Abstract
Lead is one of the most widely scattered toxic metals in the environment and used by mankind for over 9,000 years. Lead in the environment may be derived from natural or anthropogenic sources. In humans, lead can cause a wide range of biological effects depending upon the level and duration of exposure. The purpose of this study was to find out the effect of lead exposure on systolic and diastolic blood pressure, serum calcium, ionized calcium, phosphorus, parathyroid hormone and vitamin D and examine the overall effect of all these parameters on the bone mineral density of battery manufacture workers. For this study ninety battery manufacture workers were selected and divided in three groups depending upon duration of lead exposure. Group I—workers with duration of lead exposure 1–5 years, Group II—workers with duration of lead exposure 6–10 years and Group III—workers with duration of lead exposure more than 10 years. Each group consisted of thirty workers. Thirty age matched healthy control subjects were taken for comparison. Demographic, occupational and clinical data were collected by using questionnaire and interview. The venous blood samples were collected from the study groups and normal healthy control group. At the time of blood collection random urine samples were collected in amber coloured bottles. The biochemical parameters were estimated by using standard assay procedures. Statistical analysis of the data was done using independent student‘t’ test for parametric variables. Values were expressed as mean ± standard deviation (SD). P values of 0.05 or less were considered to be statistically significant. The blood lead levels and urinary lead levels of all workers were significantly increased (P < 0.001) in proportion to the duration of lead exposure as compared to controls. Systolic and diastolic blood pressure were significantly raised (P < 0.001) in all three study groups of battery manufacture workers as compared to controls. Serum Calcium, Ionized calcium, phosphorus were significantly decreased (P < 0.001) in all the three study groups. Serum vitamin D levels were lowered (P < 0.01) and serum PTH was increased (P < 0.01) in workers as compared to controls. The results of this study clearly indicate that the absorption of lead is more in these workers which adversely affects blood pressure, disturbs calcium and phosphorus metabolism which further impairs mineralization of bone resulting in decreased bone mineral density observed in these workers. Lead toxicity is still persistent in battery manufacture workers though they are using sophisticated techniques in these industries. There is a need to protect the workers from the health hazards of occupational lead exposure.
Keywords: Battery manufacture workers, Blood lead (Pb–B), Urinary lead (Pb–U), Systolic and diastolic blood pressure, Calcium, Phosphorus, Ionized calcium, Bone mineral density (BMD), Vitamin D, Parathyroid hormone (PTH)
Introduction
Lead is one of the most widely scattered toxic metals in the environment and used by mankind for over 9,000 years. Lead in the environment may be derived from natural or anthropogenic sources [1, 2]. It is rapidly taken up in blood and soft tissues (half life 28–30 days) followed by a slower redistribution to bone (half life 27 years). Dietary and airborne lead which is not absorbed in GIT is excreted in faeces [3, 4]. In humans, lead can cause a wide range of biological effects depending upon the level and duration of exposure [5].
Lead affects mineral metabolism mainly calcium and phosphorus by inhibiting 1-α-hydroxylase enzyme in renal tubules which is required for the synthesis of calcitriol. In several occupational lead exposure studies it is reported that lead inhibits the synthesis of calcitriol resulting in decrease in calcium and phosphorus absorption at intestine and renal tubules which leads to hypocalcaemia and hypophosphotaemia. Also it decreases vitamin D and parathyroid hormone level [2, 6, 7]. All these effects decrease mineralization of bone leading to decrease bone mineral density and increase the risk of osteoporosis in occupational lead exposed population. Lead also affects on cardiovascular system and increases systolic and diastolic blood pressure [8–13].
Many industries are using most sophisticated machineries to reduce lead exposure. A study of clinical signs and symptoms with blood lead level and relevant biochemical changes will help to provide important information, for making suitable changes in the working environment of industrial workers. Hence aim of the study was to find out the effects of lead exposure on blood pressure, calcium metabolism and bone mineral density of battery manufacture workers with respect to duration of lead exposure.
Subjects and Methods
This study comprises ninety lead acid battery manufacture male workers (Study group) and thirty normal healthy age and sex matched subjects (Control Group). The study group subjects were further divided in three groups depending upon the duration of lead exposure. Each group includes thirty subjects.
Group I—1 to 5 years lead exposure.
Group II—6 to 10 years lead exposure.
Group III—more than 10 years lead exposure.
All the study group subjects and controls had age in the range of 20–45 years. Prior to data and biological specimen collection, factory owners and workers were informed on the study objectives and health hazards of lead exposure. Informed consent was obtained from all workers. Demographic, occupational and clinical data were collected by using questionnaire and interview. Most of the workers had major complaints of muscle pains, itchy feeling, mild fatigue, aggressiveness, irritability, lethargy, poor concentration, abdominal discomfort, etc. The socio-economic status of all the subjects of the study groups and control was average. None of the subjects had a past history of major illness. Dietary intake and food habits of all subjects were normal. The subjects, who were on drugs for minor illnesses, were excluded from this study. Non-smokers, non-alcoholic healthy males, who are occupationally exposed to lead for more than 6 h per day with the duration of exposure from 2 to 20 years, were only selected for this study. The protocol was approved by the institutional ethical committee. The care was taken during the experimental work according to Helsinki declaration [14].
The systolic and diastolic blood pressure of the workers was measured in resting condition before the sample collection.
The venous blood samples were collected from the study groups and normal healthy control group. Each subject was prepared by cleaning the site with separate spirit swab and blood was withdrawn by anticubital vein puncture using a 5 ml sterilized disposable syringe with disposable steel needle and collected in plain screw cap polypropylene test tube for estimation of biochemical parameters from serum samples. 2 ml serum was sent to Thyrocare Laboratories Mumbai for the estimation of Vit.D and PTH. At the time of blood collection random urine samples were collected in amber colour bottles to avoid errors from the inadequate collection of 24 h urine samples from each subject.
Blood lead (Pb–B) and urinary lead (Pb–U) were estimated by a Perkin Elmer model 303 graphite furnace atomic absorption spectrophotometer using the method of Parson et al. [15]. The values were expressed as μg/dl. The biochemical parameters like serum calcium, phosphorus were estimated by using standard assay procedures. Calcium was estimated by Arsenazo III complex method [16]. Calcium reacts with a dye Arsenazo at specific pH to form bluish purple colour complex. The intensity of the colour formed is directly proportional to the amount of calcium present in the sample. The absorbance of the coloured complex is read at 620 nm and compared with the standard.
Serum ionized calcium was calculated employing the method of Mclean and Hasting as adopted by Beeler and Atrou using the following formula [17].
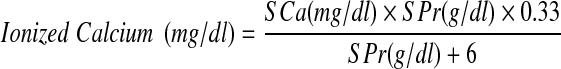 |
where SCa = Serum total calcium and SPr = Serum total protein.
The values obtained in mg/dl were converted to Mmol/L.
Serum inorganic phosphorus was estimated by Fiske and Subbarao method [18, 19]. Inorganic phosphorous combines with ammonium molybdate in the presence of strong acids to form phosphomolybdate. The formation of phosphomolybdate is measured at 340 nm and is directly proportional to the concentration of inorganic phosphorous present in the serum. Serum PTH and Vitamin D were estimated by chemiluminescent immunosorbent assay. The forearm bone mineral density was measured using dual-energy X-ray absorptiometry and expressed as the T-score [20].
Statistical analysis of the data was done using students ‘t’ test. Values were expressed as mean ± standard deviation (SD).
Results
Table 1 summarizes the mean values and SD of Pb–B, Pb–U, systolic and diastolic BP, serum Ca, ionized Ca, Phosphorus, Vitamin D, PTH and BMD in battery manufacturing workers and control group. Figure 1 shows the percentage change of Pb–B and Pb–U in three groups of battery manufacture workers with respect to controls. Figure 2 shows the percentage change of systolic B.P. and diastolic B.P. in three groups with respect to controls. Figure 3 gives the percentage change of Serum Ca, ionized Ca, Phosphorus, PTH and Vitamin D in three groups with respect to controls and Fig. 4 shows the T-score of bone mineral density of control and battery manufacturing workers groups.
Table 1.
Mean values and SD of Pb–B, Pb–U, systolic and diastolic BP, Serum Ca, Ionized Ca, Phosphorus, Vitamin D, PTH and BMD in battery manufacturing workers and control group
| Sl. No. | Parameter | Control group (N = 30) | Battery manufacturing workers (90) | ||
|---|---|---|---|---|---|
| Group I (N = 30) | Group II (N = 30) | Group III (N = 30) | |||
| 1 | PbB μg/dl | 10.2 ± 5.8 (2.0–23.0) |
58.37 ± 20.2*** (18.0–80.0) |
62.52 ± 15.4*** (26–84) |
65.5 ± 18.5*** (59–82.4) |
| 2 | PbU μg/dl | 6.28 ± 3.83 (1.0–14.0) |
19.17 ± 10.5*** (5.0–35.0) |
22.51 ± 17.4*** (6–43.4) |
28.3 ± 19.5*** (8–50.2) |
| 3 | Systolic B.P. mm/hg | 115.83 ± 10.5 (110–120) |
123.53 ± 9.43** (120–130) |
127 ± 12.41*** (120–140) |
130.8 ± 10.5*** (116–150) |
| 4 | Diastolic B.P mm/hg | 76.98 ± 8.7 (70–80) |
80.73 ± 4.3* (70–92) |
87.3 ± 7.7*** (80–110) |
90.26 ± 6.55*** (90–110) |
| 5 | Calcium mg/dl | 9.99 ± 0.43 (9.6–11.0) |
8.71 ± 0.6*** (7.0–9.0) |
8.2 ± 0.52*** (6.9–9.0) |
8.0 ± 0.76*** (6.82–8.8) |
| 6 | Ionised Ca mmol/L | 2.42 ± 0.10 (2.27–2.67) |
2.07 ± 0.14*** (1.68–2.16) |
1.9 ± 0.13*** (1.67–2.18) |
1.92 ± 0.18*** (1.67–2.14) |
| 7 | Phosphorus mg/dl | 4.5 ± 0.46 (3.3–5.5) |
3.89 ± 0.45*** (3.0–4.7) |
3.7 ± 0.36*** (3.0–4.5) |
3.65 ± 0.43*** (2.7–4.1) |
| 8 | PTH pg/ml (n = 15) | 25 ± 12 (12–40) |
28 ± 15• (10–50) |
29.5 ± 14• (12–55) |
34 ± 13* (10–54) |
| 9 | Vitamin D ng/ml (n = 15) | 13.74 ± 3.8 (9.25–17) |
12.9 ± 2.9• (9.0–18.0) |
11.96 ± 3.5• (8.25–17) |
11.76 ± 2.89• (8.0–17) |
| 10 | BMD T -Score | >−1.0 | −0.843 ± 0.39 (−0.2 to −1.9) |
−1.83 ± 0.42 (−1.1 to −2.8) |
−2.25 ± 0.62 (−1.5 to–3.4) |
Figures without parenthesis indicate Mean ± SD values and those in parenthesis are range of values. * P < 0.05, * *P < 0.01, * * *P < 0.001 as compared to controls and, •Non significant as compared to controls. PbB blood lead, PbU urinary lead, PTH parathyroid hormone, BMD bone mineral density
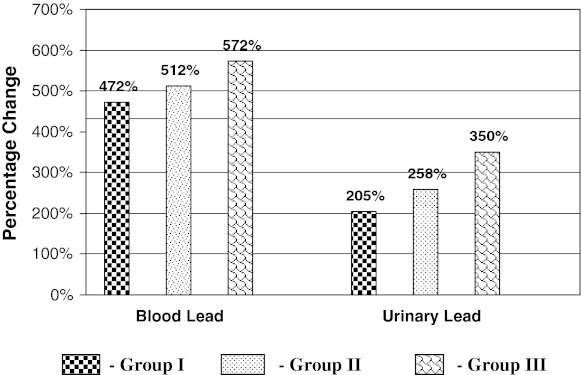
Fig. 1.
Percentage change of Pb–B and Pb–U in three groups with respect to controls
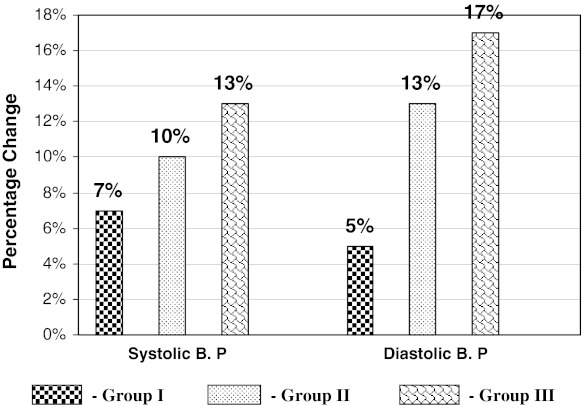
Fig. 2.
Percentage change of systolic B. P and Diastolic B. P in three groups with respect to controls
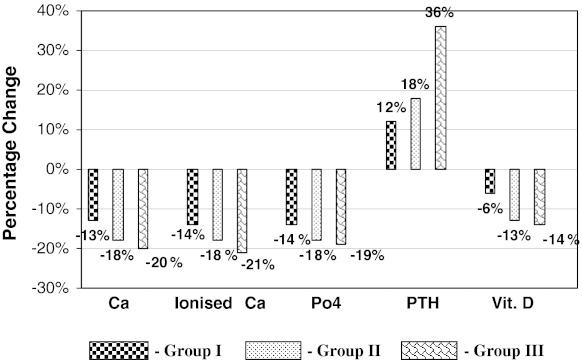
Fig. 3.
Percentage change of Ser.Ca, ionized Ca, phosphorus, PTH AND Vit. D. in three groups with respect to controls
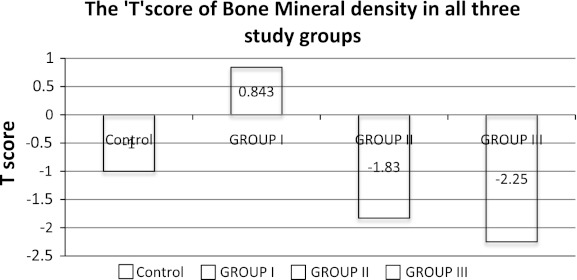
Fig. 4.
T-score of bone mineral density of control and battery manufacturing workers
Discussion
Blood lead levels were significantly increased by 472, 512 and 572 % (P < 0.001) in study groups I, II, and III respectively as compared to controls. Urinary lead excretion were significantly increased by 205, 258, and 350 % (P < 0.001) in I, II and III study groups respectively with respect to controls (Table 1 and Fig. 1). These results indicate that the absorption of lead is more in all three study groups resulting in more excretion of lead in urine. As duration of lead exposure increases it also increases body burden of lead.
Battery manufacturing involves the use of metallic lead for making grids, bearing and solder. Manufacturing process is usually manual and involves the release of lead particles and lead oxides that may cause environmental pollution and severe lead poisoning. Poor hygiene and inappropriate protection increases the risk of exposure.
Systolic blood pressure was significantly increased by 7, 10 and 13 % (P < 0.001) and diastolic blood pressure also elevated by 5, 13 and 17 % (P < 0.001) in study groups I, II, and III respectively as compared to controls (Table 1, Fig. 2). It indicates that lead affects on cardiovascular system of these workers, which resulted in the elevation of blood pressure.
It is reported that long term occupational exposure to lead is related to a slight increase of systolic and diastolic blood pressure among workers who had been exposed to higher level of lead with respect to workers exposed to lower levels of lead [21, 22]. Schuhmacher [23] showed a significant rise of blood pressure with the increases in blood lead levels in 36 male subjects who were occupationally exposed to lead. Hertz Picciotto [24] suggested a small positive association between blood lead and blood pressure in both occupational and general population. Dos Santos and Maheswaran [25, 26] noted a significant correlation between systolic and diastolic blood pressure and duration of exposure to lead. These studies suggest that the blood pressure is correlated with Pb–B and Pb–U. Our study also indicates that the blood lead levels contribute independently to the elevation of blood pressure. The effect of lead exposure on hypertension is usually because of effects of lead on kidney functions. Kidney compromises and in turn effects on high blood pressure. Hypertension is prominent in workers known to be chronically exposed to lead [8, 9]. So it is possible that lead induced nephrotoxicity is a probable cause of secondary hypertension.
Serum total calcium levels were significantly decreased by 13, 18 and 20 % (P < 0.001), and serum ionized calcium levels were also significantly decreased by 14, 18, 21 % (P < 0.001) in study groups I, II and III respectively of workers as compared to controls. Serum phosphorus levels were significantly decreased by 14, 18, and 19 % (P < 0.001) in the study groups I, II and III respectively of battery manufacture workers as compared to controls. Serum vitamin D levels were significantly decreased by 6, 13 and 14 % (P < 0.01) in study groups I, II and III respectively of battery manufacture of workers as compared to controls. Whereas serum parathyroid hormone levels were significantly elevated by 12, 18, and 36 % (P < 0.01) in study groups I, II and III respectively of battery manufacture of workers as compared to controls (Table 1, Fig. 3).
Decreased serum total Ca, ionized Ca, and phosphorus in battery manufacture workers may be due to increased blood lead because lead inhibits 1-α hydroxylase enzyme in renal tubules, which is required for calcitriol formation. Calcitriol plays a crucial role to maintain homeostasis of calcium and phosphorus metabolism, it stimulates the synthesis of calcium binding proteins in intestine, which are required for absorption of calcium across small intestine. It also facilitates absorption of calcium and phosphorus at renal tubules. In this study increased blood lead may decrease calcitriol concentration resulting in hypocalcaemia and hypophosphatemia [27, 28]. This decreased calcium level confirms earlier experimental and clinical reports and reflects perturbation of calcium metabolism due to lead [10, 29, 30]. Lead is a biochemical analogue of calcium, thus it interferes with calcium in several metabolic pathways. Our study also shows the interference of lead with the final metabolism of vitamin D to the active metabolite, calcitriol (1,25-DHCC), a hormone required for adequate calcium absorption.
The mechanism of the reduction of ionized calcium is still not clearly understood. It can be speculated that parathyroid hormone (PTH) which has a more direct effect on ionized calcium is also perturbed by the elevated lead level [10]. It indicates that lead is an endocrine modulator and thus a candidate for the endocrine disruptors group. We measured serum vitamin D and parathyroid hormone in selective 15 battery manufacture workers from each group whose serum total calcium and phosphorus drastically decreased at 1–5 years, 6–10 years and more than 10 years lead exposure. Estela Kristal-Bouneh reported that the levels of both Vit.D and PTH were significantly increased in the occupational lead exposed workers which helped in maintaining the normal serum calcium levels in those workers [28]. Our results are contrary to this report. Theoretically at high blood lead level active vitamin D3 (calcitriol) concentration decreases due to inhibition of 1-α-hydroxylase enzyme in renal tubule resulting in hypocalcaemia which further stimulates secretion of PTH. Therefore a detail research work with more samples is necessary to rule out the possibilities of decreased serum total calcium and phosphorus in this study.
T-score of bone mineral density of control was more than (−1.0). The battery manufacture workers exposed to lead from 1 to 5 years had the mean T-score of bone mineral density (−0.843), those exposed to lead from 6 to 10 years had (−1.83) and those who were exposed to lead for more than 10 years had mean T-score (−2.25) (Table 1, Fig. 4). Calcium and phosphorus are mainly required for bone mineralization. Several studies reported that elevated blood lead causes hypocalcaemia and hypophosphotaemia in occupational lead exposed population [29, 30]. Therefore we measured the bone mineral density in battery manufacture workers with 1–5 years, 6–10 years and more than 10 years lead exposure groups and found significantly decreased bone mineral density in all the three lead exposed study groups. Decreased bone mineral density in this study group clearly indicates that lead affects the mineral metabolism at absorption level.
In conclusion the study clearly indicates that Systolic and diastolic blood pressure was significantly high in battery manufacture workers as compared to controls, which indicates adverse effects of lead on cardiovascular system. Lead impairs normal kidney functions which may result in secondary hypertension. Significantly decreased total calcium, ionized calcium, phosphorus, vitamin D and bone mineral density and significantly increased PTH is observed in battery manufacture workers as compared to control group. It may be due to inhibition of 1-α hydroxylase enzyme in renal tubules. Lead causes nephrotoxicity and inhibits 1-α hydroxylase enzyme which leads to decreased calcitriol synthesis resulting in impaired calcium and phosphorus absorption across GIT and renal tubules. Significantly increased PTH in both study groups might be due to hypocalcaemia and hypophosphatemia.
This study will be useful to create awareness about health hazards related to lead exposure among occupationally lead exposed population. This study emphasizes that lead toxicity is still persistent in battery manufacture workers though sophisticated techniques are used in these industries. Lead toxicity will be present in these workers unless they take proper precautions to reduce lead exposure. Best remedy for reducing lead toxicity is by changing their occupations but it is highly impossible for these workers to leave their job because their whole family is dependent on them and getting new job is also very difficult nowadays. There is a need to protect the workers from the health hazards of occupational lead exposure.
References
- 1.World health organization. Biological indices of lead exposure and body burden. In: IPCS, Inorganic lead Environmental Health Criteria 118’, vol 165. Geneva: WHO; 1995. p. 114–118.
- 2.Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological profile for lead, US Department of Health and Human Services. Atlanta GA: US Government Printing; 2005. p. 102–225.
- 3.Casarett and Doull’s Toxicology. In: Curtis D. Klaassen, editor. The basic science of poisons, Chap. 23: Toxic effects of metals. 7th ed. New York: McGraw Hill publication; 2008. p. 943–947.
- 4.Saryan LA, Zenz C. Lead and its compounds In: Zenz CO, Dickerson B, Horvath EP, editors. Occupational medicine, Chap. 38. 3rd ed. St. Louis, MO: Mosby publishing company; 1994. p. 506–540,
- 5.IPSC Environmental health criteria 85 Lead-Environmental effect, Geneva WHO; 1989. p. 106.
- 6.Gidlow DA. “Lead toxicity” in depth review. Occup Med. 2004; 54:76–81. [DOI] [PubMed]
- 7.Dongre NN, Suryakar AN, Patil AJ, Rathi DB. Biochemical effects of occupational lead exposure to workers in small scale automobile workshops of North Karnataka (India). J Env Health Res. 2010; 10(2):27–34.
- 8.Buchot JP, Rods H, Benard A, Lauwerys R. Assessment of renal function of workers exposed to inorganic lead, cadmium or mercury vapor. J Occup Med. 1980;22:741–750. [PubMed]
- 9.Saboli I. Common mechanisms in nephropathy induced by toxic metals. Nephron Physiol. 2006;104:107–114. doi: 10.1159/000095539. [DOI] [PubMed] [Google Scholar]
- 10.Anetor JI, Akingbola TS, Adeniyi FAA, Taylor GO. Decreased total and ionized calcium levels and hematological indices in occupational lead exposure as evidence of the endocrine disruptive effect of lead. IJOEM. 2005;9(1):15–21. [Google Scholar]
- 11.Howard H, Rabinowitz M, Smith D. Bone lead as biological marker in epidemiologic studies of chronic toxicity: conceptual paradigms. Environ Health Perspect. 1998;106(1):1–8. doi: 10.1289/ehp.981061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jennifer AJ, Carla GT, Hope AW. Marginal zinc deficiency exacerbates bone lead accumulation and high dietary zinc attenuates lead accumulation at the expense of bone density in growing rats. Toxicol Sci. 2006;92(1):286–294. doi: 10.1093/toxsci/kfj201. [DOI] [PubMed] [Google Scholar]
- 13.Hu H, Aro A, Payton M, Korrick S, Sparrow D, Weiss ST, Rotnitzky A. The relationship of bone and blood lead to hypertension: the normative study. JAMA. 1996;275:1171–1176. doi: 10.1001/jama.1996.03530390037031. [DOI] [PubMed] [Google Scholar]
- 14.Declaration of Helsinki. (1964) Amended by World Medical Assembly, Venice, Italy and 1983 Br Med J. 1996; 313(70):601–605.
- 15.Parson PJ, Slavin W. A rapid Zeeman graphite furnace AAS method for determination of lead in blood. Spectrochim Acta. 1993;48 B:925–939.
- 16.Mcleans FL, Hastings AB. The state of calcium in the fluids of the body. J Biol Chem. 1935;108:285–322. [Google Scholar]
- 17.Beeler MF, Catrou PG. Disorders of calcium metabolism. In: Interpretations in clinical chemistry: a textbook approach to chemical pathology. Chicago: American Society of Clinical Pathologist; 1983. p. 34–44.
- 18.Daly JA, Ertingshausen G. Direct method for determining inorganic phosphorus in serum with the centrifichem. Clinical Chem. 1972;18:263. [PubMed] [Google Scholar]
- 19.Wang J, Chen CC, Osaki S. Optimization of the phospho- rus-UV reagent. Clin Chem. 1983;29:1255. [Google Scholar]
- 20.Alfven T, Jarup L, Elinder CG. Cadmium and lead in blood in relation to low bone mineral density and tubular proteinuria. Environ Health Persp. 2002;110(7):699–702. [DOI] [PMC free article] [PubMed]
- 21.Cramér K, Dahlberg L. Incidence of hypertension among lead workers: a follow-up study based on regular control over 20 years. Br J Ind Med. 1966;23:101–104. doi: 10.1136/oem.23.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fenga C, Cacciola A, Martino LB, Calderaro SR, Di Nola C, Verzera A, Trimarchi G, Germano D. Relationship of blood lead levels to blood pressure in exhaust battery storage workers. Ind Health. 2006;44:304–309. doi: 10.2486/indhealth.44.304. [DOI] [PubMed] [Google Scholar]
- 23.Schumacher M, Bosque MA, Domingo JL, Corbella J. Effect of chronic lead, cadmium exposure on blood pressure in occupationally exposed workers. Biol Trace Elem Res. 1994;41:269–278. doi: 10.1007/BF02917428. [DOI] [PubMed] [Google Scholar]
- 24.Hertz-Picciotto I. Review of the relation between blood lead and blood pressure. Epidemiol Rev. 1993;15:352–373. doi: 10.1093/oxfordjournals.epirev.a036125. [DOI] [PubMed] [Google Scholar]
- 25.Dos Santos AC, Colacioppo S, Dal Bo CMR, Dos Santos NAG. Occupational exposure to lead, kidney function tests and blood pressure. Am J Ind Med. 1994;26:635–643. doi: 10.1002/ajim.4700260506. [DOI] [PubMed] [Google Scholar]
- 26.Maheswaran R, Gill JS, Beevers DG. Blood pressure and industrial lead exposure. Am J Epidemiol. 1993;137:645–653. doi: 10.1093/oxfordjournals.aje.a116722. [DOI] [PubMed] [Google Scholar]
- 27.Damstra T. Toxicological properties of lead. Envrion Health Perspect. 1977;19:297–307. doi: 10.1289/ehp.7719297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kristal-Bouneh E, Froom P, Yerushalmi N, Harari G, Ribak J. Calcitropic hormones and occupational lead exposure. Am J Epidemil. 1998;147(5):458–463. doi: 10.1093/oxfordjournals.aje.a009471. [DOI] [PubMed] [Google Scholar]
- 29.Sun Y, Sun D, Zhou Z, Zhu G, Zhang H, Chalng X, Lei L, Jin T. Osteoporosis in a Chinese population due to occupational exposure to lead. Am J Ind Med. 2008;51:436–442. doi: 10.1002/ajim.20567. [DOI] [PubMed] [Google Scholar]
- 30.Potula V, Henderson A, Kaye W. Calcitropic hormones, bone turn over and lead exposure among female smelter workers. Arch Environ Occup Health. 2005;60(4):195–204. doi: 10.3200/AEOH.60.4.195-204. [DOI] [PubMed] [Google Scholar]