Abstract
Prostate specific antigen (PSA) has long been used as a biological marker for prostatic cancer. Recent studies have demonstrated that PSA synthesis can be induced by steroid hormones in several tissues of women. Menstrual cycle is regulated by the cyclic variation of estradiol and progesterone. This study was undertaken in order to study the correlation of serum PSA to both these corpus luteal hormones. 110 serum samples and 10 saliva samples were collected from healthy women aged 18–45 years of age having normal menstrual cycles. Active PSA DSL-9700 ultrasensitive kit with detection limit 0.001 ng/ml was used to analyze PSA. 38.2 % of all serum samples and 10 % of saliva samples had detectable concentrations of PSA. The serum PSA was highest during mid follicular phase (between 4th and 8th days of cycle). Variation in PSA levels seemed to follow the variations in progesterone with a lag period of 12–14 days, but did not appear to bear any relationship with the estradiol levels.
Keywords: Prostate specific antigen, Serum, Saliva, Estradiol, Progesterone, Insulin like growth factor binding protein, Insulin like growth factor, PSA, IGF, IGFBP-3
Introduction
Prostate specific antigen (PSA) is a serine protease of human glandular kallikrein family and has historically been used as the most specific and sensitive marker for prostatic cancer. However, since 1994 several studies have demonstrated the presence of PSA in several tissues and body fluids of women including serum, amniotic fluid, breast milk, saliva, urine, cord blood, endometrium, placenta, mammary glands, salivary glands and sweat glands. PSA levels in sera of normal healthy females change consistently during the menstrual cycle and in pregnancy [1–9].
PSA gene (hklk-3) is located on chromosome 19 and has a steroid hormone-binding site. It has close sequence homology with gamma-nerve-growth-factor (56 %) and epidermal growth factor binding protein (53 %) [2]. PSA is a single chain glycoprotein of approximately 33 KD with chymotrypsin like activity. In vitro studies have shown that PSA can be induced by progesterone, androgens, vitamin D3 and possibly estrogens [2–6, 10]. During normal menstrual cycle, the corpus luteal steroids are thought to stimulate the target tissues, like breast and endometrium, to produce PSA which enters blood stream and is detectable by ultra sensitive assays [9–12].
In both sexes serum PSA exists in several molecular forms in both the sexes: most of the serum PSA exists complexed with alfa-1 anti-chymotrypsin (ACT); smaller amounts of PSA are complexed with alfa-2 macroglobulin and alfa-1 antitrypsin, and the rest exists in the free form [6, 13].
In 1996, Diamandis et al. detected PSA in 50 % non pregnant females. They proposed that normal breast tissue, endometrium and salivary glands are the likely sources of serum PSA. Serum PSA was found to be higher in women of higher age, hispanic women and hirsute women [14–16].
Zarghami et al. reported a cyclic, predictable and reproducible variation in serum PSA during the menstrual cycle. They found that serum PSA levels peak during mid to late luteal phase. The increase in PSA levels follows the increase in serum progesterone, but with a lag period of 10–12 days. Once the Corpus luteum regresses, the PSA concentration decreases with a half-life of approximately 3–5 days, suggesting that PSA may be regulated by corpus luteal steroids [11]. Some other studies, however, did not find any significant difference in PSA concentrations in different phases of the menstrual cycle [17]. PSA gene hklk-3 is expressed in the human endometrium [18].
Insulin like growth factor-I possibly contributes to endometrial proliferation and differentiation processes. Endometrium expresses IGF binding protein-3 and the secretion is increased by progesterone. IGFBP-3 acts as an inhibitor of IGF action. PSA has IGFBP-3 protease action and inhibits its binding to IGF-I: it thus acts as a local regulator of uterine function [1, 19].
Mannello et al. [7] reported that women taking oral contraceptives had higher salivary PSA as compared to controls. Contrary to original views, positive PSA staining seen in normal as well as malignant salivary glands has demonstrated that salivary glands are the source of the PSA found in saliva [12, 20].
PSA plays the role of growth factor or growth factor regulator by virtue of its IGFBP-3 protease activity, as well as by activation of latent TGF-beta. In a normal menstrual cycle, PSA is credited as a local regulator of uterine function. It is plausible that PSA, like tissue kallikrein, acts as a mediator of menstrual blood flow, the cyclic proliferation of the endometrium, implantation and/or parturition [1–3].
PSA has been found to be raised in several carcinomas and other pathological conditions in both sexes. In view of the racial variations in serum PSA, this study was conducted to examine the serum PSA levels of healthy Asian females during different phases of menstrual cycle and correlating them to the serum levels of progesterone and estrogen. Additionally, 10 saliva samples from non pregnant women were also analyzed for PSA.
Materials and Methods
Subjects
The subjects included in this study were women having normal menstrual cycles. 110 venous blood samples and 10 salivary samples were collected. Normal menstrual cycle was defined as average blood losses lasting for (2–5) days and with a frequency of 27–32 days. A detailed menstrual history was taken from all subjects including the amount of and duration of losses during menstruation, the length of the cycle and the regularity of the cycle. Patient’s last menstrual period (LMP) and the day of the cycle were noted. History was taken regarding intake of oral contraceptives or any other hormonal preparation. Patient’s past obstetrical history, past medical history and history of any non-hormonal drug intake was also sought. Women with age below 18 years or above 45 years were excluded from this study. Women having menstrual disorders or malignancy were not included in the study. All women taking hormonal preparations were excluded from the study.
Sample Collection
Collection of blood samples: Vene-puncture was done in the sitting position and blood was drawn from the ante-cubital vein. In all the cases disposable 5 ml plastic syringes and 21 gauge needles were used. The blood was transferred into a clean plain glass vial. The sample collected was transported immediately to the biochemical laboratory where the blood was allowed to clot at 37 °C. Then the sample was centrifuged at 3,000 rpm for 5 min to separate out the serum. This serum was transferred to another properly labeled clean plain vial and frozen if not analyzed immediately.
Collection of saliva samples: Each participant was asked to rinse her mouth with water 5 min before collecting the sample. The saliva samples were collected in clean plain vials, transported to the biochemistry laboratory and centrifuged at 3,500 rpm for 10 min. The clear supernatant was transferred to clean plain, well labeled vials and frozen till analysis.
Estimations
All the serum samples were analyzed for estradiol, progesterone and PSA. All the sera were analyzed for estradiol, progesterone and PSA. Saliva samples were analyzed for PSA.
PSA estimation: All the samples were analyzed for PSA using Active PSA DSL-9700 ultra sensitive kit. The procedure employs a non-competitive two-site immuno-radiometric assay (IRMA) principle. The IRMA is a non-competitive assay in which the analyte to be measured is sandwiched between two antibodies. The first antibody is immobilized to the inside walls of the tubes. The other antibody is radio labeled for detection. In serum, most of the active PSA is complexed with the serum protease inhibitor at anti-chymotrypsin (ACT) and to a lesser extent, with other serum proteins including alfa 2 macroglobulins and alfa 1 antitrypsin. Serum also contains enzymatically inactive unbound PSA. This IRMA kit provides equimolar measurement of ACT bound and free forms of PSA [21].
Estradiol estimation: All serum samples were analyzed for estradiol using Immunotech kits. The procedure employs competition radioimmunoassay in which antibody-coated tubes with an 125I-labeled estradiol tracer were used. It follows the basic principle of radioimmunoassay where there is a competition between radioactive and non-redioactive antigens for a fixed number of antibody binding sites. Samples and standards are incubated for 3 h in antibody-coated tubes with an 125I-labeled estradiol tracer. After incubation the liquid contents of the tubes are aspirated and the bound radioactivity is measured. The amount of 125I-labeled analyte bound to the antibody is inversely proportional to the concentration of the analyte present. The standard curve is established and unknown values are determined by interpolation from the standard curve [22].
17-OH-Progesterone estimation: All serum samples were analyzed for 17-OH-Progesterone using Immunotech kits. The procedure employs competition radioimmunoassay. The procedure follows the basic principle of radio-immunoassay where there is competition between a radioactive and a non-radioactive antigen binding sites. The samples and standards are incubated with 125I labeled progesterone as tracer in anti-progesterone antibody coated tubes. After incubation the liquid contents of the tubes are aspirated and the bound radioactivity is measured. The amount of 125I labeled analyte bound to the antibody is inversely proportional to the concentration of the analyte present. A calibration curve is established and the unknown values are determined by interpolation from the curve [22].
Results
Out of the 110 serum samples, 85.8 % belonged to women in 20–40 years age group; 7.1 % from women younger than 20 years and 7.1 % from women above 40 years of age.
SPSS software was employed for the statistical analysis of the data. In the analysis, the difference in values was considered significant (S) if, P < 0.05. P < 0.01 is considered highly significant (HS) and P > 0.05 is considered non-significant (NS).
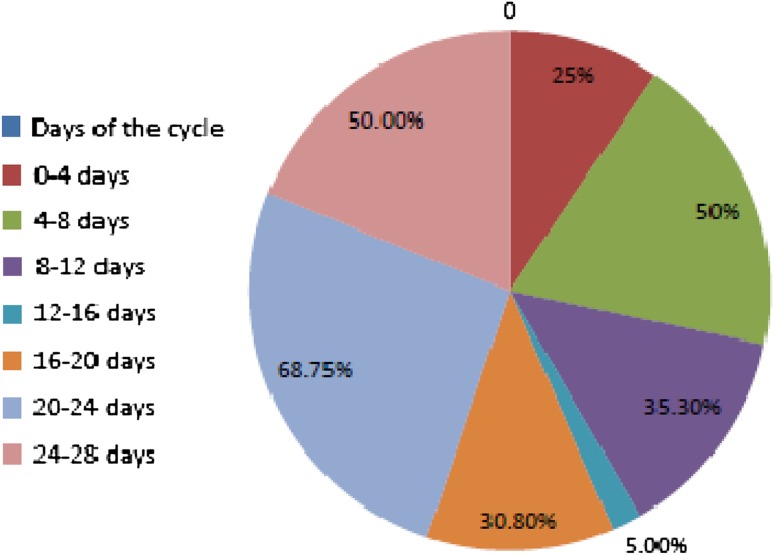
38.2 % of all samples had detectable PSA (>0.001 ng/ml). 68.75 % of the samples for 20th–24th day of cycle had detectable amounts of PSA. Only 5.6 % of all the samples for day 12th–16th had PSA >0.001 ng/ml (Fig. 1).
Fig. 1.
Percentage of serum samples with detectable PSA on different days of menstrual cycle
Estradiol levels were low to begin with (day 0th–4th), rose till immediately after ovulation (16th–20th day) and then decreased. The changes in levels of estradiol are highly significant statistically.
Progesterone levels were found to be low during follicular phase (0.49–2.16 ng/ml). It peaked soon after ovulation (12.00 ng/ml) and then started decreasing, till the end of the cycle. The changes in progesterone level were highly significant statistically.
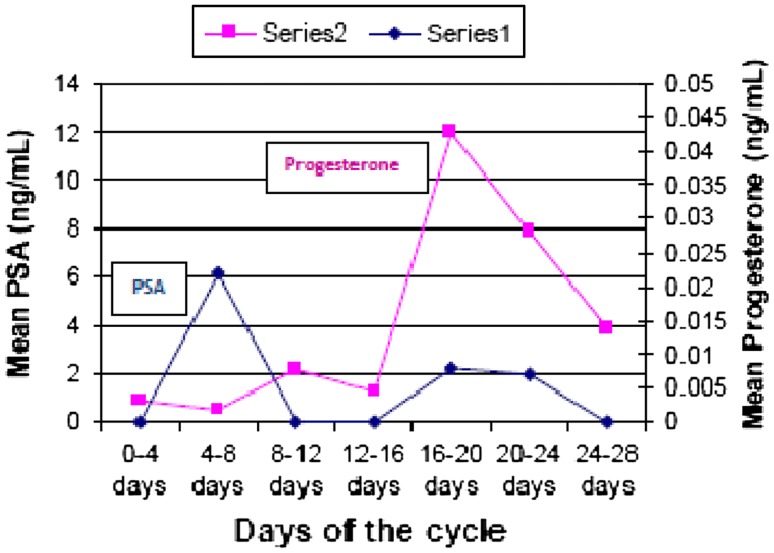
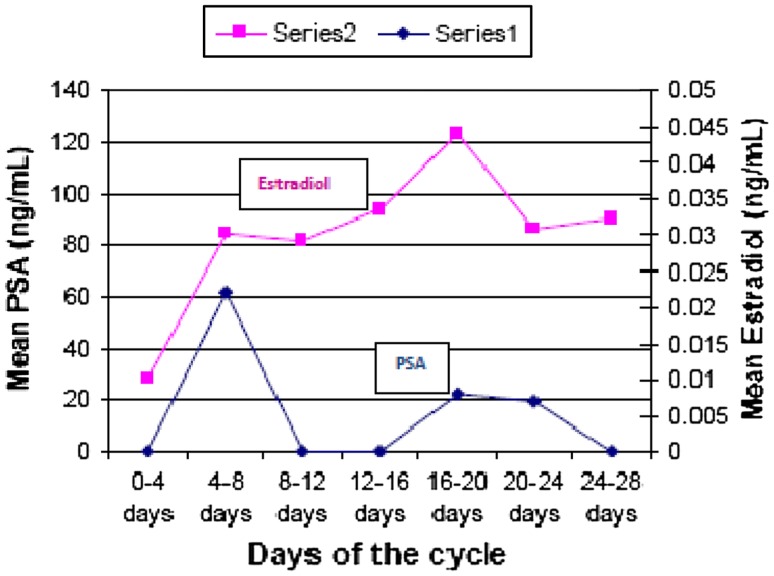
Prostate specific antigen values were found to show two peaks—one tall peak in mid-follicular phase (0.022 ng/ml) and one shorter peak in early luteal phase (0.008 ng/ml) during normal menstrual cycle. The changes in PSA levels were statistically significant. Variation in PSA levels appeared to bear no relationship with the variations in estradiol levels during menstrual cycle but seemed to follow the variations in progesterone with a lag period of 12–14 days (Figs. 2, 3; Table 1).
Fig. 2.
Changes in serum PSA versus progesterone during menstrual cycle
Fig. 3.
Changes in serum PSA versus estradiol during menstrual cycle
Table 1.
Mean serum estradiol, progesterone and PSA vis phase of menstrual cycle
| Phase (days of cycle) | Observation (n) | Mean estradiol ± SEM (pg/ml) | Mean progesterone ± SEM (ng/ml) | Mean PSA ± SEM (ng/ml) |
|---|---|---|---|---|
| Follicular (0–12) | 45 | 93.58 ± 10.08 | 1.29 ± 0.37 | 0.009 ± 0.058 |
| Ovulatory (12–16) | 20 | 123.81 ± 33.25 | 2.97 ± 1.47 | 0.000 ± 0.000 |
| Luteal (16–28) | 45 | 140.67 ± 30.95 | 8.25 ± 1.75 | 0.005 ± 0.003 |
| P value = 0.113 (NS) | P value = 0.000 (HS) | P value = 0.026 (S) |
A total of 10 saliva samples were collected from the non-pregnant group. Only 1 sample collected from a 24 years old woman on 5th day of the cycle had detectable amounts of PSA (0.020 ng/ml). Rest of the saliva samples had undetectable quantities of PSA (<0.001 ng/ml) (Table 2).
Table 2.
PSA levels in saliva on different days of menstrual cycle
| Days of the cycle | Detectable PSA >0.001 ng/ml | Total (n) |
|---|---|---|
| 0–4 | 0 | 3 |
| 4–8 | 1 | 2 |
| 8–12 | 0 | 1 |
| 12–16 | 0 | 2 |
| 20–24 | 0 | 1 |
| >32 | 0 | 1 |
| Total | 1 | 10 |
| 10 % | 100 % |
Discussion
85.8 % of the women included in this study belonged to the (20–40 years) age group. In our study the detection limit was 0.001 ng/ml and all the women were younger than 50 years (18–45 years). Out of total of 110 women included in the study, 38.2 % had detectable PSA (more than 0.001 ng/ml) (Fig. 1). Earlier studies have reported detectable PSA in 50 % non pregnant females [2, 15, 23]. Filella et al. [16] used ultrasensitive Fluoro-immunometric assay (detection limit of 0.004 μg/l) detected the presence of PSA in 58 % of the sera tested.
Progesterone levels were low in early follicular stage, started rising in late follicular phase, peaked at 16th–20th day and decreased. These changes were found to be highly significant statistically (Fig. 2). Black et al. [24] found the mean progesterone levels of 1.8 ± 1.1 ng/ml during days 0–11; 3.1 ± 1.8 during days 12–14; 12.1 ± 7.8 ng/ml during days 15–25 and 4.3 ± 3.3 ng/ml during days 26–28 of menstrual cycle. These values compare very well with the findings in our study. Progesterone is the major endocrine product of corpus luteum of menstrual cycle [11]. In vitro studies on T-47D Breast cancer cell line showed that progesterone can up-regulate PSA mRNA and protein [25].
Estradiol levels were low during early follicular phase, but increased nearly 3 folds in mid and late follicular phase. Peak was seen at 16th–20th day and second peak was seen in late luteal phase followed by rapid decrease in levels (Fig. 3). These changes were found to be highly significant statistically. The pattern of estradiol changes in our study was similar to the pattern quoted in literature [11]. Malatesta et al. [9] conducted in vitro studies on placental cell explants and found that 17-estradiol can up-regulate PSA synthesis.
Serum PSA levels PSA was detectable (>0.001 ng/ml) in 38.2 % of the serum samples. Measurable concentrations of PSA were found in samples for (4th–8th days) and (16th–24th days). Highest PSA concentrations were found during (4th–8th) days of menstrual cycle (0.022 ± 0.000 ng/ml). A second smaller peak was seen at (16th–20th) days (0.008 ± 0.008) ng/ml). Diamandis found low levels of PSA during days 10–23 and a peak at the end of the cycle or the beginning of the next cycle [20]. In our study too the peak was seen at the beginning of the cycle (Figs. 2, 3).
In this study the mean serum progesterone was low in the follicular phase (1.29 ± 0.37 ng/ml), gradually increased during ovulatory phase (2.97 ± 1.47 ng/ml) and peaked in the luteal phase (8.25 ± 1.75 ng/ml). These values were comparable to the values reported by Black et al. [24].
The mean serum estradiol levels were lowest in follicular phase (93.58 ± 10.08 pg/ml), peaked in ovulatory phase (123.81 ± 33.25 pg/ml) and then tapered off in the luteal phase (140.67 ± 30.95 pg/ml).
Mean serum PSA was highest in follicular phase (0.009 ± 0.058 ng/ml) and undetectable in ovulatory phase. In luteal phase the mean PSA was (0.005 ± 0.003 ng/ml) (Table 1).
The progesterone peak was seen on days (16th–20th) and the PSA peak was seen after 12 day (i.e. 4th–8th days). The biphasic increase in PSA levels appeared to mimic the rise and fall of progesterone (Fig. 2). We did not find any correlation between serum PSA and serum estradiol (Fig. 3). Similar findings were reported by Zarghami et al. [11] who found highest PSA concentrations during mid to late follicular phase and a difference of 10–12 days between PSA and progesterone peak. Studies in other mammals have also indicated that PSA secretion is higher in follicular phase and the authors opined that these cyclical changes in PSA are probably determined by cyclic variations in estradiol and progesterone [23].
Only one out of ten salivary samples had detectable amounts of PSA (0.020 ng/ml). This sample was collected from a 24 years old woman on the 5th day of the cycle with a serum PSA of 0.014 ng/ml. It was the only serum sample out of 5 samples collected on 5th day of cycle that had detectable PSA. 90 % of the samples had PSA levels <0.001 ng/ml (Table 2).
Mannello et al. studied saliva samples from 20 healthy females and found detectable levels of PSA in all the samples (0.048 ± 0.007 μg/l) and the PSA concentration was comparable to the plasma PSA concentration of 0.047 ± 0.008 μg/l. They reported higher salivary PSA (0.099 ± 0.016 μg/l) in women taking oral contraceptive (containing progestin and estrogen), but similar serum PSA concentrations (0.045 ± 0.006 μg/l). The authors attributed this increase in PSA levels in oral contraceptive takers to the up-regulation of PSA synthesis in salivary glands by the hormones present in the pills [6].
Aksoy et al. studied serum and salivary PSA in healthy women on days 4th, 9th, 14th, and 21st days of the menstrual cycle found that salivary PSA concentrations were positively correlated with serum PSA concentrations on all those instances, but not with the serum progesterone or estrogen concentrations. They opined that salivary PSA, rather than being produced in the salivary gland, may reflect the serum PSA during the normal menstrual cycle [12]. Van Krieken, however, reported positive PSA staining in normal as well as neoplastic salivary glands. This demonstrates that salivary glands are the source of the PSA found in saliva [20]. In our study the number of saliva samples was small (10) and only 10 % of these had detectable PSA concentration (>0.001 ng/ml).
Conclusion
38.2 % of all subjects had detectable serum PSA while only 10 % had detectable salivary PSA. Mean serum PSA in follicular phase was (0.009 ± 0.058) ng/ml, in ovulatory phase (0.000 ± 0.000) ng/ml and in luteal phase (0.005 ± 0.021) ng/ml. Serum PSA of healthy non pregnant women showed a biphasic increase: a larger peak between 4th and 8th day of cycle and a smaller peak between 16th and 20th day of cycle. It seemed to reflect the changes in serum progesterone, albeit with a lag period of 12–16 days. We propose that the PSA is produced in target tissues in response to the rising levels of progesterone seen in ovulatory phase. This PSA diffuses into blood and is detected in luteal phase. Progesterone peaks during luteal phase and PSA peak is seen in early follicular phase of the next cycle. Detectable salivary PSA corresponded to detectable levels of serum PSA. A larger study may be required to study the pattern of PSA expression in saliva.
References
- 1.Clements J, Mukhtar A. Glandular kallikreins and prostate-specific antigen are expressed in the human endometrium. J Clin Endocrinol Metab. 1994;178(6):1536–1539. doi: 10.1210/jc.78.6.1536. [DOI] [PubMed] [Google Scholar]
- 2.Yu H, Diamandis EP. Prostate-specific antigen immuno reactivity in amniotic fluid. Clin Chem. 1995;41(2):204–210. [PubMed] [Google Scholar]
- 3.Nagar R, Msalati AA. Prostate specific antigen in amniotic fluid. Lib J Med Res. 2010;7(2):4–9. [Google Scholar]
- 4.Yu H, Diamandis EP. Prostate-specific antigen in milk of lactating women. Clin Chem. 1995;41(1):54–58. [PubMed] [Google Scholar]
- 5.Nagar R, Msalati AA. Prostate specific antigen in breast milk. Lib J Med Res. 2010;7(2):41–50. [Google Scholar]
- 6.Mannello F, Bianchi G, Gazzanelli G. Immunoreactivity of prostate-specific antigen in plasma and saliva of healthy women. Clin Chem. 1996;42(7):1110–1111. [PubMed] [Google Scholar]
- 7.Mannello F, Condemi L, Cardinali A, Bianchi G, Gazzanelli G. High Concentration of prostate-specific antigen in urine of women receiving oral contraceptives. Clin Chem. 1998;44(1):181–183. [PubMed] [Google Scholar]
- 8.Mannello F, Malatesta M, Fusco E, Bianchi G, Cardinali A, Gazzanelli G. Biochemical characterization and immune-localization of prostate-specific antigen in human term placenta. Clin Chem. 1998;44(8):1735–1737. [PubMed] [Google Scholar]
- 9.Malatesta M, Mannello F, Luchetti F, Marcheggiani F, Condemi L, Papa S, Gazzanelli G. Prostate-specific antigen synthesis and secretion by human placenta: a physiological kallikrein source during pregnancy. J Clin Endocrinol Metab. 2000;85(1):317–321. doi: 10.1210/jc.85.1.317. [DOI] [PubMed] [Google Scholar]
- 10.Negri C, Tosi F, Dorizzi R, Fortunato A, Spiazzi GG, Muggeo M, Castello R, Moghetti P. Anti-androgen drugs lower serum prostate-specific antigen (PSA) levels in hirsute subject: evidence that serum PSA is a marker of androgen action in women. J Clin Endocrinol Metab. 1999;85(1):81–84. doi: 10.1210/jc.85.1.81. [DOI] [PubMed] [Google Scholar]
- 11.Zarghami N, Grass L, Sauter ER, Diamandis EP. Prostate Specific antigen in serum during the menstrual cycle. Clin Chem. 1997;43(10):1862–1867. [PubMed] [Google Scholar]
- 12.Aksoy H, Akçay F, Umudum Z, Yildirim AK, Memisogullari R. Changes of PSA concentrations in serum and saliva of healthy women during the menstrual cycle. Ann Clin Lab Sci. 2002;32(1):31–36. [PubMed] [Google Scholar]
- 13.Honda SAA, Goldstein AP, Morita T, Sugiyama C, Cody L, Rios CN, Bhagavani NV. Prostate-specific antigen concentration in serum in acute illness. Clin Chem. 1996;42(11):1785–1788. [PubMed] [Google Scholar]
- 14.Klee GG, Dodge LA, Zincke H, Oesterhng JE. Measurement of serum prostate specific antigen using IMx prostate specific antigen assay. J Urol. 1994;151:94–98. doi: 10.1016/s0022-5347(17)34879-6. [DOI] [PubMed] [Google Scholar]
- 15.Yu H, Diamandis EP. Measurement of serum prostate specific antigen levels in women and in prostatectomized men with an ultrasensitive immunoassay technique. J Urol. 1995;153:1004–1008. doi: 10.1016/S0022-5347(01)67622-5. [DOI] [PubMed] [Google Scholar]
- 16.Filella X, Molina R, Alcover J, Carretero P, Ballesta AM. Detection of non-prostatic PSA in serum and non-serum sample from women. Int J Cancer. 1996;68:424–427. doi: 10.1002/(SICI)1097-0215(19961115)68:4<424::AID-IJC4>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 17.Burelli A, Cionini R, Rinaldi E, Benelli E, Fiore E, Canale D, Bencivelli W, Nencetti C, Pinchera A, Pucci E. Serum PSA levels are not affected by the menstrual cycle or the menopause, but are increased in subjects with polycystic ovary syndrome. J Endocrinol Invest. 2006;29(4):308–312. doi: 10.1007/BF03344101. [DOI] [PubMed] [Google Scholar]
- 18.Vessella RL, Noteboom J, Lange PH. Evaluation of the Abbott IMx automated immunoassay of prostate-specific antigen. Clin Chem. 1992;38(10):2044–2054. [PubMed] [Google Scholar]
- 19.Rutanen EM, Pekonen F, Makinen T. Soluble 34 K binding protein inhibits the binding of insulin-like growth factor I to its cell receptors in human secretory phase endometrium: evidence for autocrine/paracrine regulation of growth factor action. J Clin Endocrinol Metab. 1988;66(1):173–180. doi: 10.1210/jcem-66-1-173. [DOI] [PubMed] [Google Scholar]
- 20.Van Krieken JH. Prostate marker immunoreactivity in salivary gland neoplasms: a rare pitfall in immunohistochemistry. Am J Surg Pathol. 1993;17(4):410–414. doi: 10.1097/00000478-199304000-00012. [DOI] [PubMed] [Google Scholar]
- 21.Miles LE, Lipschitz DA, Bieber CP, Cook JD. Measurement of Ferritin by a 2-site immunoradiometric assay. Anal Biochem. 1974;61(1):209–224. doi: 10.1016/0003-2697(74)90347-9. [DOI] [PubMed] [Google Scholar]
- 22.Berson SA, Yalow RS. Clinical applications of radioimmunoassay of plasma parathyroid hormone. Am J Med. 1971;50(5):623–629. doi: 10.1016/0002-9343(71)90117-3. [DOI] [PubMed] [Google Scholar]
- 23.Fochi RA, Perez APS, Bianchi CV, Rochel SS, Góes RM, Vilamaior PS, Taboga SR, Santos FC. Hormonal oscillations during the estrous cycle influence the morpho-physiology of the gerbil (Meriones unguiculatus) female prostate (Skene’s para urethral Glands) Biol Reprod. 2008;79(6):1084–1091. doi: 10.1095/biolreprod.108.070540. [DOI] [PubMed] [Google Scholar]
- 24.Black WP, Martin BT, Whyte WG. Plasma progesterone concentration as an index of ovulation and corpus luteum function in normal and gonadotropin stimulated menstrual cycle. J Obstet Gynaecol Br Commonw. 1972;79:363–372. doi: 10.1111/j.1471-0528.1972.tb15810.x. [DOI] [PubMed] [Google Scholar]
- 25.Zarghami N, Grass L, Diamandis EP. Steroid hormone regulation of prostate-specific antigen gene expression in breast cancer. Br J Cancer. 1977;75(4):579–588. doi: 10.1038/bjc.1997.101. [DOI] [PMC free article] [PubMed] [Google Scholar]