Abstract
The aim of this study was to assess the diagnostic yield of the tumour markers carcinoembryonic antigen, carbohydrate antigen 15-3, carbohydrate antigen 19-9 and carbohydrate antigen 125, in serum and bronchoalveolar lavage fluid in a group of patients with bronchogenic carcinoma. Serum and bronchoalveolar lavage fluid samples were collected in a group of 90 patients with benign or malignant pulmonary diseases. After appropriate processing, tumour markers were determined by enzyme immunoassay. The diagnostic yields (sensitivity, specificity and predictive values) in each environment (serum and bronchoalveolar lavage fluid) were obtained by using "Receivers operating characteristic" curve. Determined individually, carcinoembryonic antigen, carbohydrate antigen 19-9 and carbohydrate antigen 125, showed the greatest diagnostic accuracy in bronchoalveolar lavage fluid. Carbohydrate antigen 15-3 did so in serum. Carcinoembryonic antigen was the most relevant marker in bronchoalveolar lavage fluid. For the factors evaluated in this study, determination of carcinoembryonic antigen, carbohydrate antigen 19-9 and carbohydrate antigen 125 in bronchoalveolar lavage fluid were clinically more useful markers in comparison with serum, although the latter may also be helpful in certain situations. Although there is no specific tumour marker for lung cancer, the combination of several can be used to diagnose most patients with lung cancer and also to rule out false positive and negative cases.
Keywords: Tumour markers, Lung carcinoma, Bronchoalveolar lavage fluid, Serum
Introduction
The term lung cancer is used for tumours arising from the respiratory epithelium (bronchi, bronchioles and alveoli). According to the World Health Organization classification four major cell types make up 88 % of all primary lung neoplasm. Adenocarcinoma consists of 32 %, squamous cell carcinoma 29 %, small cell carcinoma 18 % and large cell carcinoma account for 9 % of all cases. Lung cancer accounts for 29 % of all cancer death (31 % in males and 26 % in female) in the world [1]. Emerging technologies for early detection of lung cancer includes low dose helical computed tomography scan [2], computer aided digital radiography [3–5], sputum immunostaining and sputum polymerase chain reaction based oncogene detection [5–8], auto fluorescence and virtual bronchoscopy. Many studies have targeted mass spectrometry aided secretome analysis in cancer cells for screening purpose [9–12].
Tumor markers are molecules occurring in blood or tissue that are produced by a tumour associated with a cancer or by the host in response to the cancer whose measurement or identification is useful for clinical diagnosis or patient management. Tumor markers can be used for screening a high risk population for cancer, making a diagnosis and prognosis in a specific cancer and monitoring the course in a patient in remission or while receiving surgery, radiation, or chemotherapy. The ideal marker would be a “blood test” for cancer in which a positive result would occur only in patients with malignancy, one that would correlate with stage and response to treatment and that could be easily and reproducibly measured.
Carcinoembryonic antigen (CEA) the first oncofetal antigens to be described, consists of a large family of cell surface glycoprotein (molecular weight: 150–300 kDa) found in many types of cells but associated with tumors and the developing fetus. The normal range is <2.5 μg/l in an adult non-smoker and <5.0 μg/l in a smoker. It is associated with plasma membrane of tumor cells, from which it may be released into the blood. Although CEA was first identified in colon cancer, an elevated CEA level is also found in a variety of cancers like lung, pancreas, gastric, and breast. It is also detected in benign conditions including cirrhosis, inflammatory bowel disease, chronic lung disease, and pancreatitis.
Carbohydrate antigen 125 (CA125) is a carbohydrate- related high molecular mass glycoprotein (molecular weight: >200 kDa) present in 80 % of nonmucinous ovarian carcinomas. It is defined by a monoclonal antibody (OC125) that was generated by immunizing laboratory mice with a cell line established from human ovarian carcinoma. In healthy population the upper level of CA125 is 35 U/ml. The CA125 is elevated in other cancers including lung, endometrial, pancreas, breast, and colon and in menstruation, pregnancy, endometriosis, and other gynecologic and non gynecologic conditions.
Carbohydrate antigen 19-9 (CA19-9) is a monoclonal antibody generated against a colon carcinoma cell line to detect a monosialoganglioside found in patients with gastrointestinal adenocarcinoma (molecular weight: >1,000 kDa). The upper reference range is <37 U/ml in healthy subjects. It is found it to be elevated in cases of gastric cancer, lung cancer, colon cancer and pancreatic cancer and has been proposed to differentiate benign from malignant pancreatic disease.
Cancer antigen 15-3 (CA 15-3) is a murine monoclonal antibody produced by normal breast cells (molecular weight: 300–450 kDa). In many patients with cancerous breast tumors, there is an increased production and shedding of CA 15-3 by the tumor cells. As it enters the bloodstream, is determination in blood makes it useful as a tumor marker to follow the course of the cancer. In healthy subjects the upper limit of CA 15-3 concentration is 25 U/ml. CA15-3 may also be elevated in individuals with other cancers, conditions, or diseases, such as lung cancer colorectal cancer, cirrhosis, hepatitis, and benign breast disease [13].
Levels of tumour markers in biological fluids like pleural effusion have been used to establish diagnosis of pleural malignancy [14, 15]. This study aims to evaluate the possible role of tumour markers like CEA, CA125, CA19-9 and CA15-3 in serum as well as in bronchoalveolar lavage (BAL) fluid of suspected cases in diagnosis of lung cancer.
Materials and Methods
The case control study was conducted at Calcutta National Medical College and Hospital in Kolkata, West Bengal, India, during the period August 2010–August 2011. The study was approved by the institutional ethical committee and an informed consent was taken from each participant. The study included 92 subjects in the age group 30–70 years attending the Chest Medicine department of the hospital. The cases and controls consisted each of 46 age and sex matched subjects. Patients with clinico-radiological suspicion of lung malignancy and later confirmed by computed tomography guided fine needle aspiration cytology (FNAC) of peripheral lymph node or fibre optic bronchoscopy (FOB) guided biopsy were considered as cases. Controls were individuals admitted in chest department for various other respiratory ailments like pneumonia, diffuse parenchymal lung disease, pulmonary tuberculosis (sputum smear negative) etc. in which there was indication for diagnostic bronchoscopy. Patients less than 15 years of age with history of adenocarcinoma of colon, pancreas, breast ovary, hepatocellular carcinoma, gonadal germ cell tumours, pancreatitis, hepatitis, cirrhosis of liver, inflammatory bowel disease and pregnancy were excluded from the study. Serum was separated from 3 ml of venous blood samples collected aseptically from all the cases and controls. 20 ml of BAL fluid was collected from all the diagnosed cases and controls by performing FOB under local anesthesia. The supernatant fluid was separated from the precipitated cell debris. The serum and BAL fluid from all the subjects were analyzed for estimation of levels of various tumour markers like CEA, CA-125, CA 19-9 and CA 15-3 using third generation enzyme linked immunosorbant assay (ELISA) kits (Can Ag, Canada).
Data Analysis
The statistical software namely SPSS 16.0 was used for the analysis of the obtained data. Difference between mean values of different tumour markers was assessed by ‘paired t test’.
Strength of association between different parameters was analyzed by ‘Pearson’s bivariate correlation analyses. p value was considered to be significant at value <.05, at a confidence limit of 95 %.
Effect of smoking on the levels of tumour markers in cases and controls in BAL and serum was assayed by ‘Mann–Whitney test’.
Results
After fulfilling the inclusion and exclusion criteria 46 subjects were selected as cases, while 42 healthy people were selected in the control group. Statistically the matching of age between these two groups was checked by independent t test (t = 1.61, p = .109). The cases comprised of 30 males and 16 females. The control group had 24 males and 18 females in it. The gender matching between these two groups was checked by Chi-square test (χ2 = 1.001, p > .05, Table not shown).
The study demonstrated that the levels of all the tumour markers were higher in the cases compared to the controls. Among the cases, the BAL fluid had significantly higher values for the individual markers, than that of serum. The BAL fluid and serum values for all the measured tumour markers were compared with each other and the correlation was found to be statistically significant (Table 1).
Table 1.
Age of cases and controls
| Cases | Controls | p value | |
|---|---|---|---|
| No. of subjects | 46 | 42 | |
| Mean age (years) | 56.54 ± 2.13 | 53.3 ± 5.4 | .109 |
ROC Analysis
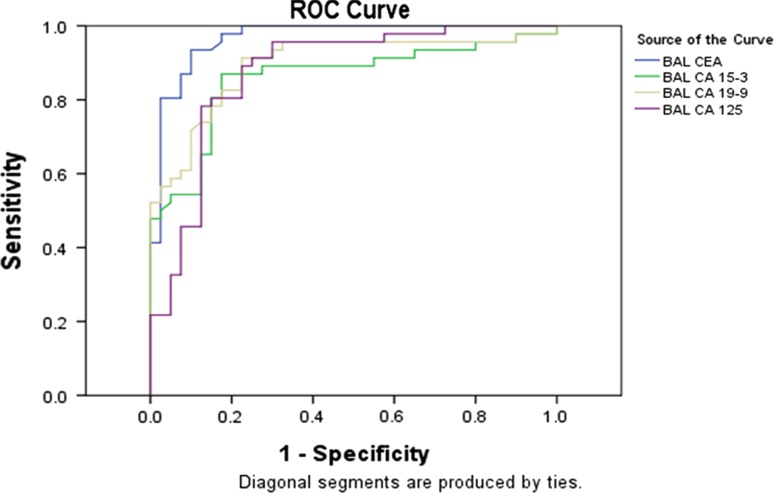
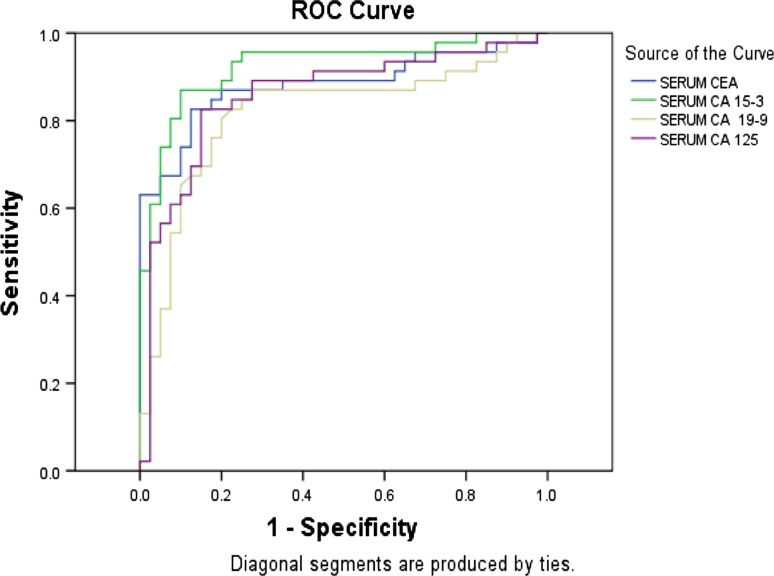
Figures 1 and 2 show the receiver operating curve (ROC) curves for BAL and serum respectively. Based on the ROC curve, cut-off values for BAL fluid and serum have been set for all the individual tumour markers (Table 7) and the respective sensitivity, specificity and predictive values have been calculated (Table 8).
Fig. 1.
Test result variable(s): ROC for CEA, CA15-3, CA19-9 and CA125 in BAL fluid
Fig. 2.
Test result variable(s): ROC for CEA, CA15-3, CA19-9 and CA125 in serum
Table 7.
Cut-off values for individual tumour markers in BAL fluid and serum
| Cut-off values | ||
|---|---|---|
| Tumour markers | BAL fluid | Serum |
| CEA | <8 μg/l | <4.5 μg/l |
| CA15-3 | <18.0 U/ml | <28.8 U/ml |
| CA 19-9 | <37.9 U/ml | <16.0 U/ml |
| CA 125 | <36.5 U/ml | <24.5 U/ml |
Table 8.
Sensitivity, specificity and predictive values for individual tumour markers based on ROC
| Parameters | CEA | CA 15-3 | CA 19-9 | CA 125 | ||||
|---|---|---|---|---|---|---|---|---|
| BAL (%) | Serum (%) | BAL (%) | Serum (%) | BAL (%) | Serum (%) | BAL (%) | Serum (%) | |
| Sensitivity | 91.3 | 89.13 | 89.13 | 93.48 | 91.3 | 89.13 | 89.13 | 91.3 |
| Specificity | 90 | 65 | 45 | 80 | 77.5 | 32.5 | 75 | 62.5 |
| Positive predictive value | 91.3 | 74.55 | 65.08 | 84.31 | 82.35 | 60.29 | 80.39 | 73.68 |
| Negative predictive value | 90.3 | 83.87 | 78.26 | 91.43 | 88.57 | 72.22 | 85.71 | 86.21 |
Discussion
In this study we have compared the BAL and serum levels of certain tumour markers such as CEA, CA15-3, CA19-9 and CA125 in lung cancer cases. The results showed definite elevation in levels of all the parameters in both BAL fluid and serum of all the cases compared to the controls (Table 2).
Table 2.
Mean levels of tumour markers in BAL and serum in cases and controls
| Mean ± SD | CEA (μg/l) | CA15-3 (U/ml) | CA19-9 (U/ml) | CA125 (U/ml) |
|---|---|---|---|---|
| Cases | ||||
| BAL | 31 ± 3.2 | 80.29 ± 10.6 | 141.83 ± 19.97 | 136.96 ± 19.03 |
| Serum | 20.55 ± 3.06 | 61.01 ± 4.53 | 62.39 ± 6.89 | 54.99 ± 4.74 |
| Controls | ||||
| BAL | 4.65 ± 3.77 | 21.66 ± 13.2 | 25.26 ± 15.1 | 29.6 ± 2.99 |
| Serum | 4.1 ± 2.1 | 21.94 ± 10.9 | 23.88 ± 18.14 | 25.9 ± 2.8 |
Among the cases, BAL fluid CEA levels are more increased in smokers. It indicates that tobacco induces cellular alterations in the bronchial cells among the case group causing increased secretion of CEA. These results are in close agreement with other studies [16]. Moreover both age and smoking history are found to influence the evaluation of CEA levels [17, 18] (Table 3).
Table 3.
Correlation data within the tumour markers in the cases
| Sl. no | Parameters | r value | p value |
|---|---|---|---|
| 1 | BAL vs. serum CEA | .113 | .001 |
| 2 | BAL vs. serum CA15-3 | .261 | .025 |
| 3 | BAL vs. serum CA19-9 | .01355 | <.001 |
| 4 | BAL vs. serum CA125 | .00658 | <.001 |
In our study Tables 4, 5, and 6, showed the effect of smoking on tumor marker levels in cases and control subjects. Among the cases, values for different tumour markers in BAL fluid were similar among smokers and non smokers except that of CA19-9 which was high only in smokers. Similarly, serum CA15-3 values were higher among smokers as compared to the non smoker cases. For the control subjects, however no such differences were observed for BAL or serum tumour marker values among the smokers and non smoker groups. When the entire case–control population were considered together, smoking was not found to alter the tumour marker values in BAL fluid and serum (p value <.001 for all).
Table 4.
Mann–Whitney test to assess the influence of smoking on tumour marker levels among cases
| Case test statisticsb | ||||||||
|---|---|---|---|---|---|---|---|---|
| Serum CEA | Serum CA 15-3 | Serum CA 19-9 | Serum CA 125 | BAL CEA | BAL CA 15-3 | BAL CA 19-9 | BAL CA 125 | |
| Mann–Whitney U | 186.000 | 143.000 | 114.000 | 171.000 | 127.500 | 115.000 | 191.000 | 143.000 |
| Wilcoxon W | 252.000 | 209.000 | 180.000 | 801.000 | 757.500 | 181.000 | 257.000 | 773.000 |
| Z | −.167 | −1.275 | −2.022 | −.554 | −1.674 | −1.996 | −.039 | −1.275 |
| Asymp. Sig. (2-tailed) | .867 | .202 | .043 | .580 | .094 | .046 | .969 | .202 |
| Exact Sig. [2*(1-tailed Sig.)] | .879a | .210a | .043a | .594a | .094a | .046a | .980a | .210a |
aNot corrected for ties
bGrouping variable: smoking
Table 5.
Mann–Whitney test to assess the influence of smoking on tumour marker levels among controls
| Control test statisticsb | ||||||||
|---|---|---|---|---|---|---|---|---|
| Serum CEA | Serum CA 15-3 | Serum CA 19-9 | Serum CA125 | BAL CEA | BAL CA 15-3 | BAL CA19-9 | BAL CA 125 | |
| Mann–Whitney U | 127.500 | 139.000 | 140.000 | 105.000 | 145.000 | 119.000 | 120.000 | 110.500 |
| Wilcoxon W | 182.500 | 604.000 | 605.000 | 570.000 | 200.000 | 174.000 | 175.000 | 575.500 |
| Z | −.703 | −.344 | −.312 | −1.406 | −.156 | −.968 | −.937 | −1.234 |
| Asymp. Sig. (2-tailed) | .482 | .731 | .755 | .160 | .876 | .333 | .349 | .217 |
| Exact Sig. [2*(1-tailed Sig.)] | .488a | .747a | .770a | .167a | .890a | .346a | .363a | .221a |
aNot corrected for ties
bGrouping variable: smoking
Table 6.
Mann–Whitney test to assess the influence of smoking on tumour marker levels among both cases and controls
| All subject statisticsa | ||||||||
|---|---|---|---|---|---|---|---|---|
| Serum CEA | Serum CA 15-3 | Serum CA 19-9 | Serum CA125 | BAL CEA | BAL CA 15-3 | BAL CA19-9 | BAL CA 125 | |
| Mann–Whitney U | 61.500 | 259.500 | 194.500 | 231.000 | 217.000 | 138.000 | 335.000 | 262.000 |
| Wilcoxon W | 881.500 | 1079.500 | 1014.500 | 1051.000 | 1037.0 | 958.000 | 1155.000 | 1082.000 |
| Z | −7.433 | −5.719 | −6.281 | −5.965 | −6.087 | −6.771 | −5.065 | −5.697 |
| Asymp. Sig.(2-tailed) | .000 | .000 | .000 | .000 | .000 | .000 | .000 | .000 |
aGrouping variable: grouping
ROC analysis is now a standard tool to asses, define and compare the diagnostic validity of tests. This statistical parameter (Figs. 1, 2) had shown higher sensitivity and specificity levels for all the markers in BAL fluid compared to controls except for CA15-3 (Table 7). BAL fluid analysis in this study possessed higher predictive values compared to serum for all the markers except CA15-3. In the present study the cut off values for maximum sensitivity and lowest false positivity for the parameters CEA, CA15-3, CA19-9 and CA125 for BAL fluid were found to be <8 μg/l, <18.0 U/ml, <37.9 U/ml and <36.5 U/ml respectively. Similar cut off values for these parameters in serum were <4.5 μg/l, <28.8 U/ml, <16.0 U/ml and <24.5 U/ml respectively as evident by the ROC curves (Figs. 1, 2); (Tables 7, 8). Numerous studies have been performed to determine diagnostic or prognostic utility of tumor markers in patients with lung cancer. Charalabopoulos et al. and Niho [19, 20] in lung cancer cases had demonstrated higher BAL fluid and serum CEA levels respectively, compared to benign lung lesion controls. Lazarev et al. [21], however have doubted the utility of estimation of biological tumour markers like CEA for its low sensitivity for diagnosis and treatment of non small cell lung cancer (NSCLC) cases. On the contrary retrospective review upon two hundred and seventy-seven diagnosed NCLC patients had shown significantly elevated serum CEA and CA125 levels at baseline [22]. Moreover CEA levels at baseline was found to correlate with overall survival time in both small and non small cell lung carcinoma cases [22, 23]. Similar studies conducted upon 51 lung cancer patients and 44 patients with a benign lung disease showed serum CEA, CA-125, CA 19-9 and CA 15-3 levels were significantly higher in the malignant group (p < .05). The results have validated the cut off values for CEA, CA15-3 and CA19-9 in BAL and serum at a level of 1.35 μg/l, 5 μg/l; 2.2 U/ml, <25 U/ml and 64 U/ml; <39 U/ml respectively [24]. In comparison to these values we found the cut off values at a higher degree of sensitivity and false positivity that might increase their diagnostic values. Studies conducted to evaluate the diagnostic usefulness of the tumor markers CA125, CEA in BAL fluid in 30 NSCLC have shown CEA and CA125 concentration in BAL fluid were significantly higher in NSCLC patients than in healthy volunteers and patients with sarcoidosis [25]. The sensitivity and specificity of CEA and CA 125 in BALF were 100 %, 84 % and 92 %, 80 %, respectively.
Similar examination conducted upon seven tumour markers CEA, AFP, CA19-9, squamous cell carcinoma antigen (SCC), neuron-specific enolase (NSE),CA125, cytokeratin 19 fragment (CYFRA) in 200 patients with adenocarcinoma have concluded that CEA had the highest positivity rate (46.5 % of patients) followed by CA125, the positivity rate increasing with advancing stage [26]. Other study results have shown that CEA and CA125 have different diagnostic yield for lung carcinoma in different environment like BAL, serum and cytosol biopsy [27].
This study has limitations that must be considered. Number of patients in the study groups was not large. Thus, care must be taken in extrapolating the present findings to other populations. Despite these limitations, we believe that our study will be helpful as assay of the above mentioned tumor markers is simple and will complement other tests in diagnosis of lung cancer.
In conclusion, the results suggest that among the chosen markers, combined measurement of CEA, CA19-9 and CA125 in BAL fluid and CA15-3 in serum is useful in diagnosis of lung cancer. These may be useful in patients in whom tumour cannot be visualized by bronchofibroscopy or to rule out false positive cases. These results need further confirmation in larger groups of patients.
References
- 1.John DM, Joan H, et al. Neoplasms of the lung. In: Fauci AS, Braunwald E, Kasper DL, Hanser SL, Longo DL, Jameson JL, et al., editors. Harrison’s principles of internal medicine. New York: McGraw Hill; 2008. pp. 2275–2304. [Google Scholar]
- 2.Henschke CI, McCauley DI, Yankelevitz DF, Naidich DP, McGuinness G, Miettinen OS, et al. Early lung cancer action project: overall design and findings from baseline screening. Lancet. 1999;354:99–105. doi: 10.1016/S0140-6736(99)06093-6. [DOI] [PubMed] [Google Scholar]
- 3.Kobayashi T, Xu XW, MacMahon H, Metz CE, Doi K. Effect of a computer-aided diagnosis scheme on radiologists’ performance in detection of lung nodules on radiographs. Radiology. 1996;199:843–848. doi: 10.1148/radiology.199.3.8638015. [DOI] [PubMed] [Google Scholar]
- 4.Kido S, Ikezoe J, Naito H, Arisawa J, Tamura S, Kozuka T, et al. Clinical evaluation of pulmonary nodules with single-exposure dual-energy subtraction chest radiography with an iterative noise-reduction algorithm. Radiology. 1995;194:407–412. doi: 10.1148/radiology.194.2.7824718. [DOI] [PubMed] [Google Scholar]
- 5.MacMahon H, Engelmann R, Behlen FM, Hoffmann KR, Ishida T, Roe C, et al. Computer-aided diagnosis of pulmonary nodules: results of a large-scale observer test. Radiology. 1999;213:723–726. doi: 10.1148/radiology.213.3.r99dc27723. [DOI] [PubMed] [Google Scholar]
- 6.Lam S, Shibuya H. Early diagnosis of lung cancer. Clin Chest Med. 1999;20:53–61. doi: 10.1016/S0272-5231(05)70126-X. [DOI] [PubMed] [Google Scholar]
- 7.Sidransky D, Tokino T, Frost P, Hamilton S, Levin B, Vogestein B. Identification of ras oncogene mutations in the stool of patients with curable colorectal tumors. Science. 1992;256:102–105. doi: 10.1126/science.1566048. [DOI] [PubMed] [Google Scholar]
- 8.Sidransky D, Von Eschenbach A, Tsai YC. Identification of p53 gene mutations in bladder cancers and urine samples. Science. 1991;252:706–709. doi: 10.1126/science.2024123. [DOI] [PubMed] [Google Scholar]
- 9.Sung HJ, Cho JY. Biomarkers for the lung cancer diagnosis and their advances in proteomics. BMB Rep. 2008;41:615–625. doi: 10.5483/BMBRep.2008.41.9.615. [DOI] [PubMed] [Google Scholar]
- 10.Andrews CO, Gora ML. Pleural effusions: pathophysiology and management. Ann Pharmacother. 1994;28:894–903. doi: 10.1177/106002809402800715. [DOI] [PubMed] [Google Scholar]
- 11.Xiao T, Ying W, Li L, Hu Z, Ma Y, Jiao L, et al. An approach to studying lung cancer-related proteins in human blood. Mol Cell Proteomics. 2005;4:1480–1486. doi: 10.1074/mcp.M500055-MCP200. [DOI] [PubMed] [Google Scholar]
- 12.Chen Y, Zhang H, Xu A, Li N, Liu J, Liu C, et al. Elevation of serum l-lactate dehydrogenase B correlated with the clinical stage of lung cancer. Lung Cancer. 2006;54:95–102. doi: 10.1016/j.lungcan.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 13.Daniel WC, Stewart S. Tumour markers. In: Burtis CA, Ashwood ER, editors. Teitz textbook of clinical chemistry 3rd edn. Philadelphia: Saunders Elsevier; 1999. pp. 733–737. [Google Scholar]
- 14.Shitrit D, Zingerman B, Shitrit AB, Shlomi D, Kramer MR. Diagnostic value of CYFRA 21-1, CEA, CA 19-9, CA 15-3, and CA 125 assays in pleural effusions: analysis of 116 cases and review of the literature. Oncologist. 2005;10:501–507. doi: 10.1634/theoncologist.10-7-501. [DOI] [PubMed] [Google Scholar]
- 15.Pasaoglu G, Zamani A, Can G, Imecik O. Diagnostic value of CEA, CA-19-9, CA 125 and CA 15-3 levels in malignant pleural fluids. Eur J Gen Med. 2007;4(4):165–171. [Google Scholar]
- 16.Pardos MC, Alvarez-Sala R, Terreros Caro FJ, Gomez L, de Gomez Terreros FJ, Villamor J. The concentrations of five tumor markers in both BAL fractions in lung cancer patients in relation to cigarette smoking. Tumori. 1999;85(6):454–457. doi: 10.1177/030089169908500606. [DOI] [PubMed] [Google Scholar]
- 17.Fukuda I, Yamakado M, Kiyose H. Influence of smoking on serum carcinoembryonic antigen levels in subjects who underwent multiphasic health testing and services. J Med Syst. 1998;22(2):89–93. doi: 10.1023/A:1022643102208. [DOI] [PubMed] [Google Scholar]
- 18.Alexander JC, Silverman NA, Chretien PB. Effect of age and cigarette smoking on carcinoembryonic antigen levels. JAMA. 1976;235(18):1975–1979. doi: 10.1001/jama.1976.03260440027017. [DOI] [PubMed] [Google Scholar]
- 19.Charalabopoulos K, Karakosta A, Bablekos G, Golias C, Charalabopoulos A, Tsanou E, et al. CEA levels in serum and BAL in patients suffering from lung cancer: correlation with individuals presenting benign lung lesions and healthy volunteers. Med Oncol. 2007;24(2):219–225. doi: 10.1007/BF02698043. [DOI] [PubMed] [Google Scholar]
- 20.Niho S, Shinkai T. Tumor markers in lung cancer. Gan To Kagaku Ryoho. 2001;28(13):2089–2093. [PubMed] [Google Scholar]
- 21.Lazarev SM, Massard Zh, Reshetov AV, Nikolaev GV, Volgin GN, Osipov EV, et al. Role of biological tumor markers CEA, Cyfra-21, NSE, TU M2-PK in diagnosis and treatment of lung cancer. Vestn Khir Im I I Grek. 2010;169(1):39–43. [PubMed] [Google Scholar]
- 22.Cedres S, Nunez I, Longo M, Martinez P, Checa E, Torrejon D, Felip E. Serum tumour markers CEA, CYFRA21-1, and CA-125 are associated with worse prognosis in advanced non-small-cell lung cancer (NSCLC) Clin Lung Cancer. 2011;12(3):172–179. doi: 10.1016/j.cllc.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 23.Yang X, Wang D, Yang Z, Qing Y, Zhang Z, Wang G, et al. CEA is an independent prognostic indicator that is associated with reduced survival and liver metastases in SCLC. Cell Biochem Biophys. 2011;59(2):113–119. doi: 10.1007/s12013-010-9121-0. [DOI] [PubMed] [Google Scholar]
- 24.Zeynep AS, Ceyda K, Tuncay G, Ozme D, Mutaf I, Bayindir U, et al. Tumor markers in blood and in bronchoalveolar lavage fluid in patients with lung cancer. Turk Respir J. 2005;6(2):73–77. [Google Scholar]
- 25.Dabrowska M, Grubek-Jaworska H, Domagała-Kulawik J, Bartoszewicz Z, Kondracka A, Krenke R, et al. Diagnostic usefulness of selected tumor markers (CA125, CEA, CYFRA 21-1) in bronchoalveolar lavage fluid in patients with non-small cell lung cancer. Pol Arch Med Wewn. 2004;111(6):652–659. [PubMed] [Google Scholar]
- 26.Ando S, Kimura H, Iwai N, Shima M, Ando M, Kuriyama T. Optimal combination of seven markers in prediction of advanced stage at first examination of patients with non-small cell lung cancer. Anticancer Res. 2001;21(4):3085–3092. [PubMed] [Google Scholar]
- 27.Pina TC, Zapata IT, Hernandez FC, Lopez JB, Paricio PP, Hernandez PM. Tumour markers in serum, bronchoalveolar lavage and biopsy cytosol in lung carcinoma: what environment lends the optimum diagnostic yield? Clin Chim Acta. 2001;305(1–2):27–34. doi: 10.1016/S0009-8981(00)00410-1. [DOI] [PubMed] [Google Scholar]