rates versus the number of vaccine doses and the coefficient of determination (r2) were generated using GraphPad Prism, version 5.04 (GraphPad Software, San Diego, California, USA, www.graphpad.com). A similar procedure was used for hospitalization rates versus age and number of vaccine doses. Additionally, the F statistic and corresponding p value were computed to test whether each linear regression slope was statistically significantly nonzero. The 95% confidence intervals (CIs) reported for hospitalization and mortality rates are based on the Poisson distribution, which for large sample sizes approximates the normal distribution. A 2-way analysis of variance (ANOVA) was performed using factors of age (in 0.1-year increments) and number of vaccine doses (2–8 doses) to investigate the percentage of variance contributed by these factors.
Results
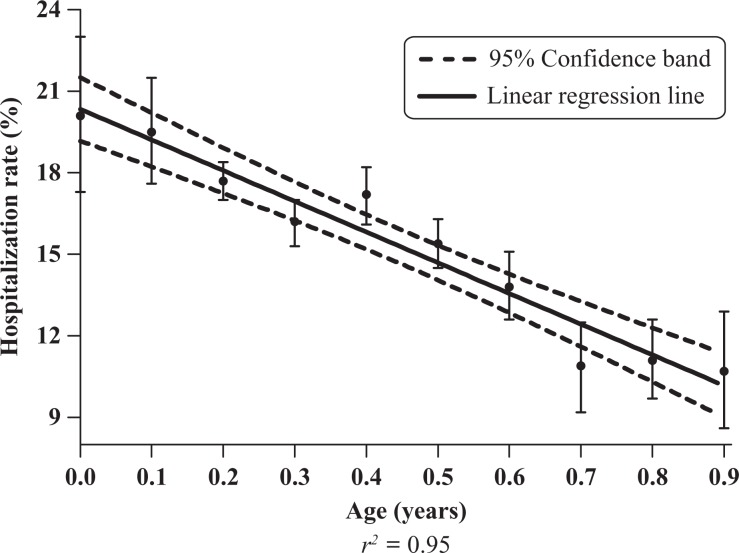
Linear regression analysis of hospitalization rates as a function of (a) the number of reported vaccine doses and (b) patient age yielded a linear relationship with r 2 = 0.91 and r 2 = 0.95, respectively (Figures 3 and 4). The hospitalization rate increased linearly from 11.0% (107 of 969) for 2 doses to 23.5% (661 of 2817) for 8 doses (Table 2), and decreased linearly from 20.1% (154 of 765) for children aged <0.1 year to 10.7% (86 of 801) for children aged 0.9 year (Table 3).
Figure 3.
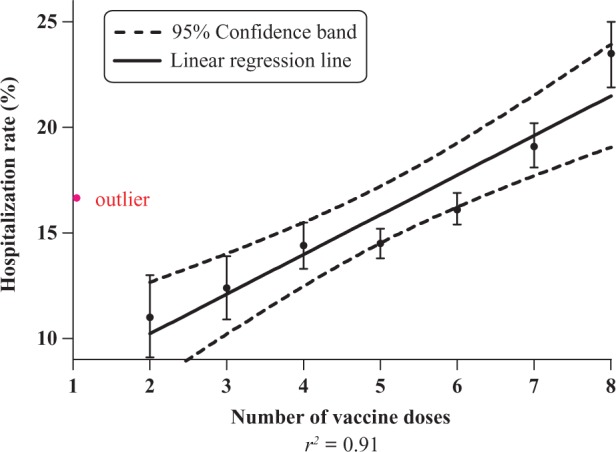
Hospitalization rate (%) versus the number of vaccine doses among infants, Vaccine Adverse Event Reporting System (VAERS), 1990–2010.
Figure 4.
Hospitalization rate (%) versus age (in 0.1 year increments) among infants receiving 1–8 vaccine doses, Vaccine Adverse Event Reporting System (VAERS), 1990–2010.
Table 2.
Hospitalization rate (%), stratified by number of vaccine doses reported among infants, VAERS 1990–2010 database
| No. of vaccine doses | Number reported hospitalized | Total no. of reports | Hospitalization rate, % | 95% confidence interval |
|---|---|---|---|---|
| 1a | 828 | 5090 | 16.3 | 15.3–17.3 |
| 2 | 107 | 969 | 11.0 | 9.1–13.0 |
| 3 | 243 | 1959 | 12.4 | 10.9–13.9 |
| 4 | 561 | 3909 | 14.4 | 13.3–15.5 |
| 5 | 1463 | 10,114 | 14.5 | 13.8–15.2 |
| 6 | 1365 | 8454 | 16.1 | 15.4–16.9 |
| 7 | 1051 | 5489 | 19.1 | 18.1–20.2 |
| 8 | 661 | 2817 | 23.5 | 21.9–25.0 |
| Totals 1–8 | 6279 | 38,801 | 16.2 | 15.8–16.5 |
VAERS: Vaccine Adverse Event Reporting System.
a One dose is considered an outlier for reasons given in the discussion section of the article.
Table 3.
Hospitalization rate (%) among infants receiving 1–8 reported vaccine doses, stratified by age (in 0.1 year increments), VAERS 1990–2010 database
| Age (years) | Number reported hospitalized | Total no. of reports | Hospitalization rate, % | 95% confidence interval |
|---|---|---|---|---|
| 0.0 | 154 | 765 | 20.1 | 17.3–23.0 |
| 0.1 | 308 | 1576 | 19.5 | 17.6–21.5 |
| 0.2 | 2210 | 12,476 | 17.7 | 17.0–18.4 |
| 0.3 | 1115 | 6897 | 16.2 | 15.3–17.0 |
| 0.4 | 806 | 4694 | 17.2 | 16.1–18.2 |
| 0.5 | 858 | 5572 | 15.4 | 14.5–16.3 |
| 0.6 | 385 | 2780 | 13.8 | 12.6–15.1 |
| 0.7 | 149 | 1372 | 10.9 | 9.2–12.5 |
| 0.8 | 208 | 1868 | 11.1 | 9.7–12.6 |
| 0.9 | 86 | 801 | 10.7 | 8.6–12.9 |
| Totals aged <1 year | 6279 | 38,801 | 16.2 | 15.8–16.5 |
VAERS: Vaccine Adverse Event Reporting System.
When the outlier associated with the hospitalization rate for 1 dose is included, the linear correlation using 1–8 doses is weakened with a reduced coefficient of determination, r 2 = 0.57 (F = 8.0; p < 0.03).
A two-way ANOVA using the number of vaccine doses (2–8) and age, ranging from 0.1 to 0.9 years in 0.1 increments, was unproductive due to the too large an interaction between age and dose, particularly with those aged 0.6–0.9 years. When restricted to ages 0.1–0.5 years, the number of vaccine doses accounted for 85.3% of the total variation (F = 25.7, p < 0.001), the age factor was not significant at 1.4% (p = 0.64), and the residual was 13.3%.
The rate ratio (RR) of the mortality rate for 5–8 vaccine doses to 1–4 vaccine doses is 1.5 (95% CI, 1.4–1.7), indicating that the mortality rate of 3.6% (95% CI, 3.2–3.9%) associated with low vaccine doses is statistically significantly lower than 5.4% (95% CI, 5.2–5.7%) associated with higher vaccine doses (Table 4).
Table 4.
Mortality rate (%) among infants, stratified by the number of vaccine doses, VAERS 1990–2010 database
| Number of vaccine doses | Number of reported deaths | Total no. of reports | Mortality rate, % (95% CI) |
|---|---|---|---|
| 1 | 197 | 5090 | 3.9 (3.3–4.4) |
| 2 | 21 | 969 | 2.2 (1.3–3.1) |
| 3 | 42 | 1959 | 2.1 (1.5–2.8) |
| 4 | 163 | 3909 | 4.2 (3.5–4.8) |
| Combined 1–4 | 423 | 11,927 | 3.6 (3.2–3.9) |
| 5 | 523 | 10,114 | 5.2 (4.7–5.6) |
| 6 | 490 | 8454 | 5.8 (5.3–6.3) |
| 7 | 320 | 5489 | 5.8 (5.2–6.5) |
| 8 | 125 | 2817 | 4.4 (3.7–5.2) |
| Combined 5–8 | 1458 | 26,874 | 5.4 (5.2–5.7) |
| Combined 1–8 | 1881 | 38,801 | 4.9 (4.6–5.1) |
VAERS: Vaccine Adverse Event Reporting System.
The RR of the mortality rate for children aged <0.5 years to those aged 0.5–0.9 years is 3.0 (95% CI, 2.6–3.4), indicating that the mortality rate of 6.1% (95% CI, 5.9–6.4%) associated with children aged <0.5 is statistically significantly higher than 2.1% (95% CI, 1.8–2.3%) associated with children aged 0.5–0.9 years (Table 5).
Table 5.
Mortality rate (%), stratified by age (0 to <1 year), VAERS 1990–2010 database
| Case age (years) | Number of reported deaths | Total no. of reports | Mortality rate, % (95% CI) |
|---|---|---|---|
| 0.0 | 55 | 765 | 7.2 (5.4–9.0) |
| 0.1 | 144 | 1576 | 9.1 (7.7–10.6) |
| 0.2 | 863 | 12,476 | 6.9 (6.5–7.4) |
| 0.3 | 355 | 6897 | 5.1 (4.6–5.7) |
| 0.4 | 206 | 4694 | 4.4 (3.8–5.0) |
| Combined <0.5 | 1623 | 26,408 | 6.1 (5.9–6.4) |
| 0.5 | 121 | 5572 | 2.2 (1.8–2.6) |
| 0.6 | 62 | 2780 | 2.2 (1.7–2.8) |
| 0.7 | 24 | 1372 | 1.7 (1.1–2.4) |
| 0.8 | 34 | 1868 | 1.8 (1.2–2.4) |
| 0.9 | 17 | 801 | 2.1 (1.1–3.1) |
| Combined 0.5–0.9 | 258 | 12,393 | 2.1 (1.8–2.3) |
| Combined <1 | 1881 | 38,801 | 4.8 (4.6–5.1) |
VAERS: Vaccine Adverse Event Reporting System.
With respect to the <1 year age group, there were 3348 males hospitalized out of 20,174 reports, and 2831 females out of 17,630 reports, yielding hospitalization rates of 16.6% (95% CI, 16.1–17.1%) and 16.1% (95% CI, 15.5–16.6%), respectively. The male-to-female RR of 1.03 (95% CI, 0.98–1.08) is not statistically significant.
The 1133 reported male deaths out of a total of 20,174 male cases and 723 reported female deaths out of 17,630 female cases yield mortality rates of 5.6% (95% CI, 5.3–5.9%) and 4.1% (95% CI, 3.8–4.4%), respectively. The male-to-female mortality RR of 1.4 (95% CI, 1.3–1.5) is statistically significant (Table 6).
Table 6.
Mortality rates (%) stratified by age (0 to <1 year) and gender, VAERS 1990–2010 databasea
| Males | Females | |||||
|---|---|---|---|---|---|---|
| Case age (years) | No. of reported deaths | Total no. of reports | Mortality rate, % (95% CI) | No. of reported deaths | Total no. of reports | Mortality rate, % (95% CI) |
| 0.0 | 32 | 406 | 7.9 | 22 | 341 | 6.5 |
| 0.1 | 82 | 786 | 10.4 | 59 | 726 | 8.1 |
| 0.2 | 535 | 6432 | 8.3 | 315 | 5776 | 5.5 |
| 0.3 | 223 | 3698 | 6.0 | 128 | 2989 | 4.3 |
| 0.4 | 112 | 2457 | 4.6 | 93 | 2122 | 4.4 |
| Combined <0.5 | 984 | 13,779 | 7.1 (6.7–7.6) | 617 | 11,954 | 5.2 (4.8–5.6) |
| 0.5 | 75 | 2918 | 2.6 | 46 | 2503 | 1.8 |
| 0.6 | 33 | 1411 | 2.3 | 29 | 1297 | 2.2 |
| 0.7 | 15 | 711 | 2.2 | 8 | 630 | 1.3 |
| 0.8 | 16 | 963 | 1.7 | 16 | 857 | 1.9 |
| 0.9 | 10 | 392 | 2.6 | 7 | 389 | 1.8 |
| Combined 0.5–0.9 | 149 | 6395 | 2.3 (2.0–2.7) | 106 | 5676 | 1.9 (1.5–2.2) |
| Combined <1 | 1133 | 20,174 | 5.6 (5.3–5.9) | 723 | 17,630 | 4.1 (3.8–4.4) |
When stratified by year, there was no correlation in hospitalization rates (r 2 = 0.03) and a weak correlation in mortality rates (r 2 = 0.40) during the studied time period, 1990–2010. The mean hospitalization rates in VAERS for the period 1990–2000 and 2001–2010 were 15.8% (3219 of 20,377) and 16.6% (3060 of 18,424), respectively. The RR is 0.95 (95% CI, 0.91–1.00), indicating a slightly lower mean hospitalization rate in VAERS for 1990–2000 relative to 2001–2010. The mean mortality rates in VAERS for the periods 1990–2000 and 2001–2010 were 5.6% (1135 of 20,377) and 4.0% (746 of 18,424), respectively. The RR is 1.38 (95% CI, 1.25–1.51), indicating a statistically significant higher mean mortality rate in VAERS for 1990–2000 relative to 2001–2010.
Discussion
In 1990, infants received a total of 15 vaccine doses prior to their first year of life: 3 DPT injections (9 vaccine doses), 3 polio, and 3 Hib vaccines—5 vaccine doses at 2, 4, and 6 months of age. By 2007, the CDC recommended 26 vaccine doses for infants: 3 DTaP, 3 polio, 3 Hib, 3 hepatitis B, 3 pneumococcal, 3 rotavirus, and 2 influenza vaccines. While each childhood vaccine has individually undergone clinical trials to assess safety, studies have not been conducted to determine the safety (or efficacy) of combining vaccines during a single physician visit as recommended by CDC guidelines. For example, 2-, 4-, and 6-month-old infants are expected to receive vaccines for polio, hepatitis B, diphtheria, tetanus, pertussis, rotavirus, Haemophilus influenzae type B, and pneumococcal, all during a single well-baby visit—even though this combination of 8 vaccine doses was never tested in clinical trials.
An article written by Guess, representing a vaccine manufacturer, claimed that it is “impractical to conduct preapproval studies of all combinations [of vaccines] in clinical practice.”1 However, a recent study by Miller and Goldman found that among the developed nations, infant mortality increased with an increase in the number of vaccine doses.2 Similar associations have also been found with respect to other serious adverse outcomes. Delong reported that the higher the proportion of children receiving recommended vaccinations, the higher the prevalence of autism or speech and language impairment.3 A CDC report on mixed exposures to chemical substances and other stressors, including prescribed pharmaceuticals, found that they may produce “increased or unexpected deleterious health effects.” In addition, “exposures to mixed stressors can produce health consequences that are additive, synergistic, antagonistic, or can potentiate the response expected from individual component exposures.”4 Administering six, seven, or eight vaccine doses to an infant during a single physician visit may certainly be more convenient for parents—rather than making additional trips to the doctor’s office—but evidence of a positive association between infant adverse reactions and the number of vaccine doses administered confirms that vaccine safety must remain the highest priority.
Single-dose outlier
There are several possible explanations why the hospitalization rate corresponding to one dose is an outlier and therefore excluded from the linear regression analysis:
The distribution of cases aged <1 year is disproportionately highest among the youngest infant age 0 to 0.1 years with 273 hospitalizations (24.4%) reported out of the total of 1115 VAERS reports in that narrow age range. Infants who receive one dose either as a newborn or as a neonate are predisposed to more hospitalizations and deaths than older infants.5
A disproportionate number of hospitalizations were due to the administration of the at-birth dose of the hepatitis B vaccination: 809 (73%) of the 1115 VAERS cases reported the receipt of hepatitis B vaccine; 242 (30%) of these 809 were reported as hospitalized. Several studies provide evidence of correlations between hepatitis B vaccination and serious adverse reactions, including pediatric multiple sclerosis.6–12 Thus, the newborn dose of hepatitis B vaccine, administered at a time when the immune system is most immature, may be contributing to increased vulnerability to serious adverse reactions causing disproportionately high rates of hospitalizations during the neonatal period.
Infants who were sensitive to their first vaccine might have been urged by the child’s physician or concerned family members to avoid subsequent vaccines, especially multiple doses administered concomitantly.
Physicians might have failed to report that two or more doses were actually given.
Accuracy of reports
VAERS is a passive surveillance system, and the large number of reports to VAERS increases the likelihood that some reports may not be adequately checked for accuracy, especially the less serious ones. Some reports to VAERS do not include full medical record documentation and may contain errors. The VAERS forms often have missing or incorrect data, including age, sex, vaccines administered, and adverse events.
Overreporting
Anyone can file a VAERS report regardless of a true cause-and-effect relationship between the administration of a vaccine and an adverse event that succeeds it. Thus, some reports are likely to be unrelated to vaccinations. In addition, erroneous diagnoses may cause some adverse events in the VAERS database to be inaccurate descriptions of the event that occurred. For example, a seizure may be reported as a simple case of fainting and vice versa.
For limited periods of time (with specific starting and stopping points), the FDA required a select group of physicians and vaccine manufacturers to participate in ‘phase 4’ postmarketing, active surveillance of possible adverse vaccine reactions, resulting in temporary spikes of reported VAERS cases. Such mandatory reporting often follows the release of a new vaccine and changes to the recommended childhood immunization schedule. Some of the variations in annual reported cases (Figures 1 and 2) are due to these factors.
Underreporting
Since VAERS is a passive system, it is inherently subject to underreporting. For example, a confidential study conducted by Connaught Laboratories, a vaccine manufacturer, indicated that “a fifty-fold underreporting of adverse events” is likely.13 According to David Kessler, former commissioner of the FDA, “only about one percent of serious events [adverse drug reactions] are reported.”14 Less serious vaccine adverse events (e.g., swelling, fever, or redness at the vaccination site) are more underreported than more serious vaccine adverse events (e.g., hospitalizations and death).15 The current analysis made no attempt to quantify underreporting due to age, type of adverse event, or other factor since only relative trends were utilized.
According to Ottaviani et al., ‘Any case of sudden unexpected death occurring … in infancy, especially soon after a vaccination, should always undergo a full necropsy study,’ otherwise a true association between vaccination and death may escape detection.16 A recent study by Kuhnert et al. demonstrated a 16-fold increase in unexplained sudden unexpected death after the fourth dose of a penta- (5-in-1) or hexavalent (6-in-1) vaccine.17 Similarly, Zinka et al. reported 6 cases of sudden infant death syndrome that occurred within 48 hours following the administration of a hexavalent vaccine. At postmortal examination, these cases showed “unusual findings in the brain” that appeared compatible with an association between hexavalent vaccination and sudden infant death syndrome.18 These examples provide additional evidence that cases of vaccine-related mortality are likely underreported in VAERS.
Methodological limitations
The methodological limitations inherent to the VAERS database are discussed in detail in other studies.19–21 The correlation of increasing hospitalizations and deaths with increasing number of vaccine doses is based solely on the number of vaccine doses associated with each VAERS case report, without differentiating between the types or composition of vaccines administered. Common vaccine substances include antigens (attenuated viruses, bacteria, and toxoids), preservatives (thimerosal, benzethonium chloride, 2-phenoxyethanol, and phenol), adjuvants (aluminum salts), additives (ammonium sulfate, glycerin, sodium borate, polysorbate 80, hydrochloric acid, sodium hydroxide, and potassium chloride), stabilizers (fetal bovine serum, monosodium glutamate, human serum albumin, and porcine gelatin), antibiotics (neomycin, streptomycin, and polymyxin B), and inactivating chemicals (formalin, glutaraldehyde, and polyoxyethylene). For the purposes of this study, all vaccine doses were equally weighted.
Approximately 85% of the variation in mean hospitalization rates for children aged 0.1–0.5 years was accounted for on the basis of the number of vaccine doses. If the quantity and severity of adverse vaccine events is, in fact, related to the accumulated total number of vaccine doses, then methodology that includes the complete age-specific vaccination history of the patient might enhance the analysis. Furthermore, while vaccines may appear to be the causal factor leading to adverse vaccine events, other underlying patient medical conditions, including latent mitochondrial disease or vitamin deficiencies, may ultimately play a role. Some reports have postulated that environmental factors, including vaccine administration, can trigger an adverse reaction due to its various components or agents that deplete body resources and/or cause immune insults.22–24
Unfortunately, VAERS does not provide information regarding background incidence of adverse events in the general population nor does it provide historical information such as the age-specific vaccine doses actually administered to the patient. These methodological limitations require supplementary information from vaccine manufacturers that is often proprietary, or novel approaches to handling VAERS data. Thus, in our analysis the total number of VAERS reports used in the rate calculations serves as a surrogate denominator that is proportionately related to the actual number of vaccine doses distributed or administered.25 The 1.5 male-to-female ratio demonstrated in cases of sudden infant death syndrome reported in other studies26 ,27 closely compares with the 1.4 (95% CI, 1.3–1.5) ratio demonstrated in VAERS among infants. This correspondence with other studies is a strong evidence that the total number of adverse events reported to VAERS, used in the denominator of the rate calculations, credibly reflects the number of vaccine doses administered.
Conclusion
VAERS is one of the largest databases containing adverse reactions reported in temporal association with vaccination. While some adverse events that are reported to VAERS may be unrelated to the recent vaccination, the VAERS database is an important postmarketing safety surveillance tool that is periodically analyzed by the CDC, FDA, and other vaccine researchers to discover potentially adverse vaccination trends. Using linear regression, several statistically significant trends were derived from the VAERS database: (a) a positive correlation between hospitalization rates and the number of vaccine doses (r 2 = 0.91); (b) a negative correlation between hospitalization rates and age (r 2 = 0.95); (c) an increased mortality rate associated with 5–8 vaccines relative to 1–4 vaccines; (d) a decreased mortality rate associated with children aged 0.5 to <1 year relative to those aged <0.5 year; and (e) a 1.4 male-to-female infant mortality ratio. These trends not only have a biological plausibility but are supported by evidence from case reports, case series, and other studies using entirely different methodologies and specific population cohorts.
Studies have not been conducted to determine the safety (or efficacy) of administering multiple vaccine doses in a variety of combinations as recommended by CDC guidelines. Our findings show a positive correlation between the number of vaccine doses administered and the percentage of hospitalizations and deaths reported to VAERS. In addition, younger infants were significantly more likely than older infants to be hospitalized or die after receiving vaccines. Since vaccines are administered to millions of infants every year, it is imperative that health authorities have scientific data from synergistic toxicity studies on all combinations of vaccines that infants are likely to receive; universal vaccine recommendations must be supported by such studies.
Adverse reaction trends detected in VAERS have important implications for vaccine recipients and health care providers. Finding ways to increase vaccine safety should be the highest priority. Further inspection of potential correlations between increasing vaccine doses, hospitalizations, and death is essential. Health care policy makers have an obligation to determine whether immunization schedules are achieving their desired goals.
Acknowledgements
The authors wish to thank Walter Schumm, PhD, and Paul G King, PhD, for their evaluations.
Conflict of Interest Statement: Neil Z Miller is associated with the ‘Think Twice Global Vaccine Institute’.
Funding: This work is funded by the National Vaccine Information Center (NVIC) who donated $2,500 towards the SAGE Choice Open Access fee for this article.
References
- 1. Guess HA. Combination vaccines: issues in evaluation of effectiveness and safety. Epidemiol Rev 1999; 21(1): 93. [DOI] [PubMed] [Google Scholar]
- 2. Miller NZ, Goldman GS. Infant mortality rates regressed against number of vaccine doses routinely given: is there a biochemical or synergistic toxicity? Hum Exp Toxicol 2011; 30(9): 1420–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Delong G. A positive association found between autism prevalence and childhood vaccination uptake across the U.S. population. J Toxicol Environ Health A 2011; 74(14): 903–916. [DOI] [PubMed] [Google Scholar]
- 4. Castranova V, Graham J, Hearl F, Herrick R, Hertzberg R, Hoover MD, et al. Mixed exposures research agenda: a report by the NORA Mixed Exposures Team. Department of Health and Human Services (DHHS), Centers for Disease Control and Prevention (CDC), National Institute for Occupational Safety and Health (NIOSH); DHHS (NIOSH) 2004; December 2005. p.106:vi. [Google Scholar]
- 5. Oestergaard MZ, Inoue M, Yoshida S, Mahanani WR, Gore FM, Cousens S, et al. Neonatal mortality levels for 193 countries in 2009 with trends since 1990: a systematic analysis of progress, projections, and priorities. PLoS Med 2011; 8(8): e1001080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gran B, Bielekova B, McFarland HF, Martin R. Development of multiple sclerosis after hepatitis B vaccination. Neurology 2000; 54(suppl 3): A164. [Google Scholar]
- 7. Hernán MA, Jick SS, Olek MJ, Jick H. Recombinant hepatitis B vaccine and the risk of multiple sclerosis: a prospective study. Neurology 2004; 63: 838–842. [DOI] [PubMed] [Google Scholar]
- 8. Terney D, Beniczky S, Barsi P, Kondákor I, Perényi J, Faludi B, et al. Multiple sclerosis after hepatitis B vaccination in a 16-year-old patient. Chin Med J 2006; 119(1): 77–79. [PubMed] [Google Scholar]
- 9. Mikaeloff Y, Caridade G, Suissa S, Tardieu M. Hepatitis B vaccine and the risk of CNS inflammatory demyelination in childhood. Neurology 2009; 72(10): 873–880. [DOI] [PubMed] [Google Scholar]
- 10. Ness JM, Bale JF., Jr Hepatitis vaccines and pediatric multiple sclerosis. Does timing or type matter? Neurology 2009; 72(10): 870–871. [DOI] [PubMed] [Google Scholar]
- 11. Sinsawaiwong S, Thampanitchawong P. GuillainBarré syndrome following recombinant hepatitis B vaccine and literature review. J Med Assoc Thai 2000; 83(9): 1124–1126. [PubMed] [Google Scholar]
- 12. Konstantinou D, Paschalis C, Maraziotis T, Dimopoulos P, Bassaris H, Skoutelis A. Two episodes of leukoencephalitis associated with recombinant hepatitis B vaccination in a single patient. Clin Inf Dis 2001; 33: 1772–1773. [DOI] [PubMed] [Google Scholar]
- 13. Institute of Medicine. Vaccine safety committee proceedings. Washington, DC: National Academy of Sciences, 11 May 1992, pp.40–41. [Google Scholar]
- 14. Kessler DA, the Working Group, Natanblut S, Kennedy D, Lazar E, Rheinstein P, et al. Introducing MEDWatch: a new approach to reporting medication and device adverse effects and product problems. JAMA 1993; 269(21): 2765. [DOI] [PubMed] [Google Scholar]
- 15. Rosenthal S, Chen RT. Reporting sensitivities of two passive surveillance systems for adverse events. Am J Public Health 1995; 85: 1706–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ottaviani G, Lavezzi AM, Matturri L. Sudden infant death syndrome (SIDS) shortly after hexavalent vaccination: another pathology in suspected SIDS? Virchows Arch 2006; 448(1): 100–104. [DOI] [PubMed] [Google Scholar]
- 17. Kuhnert R, Hecker H, Poethko-Müller C, Schlaud M, Vennemann M, Whitaker HJ, et al. A modified self-controlled case series method to examine association between multidose vaccinations and death. Stat Med 2011; 30(6): 666–677. [DOI] [PubMed] [Google Scholar]
- 18. Zinka B, Rauch E, Buettner A, Rueff F, Penning R. Unexplained cases of sudden infant death shortly after hexavalent vaccination. Vaccine 2006; 24 (31-32): 5779–5780. [DOI] [PubMed] [Google Scholar]
- 19. Geier DA, Geier MR. A review of the Vaccine Adverse Event Reporting System database. Expert Opin Pharmacother 2004; 5(3): 691–698. [DOI] [PubMed] [Google Scholar]
- 20. Varricchio F, Iskander J, Destefano F, Ball R, Pless R, Braun MM, et al. Understanding vaccine safety information from the Vaccine Adverse Event Reporting System. Pediatr Infect Dis J 2004; 23(4): 287–294. [DOI] [PubMed] [Google Scholar]
- 21. Zhou W, Pool V, Iskander JK, English-Bullard R, Ball R, Wise RP, et al. Surveillance for safety after immunization: Vaccine Adverse Event Reporting System (VAERS)—United States, 1991–2001. MMWR Surveill Summ 2003; 52(1): 1–24. [PubMed] [Google Scholar]
- 22. Dettman G, Kalokerinos A, Dettman I. Vitamin C, Nature's Miraculous Healing Missile! Melbourne: Frederick Todd, 1993, pp.1–422. [Google Scholar]
- 23. Prandota J. Possible pathomechanisms of sudden infant death syndrome: key role of chronic hypoxia, infection/inflammation states, cytokine irregularities, and metabolic trauma in genetically predisposed infants. Am J Ther 2004; 11(6): 517–546. [DOI] [PubMed] [Google Scholar]
- 24. Poling JS, Frye RE, Shoffner J, Zimmerman AW. Developmental regression and mitochondrial dysfunction in a child with autism. J Child Neurol 2006; 21(2): 170–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Braun M. Vaccine Adverse Event Reporting System (VAERS): usefulness and limitations. Johns Hopkins Bloomburg School of Public Health, http://www.vaccinesafety.edu/VAERS.htm (2006, accessed 9 November 2011). [DOI] [PubMed] [Google Scholar]
- 26. Mage DT, Donner EM. The fifty percent male excess of infant respiratory mortality. Acta Paediatr 2004; 93(9): 1210–1215. [PubMed] [Google Scholar]
- 27. Moon R, Fu L. Sudden infant death syndrome. Pediatr Rev 2007; 28: 209–214. [DOI] [PubMed] [Google Scholar]