Abstract
The first trimester screening programme offers a noninvasive option for the early detection of aneuploidy pregnancies. This screening is done by a combination of two biochemical markers i.e. serum free β-human chorionic gonadotrophin (free β-hCG) and pregnancy associated plasma protein A (PAPP-A), maternal age and fetal nuchal translucency (NT) thickness at 11 + 0–13 + 6 weeks of gestation. A beneficial consequence of screening is the early diagnosis or trisomies 21, 18 and 13. At 11 + 0–13 + 6 weeks, the relative prevalence of trisomies 18 and 13 to trisomy 21 are found to be one to three and one to seven, respectively. All three trisomies are associated with increased maternal age, increased fetal NT and decreased PAPP-A, but in trisomy 21 serum free β-hCG is increased whereas in trisomies 18 and 13 free β-hCG is decreased.
Keywords: PAPP-A, Free β-hCG, Nuchal translucency, Down syndrome, Patau syndrome, Edward syndrome
Prenatal screening for trisomies based on the analysis of biochemical markers in maternal serum has become an established part of obstetric practice in many countries. Resent interest in prenatal screening for trisomies has focused on the first trimester. Of the biochemical markers that have been investigated, only maternal serum free β-human chorionic gonadotrophin (free β-hCG) and pregnancy associated plasma protein-A (PAPP-A) has been shown to be of value. The goal of first trimester maternal serum screening programs is to identify women at increased risk of having a baby affected with Down syndrome, Patau syndrome, and Edward syndrome defects and those that will benefit from the testing.
The association between advancing maternal age [1] and increased risk of trisomy 21 (Table 1) is well known, and pregnant women older than 35 years at delivery are routinely offered invasive prenatal diagnostic testing. The most commonly used test for genetic diagnosis is amniocentesis, but the rate of spontaneous fetal loss related to amniocentesis averages about one in every 200 [2] procedures. Because of this risk, serum analyte testing has become an important, noninvasive first step in detecting patients at risk for congenital abnormalities.
Table 1.
Prevalence of trisomy 21 by maternal age and gestational age
| Maternal age (years) | Gestational age (weeks) | |||||
|---|---|---|---|---|---|---|
| 10 | 12 | 14 | 16 | 20 | 40 | |
| 20 | 1:983 | 1:1,068 | 1:1,140 | 1:1,200 | 1:1,295 | 1:1,527 |
| 25 | 1:870 | 1:946 | 1:1,009 | 1:1,062 | 1:1,147 | 1:1,352 |
| 30 | 1:576 | 1:626 | 1:668 | 1:703 | 1:759 | 1:895 |
| 31 | 1:500 | 1:543 | 1:580 | 1:610 | 1:658 | 1:776 |
| 32 | 1:424 | 1:461 | 1:492 | 1:518 | 1:559 | 1:659 |
| 33 | 1:352 | 1:383 | 1:409 | 1:430 | 1:464 | 1:547 |
| 34 | 1:287 | 1:312 | 1:333 | 1:350 | 1:378 | 1:446 |
| 35 | 1:229 | 1:249 | 1:266 | 1:280 | 1:302 | 1:356 |
| 36 | 1:180 | 1:196 | 1:209 | 1:220 | 1:238 | 1:280 |
| 37 | 1:140 | 1:152 | 1:163 | 1:171 | 1:185 | 1:218 |
| 38 | 1:108 | 1:117 | 1:125 | 1:131 | 1:142 | 1:167 |
| 39 | 1:82 | 1:89 | 1:95 | 1:100 | 1:108 | 1:128 |
| 40 | 1:62 | 1:68 | 1:72 | 1:76 | 1:82 | 1:97 |
| 41 | 1:47 | 1:51 | 1:54 | 1:57 | 1:62 | 1:73 |
| 42 | 1:35 | 1:38 | 1:41 | 1:43 | 1:46 | 1:55 |
| 43 | 1:26 | 1:29 | 1:30 | 1:32 | 1:35 | 1:41 |
| 44 | 1:20 | 1:21 | 1:23 | 1:24 | 1:26 | 1:30 |
| 45 | 1:15 | 1:16 | 1:17 | 1:18 | 1:19 | 1:23 |
[1]
Current studies done on first trimester maternal serum screening has shown that the double marker test helps to identify 90 % of women at risk for Down syndrome, 94 % of all major chromosomal defects such as Patau syndrome, Edward syndrome, triploidy and Turner syndrome, and 60 % of other chromosomal defects, such as deletions, partial trisomies, unbalanced translocations, and sex chromosome aneuploidies other than turners [3]. Some of the advantages of first-trimester biochemical screening over second trimester biochemical screening include providing clinicians and patients with the substantial advantage of an earlier diagnosis, higher detection rates for fetal Down syndrome i.e; 90 % [4] or even higher, compared to 80 % for the second trimester quadruple test [5, 6] and 70 % for the older triple screening test [7], and detection of most major chromosome abnormalities other than trisomy 21. It also acts as a nonspecific marker for other birth defects including some major cardiac defects and syndromic conditions. It can detect a number of major structural birth defects associated with normal chromosomes.
Maternal serum screening has some limitations. One disadvantage is that neural tube defect detection would require either a separate AFP test after 15 weeks or reliance on the fetal anomaly scan at 18–22 weeks also known as the morphology scan. Another disadvantage is that early screening preferentially identifies those chromosomally abnormal pregnancies that are destined to miscarry. Approximately 30 % of affected fetuses die between 12 weeks of gestation and term [1]. Thus, women are unnecessarily forced to decide to terminate a pregnancy that is going to miscarry. One should remember that the double marker test is only a screening test which provides a risk for the genetic disorder, but not the diagnosis.
Combined First Trimester Screening Test
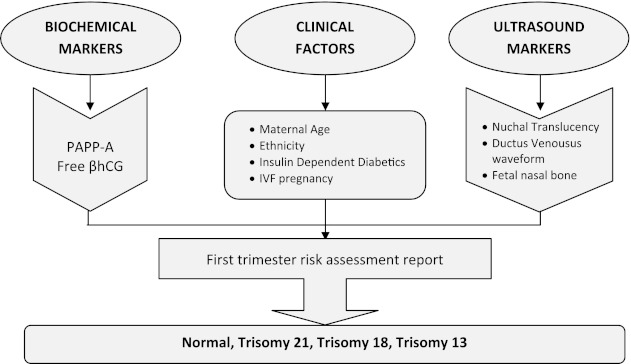
First trimester screening is performed between 10 and 14 weeks of gestation (Fig. 1). The markers used for the risk calculation are 2 serum markers: PAPP-A and free β-hCG). Decreased levels of PAPP-A before the 14th week of gestation are associated with an increased risk for Down syndrome and trisomy 18. Whereas increased levels of hCG are associated with an increased risk of Down syndrome. The third marker is the fetal nuchal translucency (NT, a fluid-containing area behind the fetal neck) which is performed by ultrasound. The nuchal translucency measurement needs to be performed by experienced sonographers and should be obtained between 10 and 13 weeks 6 days of gestation. The majority of fetuses with Down syndrome have an increase NT measurement when compared to normal fetuses of the same gestational age.
Fig. 1.
First trimester maternal serum screening
Maternal Blood Proteins in First Trimester Screening
A number of proteins in the maternal circulation have been found during the time of pregnancy. Many of these proteins are made or modified by the placenta. Differences in levels of some of the proteins have been observed in patients carrying a fetus with Down syndrome and certain other chromosome abnormalities. The discovery of these slight differences in protein levels forms the basis for using them in screening protocols. These are referred to as biochemical markers. Certain patterns of biochemical markers have been associated with fetal Down syndrome as well as other conditions. The levels of these proteins change during pregnancy, so interpretation requires knowledge of the gestational age. Also, the effectiveness of these proteins varies with gestational ages. For example, differences in protein levels may be observed during the second trimester but not the first, while other proteins show differences during the first trimester but not the second.
Maternal Serum PAPP-A
PAPP-A is one of the two maternal serum markers currently used for screening between 10 and 14 weeks. It is produced by the placental syncytiotrophoblast and deciduas. It increases the bioavailability of insulin like growth factor, which in turn mediate trophoblast invasion and modulates glucose and amino acids transport in the placenta. PAPP-A is also expressed in ovarian granulose cells, and in non-reproductive tissues, such as fibroblasts, osteoblasts and vascular smooth muscle cells.
Chemistry of PAPP-A
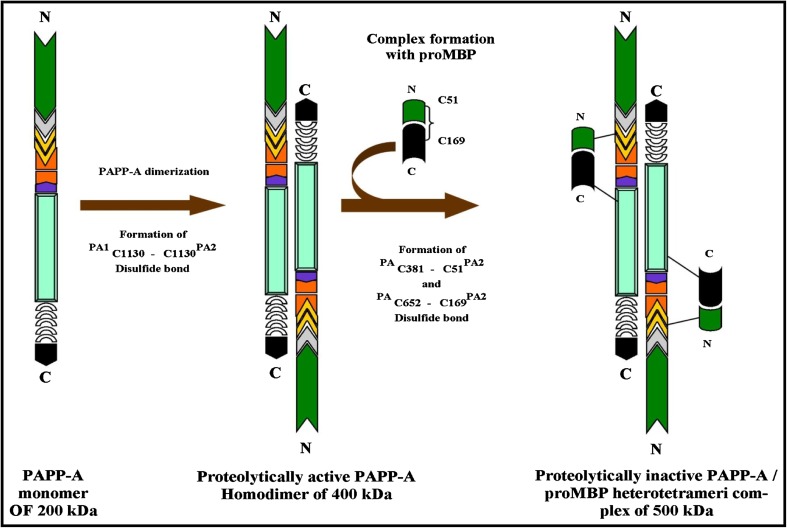
PAPP-A is a glycoprotein which was originally found by Lin et al. [8] in the plasma of pregnant woman. The protein is secreted as a disulfide-bound homodimer (Fig. 2) with a molecular weight of 400 kDa. Each subunit is derived from prepro PAPP-A containing 1,627 residue proteins [9–11] which is processed into mature PAPP-A of 1,547 amino acid residues and 14 putative N-glycosylation sites [9, 10]. It is secreted as an active protease, following intracellular cleavage of the C-terminal side of PAPP-A polypeptide [9]. It is a zinc binding metalloproteinase belonging to the metzincin superfamily of metalloproteinases [8]. In the plasma, PAPP-A circulates either as a free form or as a heterotetrameric complex of the proform of eosinophil major basic protein (proMBP) forming an approximately 500 kDa and called PAPP-A/proMBP (Fig. 2) [12, 13] ProMBP is substituted with both N- and O-linked carbohydrate in addition to a single heparan sulfate moiety, which is believed to occupy the cell-surface binding site of PAPP-A in the circulating PAPP-A/proMBP complex [14, 15]. The PAPP-A gene has been assigned to human chromosome 9q33.1 span over 200 kb of DNA, and contains 22 exons [13].
Fig. 2.
Pregnancy associated plasma protein A or PAPP-A as a disulfide-bound homodimer in free form and as a heterotetrameric complex of proform of eosinophil major basic protein (ProMBP) i.e PAPP-A/ProMBP in pregnancy
PAPP-A in Pregnancy
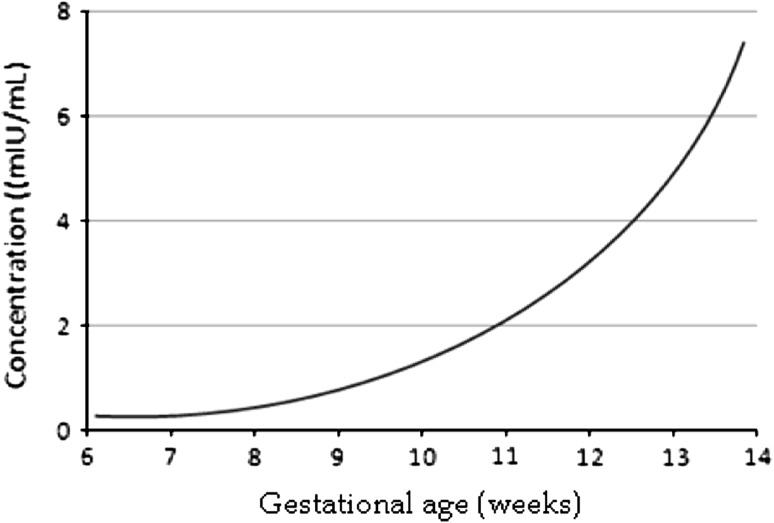
During pregnancy, both PAPP-A and proMBP are expressed abundantly in human placenta but in different cell types. Most of the PAPP-A is synthesized in the placental syncytiotrophoblast [16] and all proMBP is synthesized in extravillous cytotrophoblasts [17] from where it is secreted without propeptide cleavage. The process of PAPP-A/proMBP complex formation occurs in the extracellular, probably at the surface of the syncytiotrophoblast. In normal pregnancy, the concentration of PAPP-A in maternal circulation increases with gestational age. Its concentration increases exponentially with a doubling time of 3–4 days during the first trimesters (Fig. 3), then the level continues to rise throughout pregnancy until delivery. The rapid increase in PAPP-A levels during the first trimester causes the interpretation of a given value to be very dependent on gestational age. Common practice is therefore to use the unit multiple of median (MoM) as a gestational age-dependent expression of PAPP-A concentration. The average half life of PAPP-A after normal delivery is 53 ± 26 h (mean plus SD) [18]. PAPP-A concentrations are 100-fold and 1,000-fold lower in fetal blood and in amniotic fluid, respectively, compared with maternal blood [19].
Fig. 3.
Exponential rise of PAPP-A in the first trimester of normal pregnancy
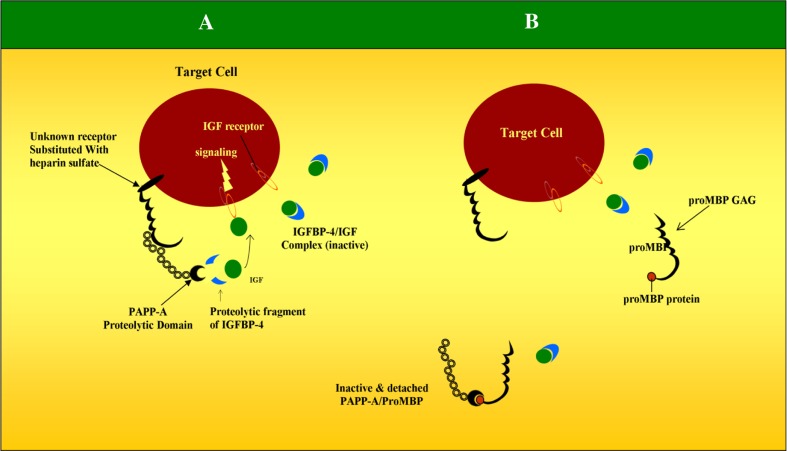
About 99 % of PAPP-A is covalently bound in a 2:2 complex to the proform of eosinophil major basic protein (proMBP) during pregnancy. The proMBP functions as an endogenous inhibitor of the proteolytic activity of PAPP-A (a protease inhibitor in vivo) whose mechanism of inhibition is currently unknown. Substrates of PAPP-A include insulin-like growth factor binding proteins (IGFBPs) 4 and 5, whose role is in the inhibition of the biological activities of insulin-like growth factors 1 and 2. PAPP-A acts as a ‘protease’ for IGFBPs [20] there by helping in the release of IGF from these binding proteins so that it is free to interact with its cell receptor in an autocrine or paracrine manner (Fig. 4). IGF is thought to play an important role in trophoblast invasion and hence the early development and vascularization of the placenta and the placental bed.
Fig. 4.
Proteolytic activity of PAPP-A in humans. Relationship between PAPP-A, IGF and IGFBP-4. a Heparin sulfate substituted receptors at the target cell immobilizes PAPP-A in a position close to the IGF receptor. PAPP-A than cleaves IGF from IGFBP-4 in close proximity of IGF receptor. Free IGF than binds IGF receptor there by inducing cell proliferation, trophoblast invasion, glucose and amino acid transport into the placenta. b GAG chain of ProMBP binds to PAPP-A domain there by releasing PAPP-A from cell surface. ProMBP inactivates the proteolytic activity of PAPP-A
Decreased levels of PAPP-A are found in association with abnormal placental function which has formed the basis for the first trimester screening of fetal Down syndrome [13, 16]. Low first trimester maternal serum levels are found not only in trisomy 21, trisomy 18 and trisomy 13, but also in non-Down syndrome fetal aneuploidies. PAPP-A is also said to have a positive relationship with birth weight [21]. As PAPP-A decreases, the risk of small-for-gestational-age infants increases. PAPP-A levels in blood stream are found to be depressed in ectopic gravidity. Some of the complications associated with an unexplained isolated low PAPP-A are preterm delivery, intrauterine growth restriction, gestational hypertension, gestational hypertension with proteinuria [22].
Studies have shown that down regulation of insulin-like growth factor-II availability due to a decreased PAPP-A serum level may be one of the causes of spontaneous abortion in these women [23]. The decrease in maternal serum PAPP-A is not associated with any change in placental synthesis of this protein, since PAPP-A mRNA expression is not significantly decreased in Down’s syndrome placentas. Furthermore, the correlation between serum and tissue expression levels of PAPP-A is lost in Down’s pregnancies. These observations suggest that the decrease in maternal serum PAPP-A is posttranslational and may be caused by an alteration of the placenta-releasing mechanisms or by a modification of the stability of the secreted protein [24]. PAPP-A is found to be significantly higher in twin pregnancy. On an average PAPP-A values are 1.86 times greater in twins than in singletons. No significant obstetrical outcomes have been described with elevated PAPP-A in the first trimester [22]. Measurable levels have been found in males and non pregnant females. In non-pregnancy individuals PAPP-A is found as a 400 kDa homodimer.
Maternal serum free β-hCG hormone
Human chorionic gonadotropin is a 39,500-Da glycoprotein hormone normally found in blood and urine only during pregnancy. In 1987, Bogart et al. [25] reported an elevated levels of maternal serum hCG in Down’s syndrome pregnancies, and since then hCG has been introduced in most screening programs. For the initiation and maintenance of pregnancy, hCG mediates multiple placental, uterine and fetal functions. Some of these include development of syncytiotrophoblast cells, mitotic growth and differentiation of the endometrium, localized suppression of the maternal immune system, modulation of uterine morphology and gene expression and coordination of intricate signal transduction between the endometrium [26].
Chemistry
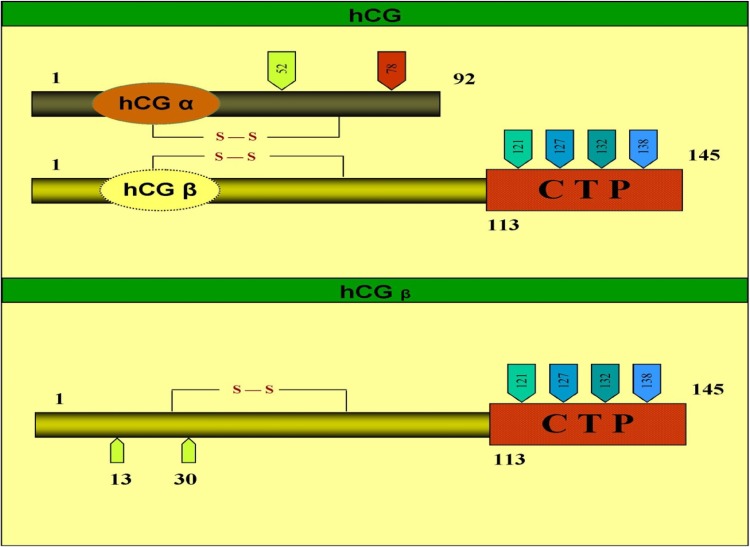
Human chorionic gonadotropin hormone is composed of two noncovalently linked subunits, α and β, and is produced by syncytiotrophoblast cells of the placenta. hCG has a single β-subunit which contains 145 amino acids linked by six disulfide bridges and an α-subunit which contains 92 amino acids linked by 5 disulfide bridges, which is also shared by three other glycoprotein hormones: LH, FSH, and TSH. The β-subunit is unique, and distinguishes hCG from the other glycoprotein hormones (Fig. 5). It contains two N-linked oligosaccharide side chains, attached to residues 13 and 30 [27]. It also has four O-linked oligosaccharide units, located in the unique proline- and serine-rich C-terminal extension (residues 122–145). The α subunit has two N-linked oligosaccharide side chains, attached at amino acid residues 52 and 78 [27]. Five hCG-related molecules are present in maternal serum: nonnicked hCG, which represents the active hormone; nicked hCG; free α-subunit; free β-subunit; and the nicked free β subunit [28]. The free β-subunit can derive from three sources, i.e., direct trophoblast cell production, dissociation of hCG into free α- and free β-subunits, and by macrophage or neutrophil enzymes nicking the hCG molecule [29]. The free β-hCG circulating in maternal serum corresponds to only about 0.3–4 % of the total hCG [29]. A single gene on chromosome 6 codes for the α subunit of all four glycoprotein hormones (TSH, LH, FSH and CG). Chromosome 19 contains a family of genes that encodes the CGβ subunit [29]. Separate messenger RNAs (mRNAs) are transcribed from each. The subunits spontaneously combine in the rough endoplasmic reticulum and are then continuously secreted into the maternal circulation.
Fig. 5.
Structure of intact hCG and hCGβ
Free β-hCG in Pregnancy
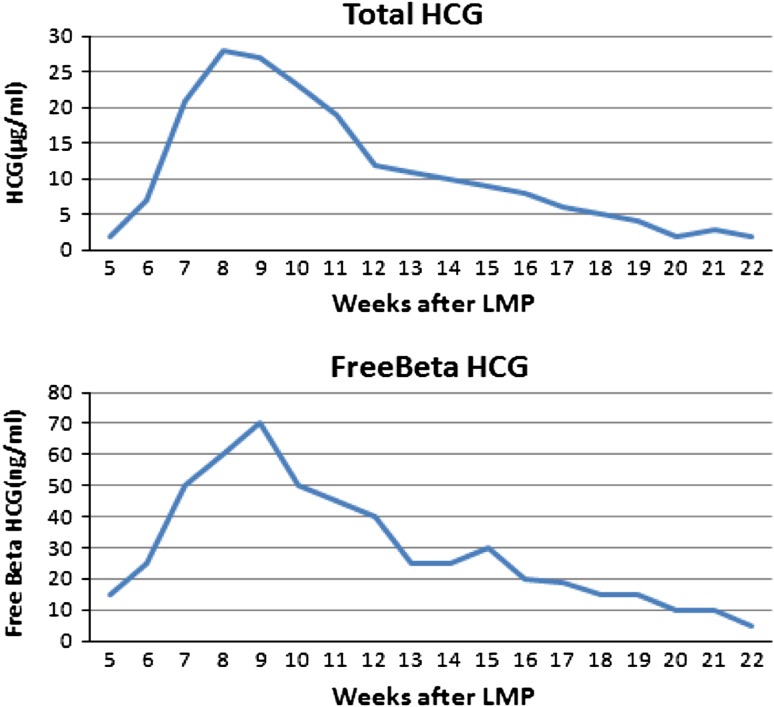
Maternal serum hCG peaks at 8–10 weeks and then declines to reach a plateau at 18–20 weeks (Fig. 6) of gestation and remains quiet constant until term. β-hCG lacks hCG activity, but several lines of study indicate that it exerts growth promoting activity. It has been speculated that β-hCG interferes with the growth-inhibiting effect of transforming growth factor-β, platelet-derived growth factor-B and nerve growth factor [30]. The half-life of injected hCG is biphasic; the rapid phase has a half-life of 5–6 h whereas that of the slower phase is 24–33 h. The half-life of purified β-hCG injected into human is 0.7 and 10 h which is shorter than that of hCG. However, after term pregnancy or an abortion, β-hCG actually disappears more slowly than that of hCG. Thus the proportion of β-hCG of total hCG immunoreactivity increases from 0.8 % at term to 27 % after 3 weeks. A Molecular biology studies have demonstrated that trisomy 21 trophoblasts show a marked increase in β-hCG mRNA and a smaller increase in α-hCG mRNA, suggesting that one of the causes of high hCG levels in maternal serum is the increased hCG production and secretion by the placenta [31]. These observations are supported by the relative immaturity of the placenta, which continues to release large amounts of hCG as in the first trimester [32].
Fig. 6.
Total hCG and free beta hCG during first trimester of pregnancy
Methods Used to Measure PAPP-A and Free β-hCG
The new technology for biochemical analysis, which provides automated, precise and reproductive measurement within short time of obtaining a blood sample makes it possible to combine biochemical and ultrasonographic testing for early assessment so that patients assessment and stress may be reduced. Separation of the sera for Down syndrome screening in 4 h after withdrawal is necessary. Cooling during any storage, including transportation is highly recommended as the preanalytical phase has a high impact for the analysis.
Serum samples for free β-hCG and PAPP-A are stable at 4 °C for whole blood or separated serum for 1 week. Reliable results are obtained if separated serum samples are stored at 20 °C up to 2 days and 1 day for whole blood. At 30 °C reliable results were obtained only if the samples were analyzed within 2 h collection.
In whole blood, free β-hCG levels increased more rapidly compared to serum, especially at 30 °C [33]. Several studies have reported that a high storage temperature and a long interval between collection and analysis of the sample produce an increase in the concentration of free β-hCG because it is liberated by the dissociation or degradation of intact hCG [34]. In whole blood kept at room temperature, the mean serum concentration of free β-hCG was reported to increase by 10–15 % after 24 h, by about 25 % after 3 days and by 45 % after 4 days [34]. Another study showed that PAPP-A levels are stable in serum for 142 days at 2–8°, 37 days at room temperature and 20 days at 30 °C. There was no significant change with either analyte after −20 °C storage for up to 240 days or after six repeated freeze-thaw cycles [33]. Presently, virtually all commercial assays are the enzyme amplified chemiluminescence technology. Maternal serum PAPP-A and free β-hCG can also be measured using a random access immunoassay analyzer using time-resolved amplified cryptate emission technology.
Nuchal Translucency
The most important ultrasound marker in first-trimester screening for chromosomal abnormalities is the measurement of NT thickness between 10 and 14 weeks of gestation which was first reported by Schulte-Valentine and Schindler in 1992 [35]. NT measures the subcutaneous fluid-filled space between the back of the spine and the skin in the fetal neck (Fig. 7). NT increases with gestational age at about 17 % a week. The translucent area disappears after 14 weeks gestational age, when the subcutaneous tissue becomes more echogenic. NT is therefore a transient phenomenon [36]. Using NT measurement and maternal age alone 81 % (Table 2) of affected pregnancies can be identified with a 5 % false-positive rate [37]. The nuchal translucency measurement needs to be performed by experienced sonographers and should be obtained between 10 and 13 weeks 6 days of gestation which is equivalent to a crown-rump length between 38 and 84 mm. NT can be detected in 99 % of fetuses at the end of the first trimester. The 50th percentile NT increases from 1.2 mm in week 11 + 0 (crown-rump length 45 mm) to 1.5 mm in week 13 + 6 (crown-rump length 82 mm). The 95th percentile ranges from 2 mm in week 11 to 2.6 mm in week 13 + 6. The majority of fetuses with Down syndrome have an increase NT measurement when compared to normal fetuses of the same gestational age. Furthermore, increased NT is attributed to aortic isthmus narrowing, cardiovascular defects which cause overperfusion of the head and neck, or abnormal/delayed development of the lymphatic system [38, 39]. Increased NT measurement may also be associated with miscarriage [36, 40] and abnormal karyotypes [41].
Fig. 7.
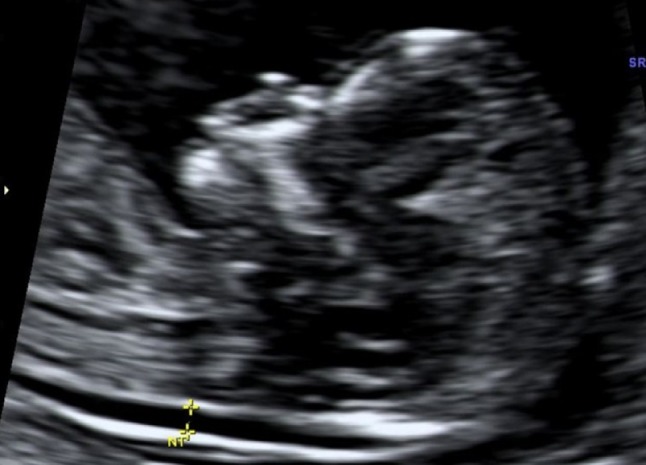
Nuchal translucency measurement
Table 2.
Detection rate of trisomy 21, 18 and 13 for given false positive rate (FPR)
| Screening | Trisomy 21 FPR: 5.0 % |
Trisomy 18 FPR: 0.5 % |
Trisomy 13 FPR: 0.5 % |
|---|---|---|---|
| Maternal age (MA) and fetal NT | 81 | 68 | 61 |
| MA and serum free β-hCG and PAPP-A | 67 | 80 | 59 |
| MA, NT and serum free β-hCG and PAPP-A | 91 | 97 | 84 |
[37]
Patient Specific Risk of Chromosomal Abnormality
Every woman has some risk that her fetus may be affected by a chromosomal defect. In order to calculate this individual risk, it is necessary first to take into account the woman’s a priori risk based on her age and gestational age (Table 1). This a priori risk is then multiplied by a likelihood ratio, calculated from her ultrasound findings and/or serum biochemistry results obtained during the course of the current pregnancy. The product of the a priori risk and the likelihood ratio yields the patient specific risk.
Multiple of Median (MoM)
In screening using maternal serum biochemical markers, the measured concentration of the markers is converted into MoM of unaffected pregnancies at the same gestation. This is to allow for the fact that marker levels vary with gestational age. MoM values are calculated by dividing an individual’s marker level by the median level of that marker for the entire population at that gestational age in that laboratory. Using MoM values, rather than absolute levels, also allows results from different laboratories to be interpreted in a consistent way. In euploid pregnancies, the average adjusted value for both free β-hCG and PAPP-A is 1.0 MoM at all gestations. In a study done by Spencer et al. [42] on variation in first trimester biochemical marker levels in Asian woman (Indian, Pakistan, Bangladesh) compared to Caucasian woman (reference group) it was found that in asian population, median PAPP-A was increased by 9 % and free β-hCG by 6 % from the reference group (Table 3). The Asian population was found to be 6.9 kg lighter than Caucasian woman. In trisomies low PAPP-A values are seen, which typically are 0.15 MoM for trisomy 21, that is, Down’s syndrome; 0.25 MoM for trisomy 13 or Patau syndrome; 0.18 MoM for trisomy 18 or Edward’s syndrome; and 0.49 for Turner’s syndrome (Table 4).
Table 3.
Variation in first-trimester biochemical marker levels in Asian woman compared to Caucasian woman
| Marker (MoM) | Caucasian | Indian | ||
|---|---|---|---|---|
| Non weight correlation | Weight correlation | Non weight correlation | Weight correlation | |
| Free β-hCG | 1.53 | 1.038 | 1.002 | 0.925 |
| PAPP-A | 1.032 | 1.029 | 1.187 | 1.082 |
MOM multiple of median; β-hCG human chorionic gonadotropin; PAPP-A pregnancy-associated plasma protein-A. [42]
Table 4.
Marker pattern in multiple of median and detection rates in different aneuploidies
| Aneuploidy | NT | Free β-hCG | PAPP-A | Detection rate (%) |
|---|---|---|---|---|
| Trisomy 21 | 2.67 MOM | 2.15 MOM | 0.15 MOM | 90 |
| Trisomy 13 | 2.87 MOM | 0.50 MOM | 0.25 MOM | 90 |
| Trisomy 18 | 3.27 MOM | 0.28 MOM | 0.18 MOM | 89 |
| 45, x | 4.76 MOM | 1.11 MOM | 0.49 MOM | >90 |
| Triploidy maternal | 0.88 MOM | 0.18 MOM | 0.06 MOM | >90 |
| Triploidy paternal | 2.76 MOM | 8.04 MOM | 0.75 MOM | >90 |
MOM multiple of median, β-hCG human chorionic gonadotropin, NT nuchal translucency, PAPP-A pregnancy-associated plasma protein-A. [49]
The laboratory uses different mathematical model to calculate a woman’s risk of having a baby with Down syndrome, trisomy 18 and trisomy 13. Some of the mathematical models include Prisca, Viewpoint, Astria etc. These mathematical model takes into consideration the maternal age, the serum levels of various biochemical markers and the fetus ultrasound measurements. In addition, a number of factors play an important role in the calculation of the risk as they will affect the values of the maternal serum biochemical analytes. This includes gestational age, weight, race, smoking, diabetic status of the individual, the number of fetuses present, and whether IVF treatment was used for conceiving. In some rare cases like ovum donor pregnancy aneuploidy risk calculations, the use of the age of the ovum donor instead of the ovum recipient reduces the false-positive rate and improves screening efficacy.
Inaccurate information can lead to significant alterations in the estimated risk. Hence it is very important that, accurate information is provided when the sample is submitted to the laboratory for analysis. For first trimester serum screening, a screen-positive or screen-negative result is based on the laboratory-specific cutoffs. Using maternal age the estimated risks for fetal trisomies 21, 18 and 13 for a woman aged 20 years at 12 weeks of gestation are about 1 in 1,000, 1 in 2,500 and 1 in 8,000, respectively, and the risk of such woman delivering an affected baby at term are 1 in 1,500, 1 in 18,000 and 1 in 42,000, respectively. The respective risks for these aneuploidies for a woman aged 35 years at 12 weeks of gestation are about 1 in 250, 1 in 600 and 1 in 1,800, and the risk of such woman delivering an affected baby at term are 1 in 350, 1 in 4,000 and 1 in 10,000.
In screening for trisomy 21 by maternal age and serum free β-hCG and PAPP-A, the detection rate is about 65 % for a false-positive rate of 5 %. The performance is better at 9–10 weeks than at 13 weeks because the difference in PAPP-A between trisomic and euploid pregnancies is greater in earlier gestations [43, 44]. Although the difference in free β-hCG between trisomic and euploid pregnancies increases with gestation, the magnitude of the difference is smaller than that of the opposite relation of PAPP-A. In trisomies 18 and 13, maternal serum free β-hCG and PAPP-A are decreased [45, 46]. In cases of sex chromosomal anomalies, maternal serum free β-hCG is normal and PAPP-A is low [46]. The overall performance of screening by combined test is better at 11 weeks than 13 weeks, with a greater contribution from PAPP-A at 11 weeks and from free β-hCG at 13 weeks. In trisomy 21 pregnancies the median MoM free β-hCG increases from 1.8 at 11 weeks to 2.09 at 13 weeks, and the respective values for PAPP-A are 0.38 and 0.65 MoMs.
Screening for biochemical testing and ultrasound scanning can also be carried out in two separate visits, with the first done at 9–10 weeks and the second at 12 weeks [44, 47, 48]. It has been estimated that this approach would improve the detection rate from 90 % to 93 to 94 %. Another option would be to perform the scan at 12 weeks and optimize the performance of biochemical testing by measuring PAPP-A at 9 weeks and free β-hCG at the time of the scan at 12 weeks or even later with an estimated detection rate of 95 %.
References
- 1.Snijders RJM, Sundberg K, Holzgreve W, Henry G, Nicolaides KH. Maternal age- and gestation-specific risk for trisomy 21. Ultrasound Obstet Gynecol. 1999;13:167–170. doi: 10.1046/j.1469-0705.1999.13030167.x. [DOI] [PubMed] [Google Scholar]
- 2.Wilson RD. Amended Canadian Guideline for prenatal diagnosis (2005) change to 2005—techniques for prenatal diagnosis. SOGC Clinical Practice Guidelines, No. 168, November 2005. J Obstet Gynaecol Can. 2005;27:1048–1054. doi: 10.1016/s1701-2163(16)30506-0. [DOI] [PubMed] [Google Scholar]
- 3.Bindra R, Liao VHA, Spencer K, Nicolaides KH. One stop clinic for assessment of risk for trisomy 21 at 11–14 weeks: a prospective study of 15030 pregnancies. Ultrasound Obstet Gynecol. 2002;20:219–225. doi: 10.1046/j.1469-0705.2002.00808.x. [DOI] [PubMed] [Google Scholar]
- 4.Nicolaides KH. Screening for fetal aneuploides at 11 to 13 weeks. Prenat Diagn. 2011;1(31):7–15. doi: 10.1002/pd.2637. [DOI] [PubMed] [Google Scholar]
- 5.Dugoff L, Hobbins JC, Malone FD, et al. Quad screen as a predictor of adverse pregnancy outcome. Obstet Gynecol. 2005;106:260–267. doi: 10.1097/01.AOG.0000172419.37410.eb. [DOI] [PubMed] [Google Scholar]
- 6.Nicholas JW, Wayne JH, Allan KH. Antenatal screening for Down’s syndrome with the quadruple test. Lancet. 2003;361(9360):835–836. doi: 10.1016/S0140-6736(03)12680-3. [DOI] [PubMed] [Google Scholar]
- 7.Wald NJ, Kennard A, Hackshaw A, McGuire A. Antenatal screening for Down’s syndrome published erratum appears in. J Med Screen. 1998;5:110. doi: 10.1177/096914139700400402. [DOI] [PubMed] [Google Scholar]
- 8.Petrovic I, Marina D, Juan CK. The role of pregnancy associated plasma protein –A (PAPP-A) in the identification of coronary artery disease activity. Acta Fac Med Naissensis. 2007;24(4):183–188. [Google Scholar]
- 9.Fialova L, Malbohan IM. Pregnancy-associated plasma protein-A (PAPP-A): theoretical and clinical aspects. Bratisl Lek Listy. 2002;103:194–205. [PubMed] [Google Scholar]
- 10.Overgaard MT, Oxvig C, Christiansen M, Lawrence JB, Conover CA, Gleich GJ. Messenger ribonucleic acid levels of pregnancy-associated plasma protein-A and the proform of eosinophil major basic protein: expression in human reproductive and non reproductive tissues. Biol Reprod. 1999;61:1083–1089. doi: 10.1095/biolreprod61.4.1083. [DOI] [PubMed] [Google Scholar]
- 11.Overgaard MT, Boldt HT, Laursen LS, Sottrup-Jensen L, Conover CA, Oxvig C. Pregnancy-associated plasma protein-A2 (PAPP-A2), a novel insulin-like growth factor-binding protein-5 proteinase. J Biol Chem. 2001;276:21849–21853. doi: 10.1074/jbc.M102191200. [DOI] [PubMed] [Google Scholar]
- 12.Qin Q-P, Kokkala S, Lund J, Tamm N, Voipio-Pulkki LM, Pettersson K. Molecular distinction of circulating pregnancy-associated plasma protein-A in myocardial infarction and pregnancy. Clin Chem. 2005;51:75–83. doi: 10.1373/clinchem.2004.036467. [DOI] [PubMed] [Google Scholar]
- 13.Overgaard MT, Sorensen ES, Stachowiak D, Boldt HB, Kristensen L, Sottrup-Jensen L, et al. Complex of pregnancy-associated plasma protein-A and the proform of eosinophil major basic protein disulfide structure and carbohydrate attachment sites. J Biol Chem. 2003;278:2106–2117. doi: 10.1074/jbc.M208777200. [DOI] [PubMed] [Google Scholar]
- 14.Oxvig C, Sand O, Kristensen T, Kristensen L, Sottrup-Jensen L. Isolation and characterization of circulating complex between human pregnancy-associated plasma protein-A and proform of eosinophil major basic protein. Biochim Biophys Acta. 1994;1201:415–423. doi: 10.1016/0304-4165(94)90071-X. [DOI] [PubMed] [Google Scholar]
- 15.Oxvig C, Haaning J, Hojrup P, Sottrup-Jensen L. Location and nature of carbohydrate groups in proform of human major basic protein isolated from pregnancy serum. Biochem Mol Biol Int. 1994;33:329–336. [PubMed] [Google Scholar]
- 16.Handschuh K, Guibourdenche J, Guesnon M, Laurendeau I, Evain-Brion D, Fournier T. Modulation of PAPP-A expression by PPARgamma in human first trimester trophoblast. Placenta. 2006;27(Suppl A):S127–S134. doi: 10.1016/j.placenta.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 17.Overgaard MT, Haaning J, Boldt HB, Olsen IM, Laursen LS, Christiansen M, Gleich GJ, et al. Expression of recombinant human pregnancy-associated plasma protein-A and identification of the proform of eosinophil major basic protein as its physiological inhibitor. J Biol Chem. 2000;275(40):31128–31133. doi: 10.1074/jbc.M001384200. [DOI] [PubMed] [Google Scholar]
- 18.Bischof P, DuBerg S, Herrmann W, Sizonenko PC. Pregnancy-associated plasma protein-A (PAPP-A) and hCG in early pregnancy. Br J Obstet Gynaecol. 1981;88:973–975. doi: 10.1111/j.1471-0528.1981.tb01683.x. [DOI] [PubMed] [Google Scholar]
- 19.Bischof P, Amandruz M, Weil-Franck C, Baeriswyl JP, Weil A, Hermann WL, et al. The disappearance rate of pregnancy-associated plasma protein-A (PAPP-A) after the end of normal and abnormal pregnancies. Arch Gynecol. 1984;236:93–98. doi: 10.1007/BF02134005. [DOI] [PubMed] [Google Scholar]
- 20.Boldt HB, Conover CA. Pregnancy-associated plasma protein-A (PAPP-A): a local regulator of IGF bioavailability through cleavage of IGFBPs. Growth Horm IGF Res. 2007;17:10–18. doi: 10.1016/j.ghir.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 21.Peterson SE, Simhan HN. First-trimester pregnancy-associated plasma protein A and subsequent abnormalities of fetal growth. Am J Obstet Gynecol. 2008;198(5):43–5. [DOI] [PubMed]
- 22.Gagnon A, Douglas RW. Obstetrical complications associated with abnormal maternal serum markers analytes. J Obstet Gynaecol Can. 2008;30(10):918–932. doi: 10.1016/S1701-2163(16)32973-5. [DOI] [PubMed] [Google Scholar]
- 23.Santolaya-Forgas I, Leon JAD, Cullen Hopkins R, Castracane VD, Kauffman RP, Sifuentes GP. Low pregnancy-associated plasma protein-A at 10 + 1 to 14 + 6 weeks of gestation and a possible mechanism leading to miscarriage. Fetal Diagn Ther. 2004;19:456–461. doi: 10.1159/000079000. [DOI] [PubMed] [Google Scholar]
- 24.Brizot ML, Hyett JA, Mckie AT, Bersinger NA, Farzaneh F, Nicolaides KH. Gene expression of human pregnancy-associated plasma protein-A in placenta from trisomic pregnancies. Placenta. 1996;17:33–36. doi: 10.1016/S0143-4004(05)80641-1. [DOI] [PubMed] [Google Scholar]
- 25.Bogart MH, Pandian MR, Jones OW. Abnormal maternal serum chorionic gonadotropin levels in pregnancies with fetal chromosome abnormalities. Prenat Diagn. 1987;7:623–630. doi: 10.1002/pd.1970070904. [DOI] [PubMed] [Google Scholar]
- 26.Banerjee P, Fazleabas AT. Extragonadal actions of chorionic gonadotropin. Rev Endocr Metab Disord. 2011;12(4):323–332. doi: 10.1007/s11154-011-9193-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ulf-Håkan S, Aila T, Henrik A, Leena V. The classification, functions and clinical use of different isoforms of hCG. Hum Reprod Update. 2006;12(6):769–784. doi: 10.1093/humupd/dml029. [DOI] [PubMed] [Google Scholar]
- 28.Trenti T, Aloe R, Cervellin G, Lippi G. Human chorionic gonadotropin in pregnancy diagnostic. Clin Chim Acta. 2011;412(17–18):1515–1520. doi: 10.1016/j.cca.2011.05.025. [DOI] [PubMed] [Google Scholar]
- 29.Fernando MR, Donato D, Felice P. Predictive value of hormone measurements in maternal and fetal complications of pregnancy. Endocrine. 2006;23(2):230–57. [DOI] [PubMed]
- 30.Butler SA, Iles RK. The free monomeric beta subunit of human chorionic gonadotrophin (hCG beta) and the recently identified homodimeric beta–beta subunit (hCG beta beta) both have autocrine growth effects. Tumour Biol. 2004;25:18–23. doi: 10.1159/000077719. [DOI] [PubMed] [Google Scholar]
- 31.Eldar-Geva T, Hochberg A, deGroot N, Weinstein D. High maternal serum chorionic gonadotropin level in Downs’ syndrome pregnancies is caused by elevation of both subunits messenger ribonucleic acid level in trophoblasts. J Clin Endocrinol Metab. 1995;80:3528–3531. doi: 10.1210/jc.80.12.3528. [DOI] [PubMed] [Google Scholar]
- 32.Oberweis D, Gillerot Y, Koulischer L, Hustin J, Philippe E. The placenta in trisomy in the last trimester of pregnancy. J Gynecol Obstet Biol Reprod. 1993;12:345–349. [PubMed] [Google Scholar]
- 33.Cowans NJ, Stamatopoulou A, Hellström J, Mäkelä MM, Spencer K. PAPP-A and free ss-hCG stability in first trimester serum using PerkinElmer AutoDELFIA and DELFIA Xpress systems. Prenat Diagn. 2010;30(2):127–132. doi: 10.1002/pd.2423. [DOI] [PubMed] [Google Scholar]
- 34.Cruz J, Cruz G, Minerkawa R, Mazi N, Nicolaides KH. Effect of temperature on free β-human chorionic gonadotropin and pregnancy-associated plasma protein-A concentration. Ultrasound Obstet Gynecol. 2010;36:141–146. doi: 10.1002/uog.7688. [DOI] [PubMed] [Google Scholar]
- 35.Schulte-Valentin M, Schindler H. Non-echogenic nuchal oedema as a marker for trisomy 21 screening. Lancet. 1992;339:1053. doi: 10.1016/0140-6736(92)90574-M. [DOI] [PubMed] [Google Scholar]
- 36.Roberts LJ, Bewley S, Mackinson AM, Rodeck CH. First trimester fetal nuchal translucency: problems with screening the general population. J Obstet Gynaecol. 1995;102:381–385. doi: 10.1111/j.1471-0528.1995.tb11289.x. [DOI] [PubMed] [Google Scholar]
- 37.Karl OK, Dave W, Caalina V, Nerea M, Nicolaides KH. Screening for trisomy 21, 18 and 13 by maternal age, fetal NT, fetal heart rate, free beta hCG and PAPP-A. Hum Reprod. 2008;23(9):1968–1975. doi: 10.1093/humrep/den224. [DOI] [PubMed] [Google Scholar]
- 38.Hyett JA, Moscoso G, Nicolaides K. Abnormalities of the heart and great arteries in first trimester chromosomally abnormal fetuses. Am J Med Genet. 1997;69:207–216. doi: 10.1002/(SICI)1096-8628(19970317)69:2<207::AID-AJMG18>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 39.von Kaisenberg CS, Nicolaides KH, Brand-Saberi B. Lymphatic vessel hypoplasia in fetuses with Turner syndrome. Hum Reprod. 1999;14:823–826. doi: 10.1093/humrep/14.3.823. [DOI] [PubMed] [Google Scholar]
- 40.Hyett JA, Sebire NJ, Snijders RJ, Nicolaides KH. Intrauterine lethality of trisomy 21 fetuses with increased nuchal translucency thickness. Ultrasound Obstet Gynecol. 1996;7:101–103. doi: 10.1046/j.1469-0705.1996.07020101.x. [DOI] [PubMed] [Google Scholar]
- 41.Khalil Asma, Pandya Pranav. Screening for Down syndrome. J Obstet Gynecol India. 2006;56(3):205–211. [Google Scholar]
- 42.Spencer K, Heath V, El-Sheikhah A, Ong CYT, Nicolaides KH. Ethnicity and need for correction of biochemical and ultrasound markers of chromosomal anomalies in the first trimester: a study of oriental Asian and Afro-Caribbean population. Prenat Diagn. 2005;25:365–369. doi: 10.1002/pd.1153. [DOI] [PubMed] [Google Scholar]
- 43.Kagan KO, Wright D, Spencer K, Molina FS, Nicolaides KH. First-trimester screening for trisomy 21 by free beta-human chorionic gonadotropin and pregnancy-associated plasma protein-A: impact of maternal and pregnancy characteristics. Ultrasound Obstet Gynecol. 2008;31:493–502. doi: 10.1002/uog.5332. [DOI] [PubMed] [Google Scholar]
- 44.Wright D, Spencer K, Kagan KO, Torring N, Petersen OB, Christou A, et al. First-trimester combined screening for trisomy 21 at 7–14 weeks gestation. Ultrasound Obstet Gynecol. 2010;36:404–411. doi: 10.1002/uog.7755. [DOI] [PubMed] [Google Scholar]
- 45.Tul N, Spencer K, Noble P, Chan C, Nicolaides KH. Screening for trisomy 18 by fetal nuchal translucency and maternal serum free beta hCG and PAPP-A at 10–14 weeks of gestation. Prenat Diagn. 1999;19:1035–1042. doi: 10.1002/(SICI)1097-0223(199911)19:11<1035::AID-PD694>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 46.Spencer K, Tul N, Nicolaides KH. Maternal serum free beta hCG and PAPP-A in fetal sex chromosome defects in the first trimester. Prenat Diagn. 2000;20:390–394. doi: 10.1002/(SICI)1097-0223(200005)20:5<390::AID-PD824>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 47.Borrell A, Casals E, Fortuny A, Farre MT, Gonce A, Sanchez A, et al. First-trimester screening for trisomy 21 combining biochemistry and ultrasound at individually optimal gestational ages: an interventional study. Prenat Diagn. 2004;24:541–545. doi: 10.1002/pd.949. [DOI] [PubMed] [Google Scholar]
- 48.Kagan KO, Wright D, Baker A, Sahota D, Nicolaides KH. Screening for trisomy 21 by maternal age, fetal nuchal translucency thickness, free beta-human chorionic gonadotropin and pregnancy-associated plasma protein-A. Ultrasound Obstet Gynecol. 2008;31:618–24. [DOI] [PubMed]
- 49.Bernd E, Glaubitz Ralf. First-trimester screening: an overview. J Histochem Cytochem. 2005;53(3):281–283. doi: 10.1369/jhc.4B6420.2005. [DOI] [PubMed] [Google Scholar]