Abstract
Inflammatory pathways have garnered considerable interest in the recent past as an important mediator of the molecular mechanisms leading to carcinogenesis. The present study was conducted to evaluate the correlation of levels of IL-6 with tumor burden and receptor status in patients of locally advanced carcinoma breast. This prospective study was conducted by the collaborative efforts of the departments of Surgery and Biochemistry, Maulana Azad Medical College and associated Lok Nayak Hospital and GB Pant Hospitals, New Delhi. The study population comprised of 30 cases of locally advanced breast carcinoma recruited from the surgical outpatient department. The various parameters that were evaluated include detailed clinico-pathological profile and IL-6 levels. Tissue specimens received after surgeries were examined for the various characteristics indicative of tumor prognosis. Majority of the patients was in the age group of 41–50 years and was postmenopausal. The serum level of IL-6 increased as the disease progressed from T3N1M0 to T4dN2M0 (41.4 ± 31.9 vs. 164.0 ± 31.1 pg/ml respectively). There was significant correlation of IL-6 levels with lymph node involvement, tumor grade, mitotic index and adipose tissue invasion. Emerging molecular markers are being investigated for breast cancer prognosis assessment and prediction of response to chemotherapy including selection of best possible treatment modality. Our study showed that there is progressive increase in IL-6 levels as the stage of disease progresses.
Keywords: IL-6, Carcinoma breast, Inflammation
Introduction
Breast cancer has emerged as the most common malignancy observed in females worldwide. Epidemiological profile of the patients sheds light on the multifactorial etiology of breast carcinoma. The disease is no longer confined to sexagenarian nulliparous women. Inflammatory pathways have garnered considerable interest in the recent past as an important mediator of the molecular mechanisms leading to carcinogenesis [1, 2].
Locally advanced breast cancer (LABC) is a very common clinical presentation of breast cancer in developing countries (30–60 %). LABC is a heterogeneous group of tumors of varying clinical presentations and biological behavior whose only common bonds are the presence of a large primary tumor, or extensive regional lymph node involvement, and the absence of any evidence of distant metastases. Patients with stage IIB, III and IV of the TNM classification are included in LABC. In this classification system patients are included if they have T3 or T4 tumors with any N stage, or any T category with N2 or N3 [3].
Cytokines regulate function of many cells in an additive, synergistic or antagonistic manner. Interleukins constitute one of the groups of cytokines. Interleukin-6 (IL-6) is mainly secreted by fibroblasts, macrophages and lymphocytes (mainly TH2 cells). It promotes tumor growth by up-regulating antiapoptotic and angiogenic proteins in tumor cells [4–6]. Serum interleukin-6 values have been shown to be significantly more in patients with breast cancer as compared to normal healthy women, which was correlated with clinical stage of disease and poor survival [7–9]. The present study was conducted to evaluate the correlation of levels of IL-6 with tumor burden and receptor status in north Indian patients of locally advanced carcinoma breast.
Materials and Methods
This prospective study was conducted by the collaborative efforts of the departments of Surgery, Biochemistry and Pathology, Maulana Azad Medical College and associated Lok Nayak Hospital and GB Pant Hospitals, New Delhi. The study population comprised of 30 cases of locally advanced breast carcinoma recruited from the surgical outpatient department. Pregnant and lactating patients were excluded from the study population. The study was commenced following detailed patient consent and clearance by the institutional ethical committee.
The various parameters that were evaluated include detailed clinico-pathological profile and IL-6 levels. Clinical staging using TNM classification was performed in all enrolled patients and was based on all information available before first treatment. This included documenting tumor size (on USG), node status and metastatic workup including USG abdomen and chest X-ray. The American Joint Committee on Cancer (AJCC) TNM staging system was used for staging of the patients. Trucut biopsy was done for histopathological examination and hormone receptor status of tumor.
Blood samples were collected before start of any treatment. 5 ml of blood was drawn from the antecubital vein of the patients after applying tourniquet to arm with 24 gauge needle in the morning. Venous blood was collected in a plain vial. Blood was centrifuged within 30 min and serum kept at −80 °C until analysis for IL-6. IL-6 was quantified by ELISA.
After enrollment into the study, each patient underwent Modified radical mastectomy after receiving neoadjuvant chemotherapy (three cycles). Paraffin embedded Pathologic breast specimen was reviewed after hematoxylin and eosin staining. Tissue specimens were examined for the various characteristics indicative of tumor prognosis. These include evidence of invasion, tumor size, mitotic index, histologic grade, receptor status, axillary lymph node status and adipose tissue invasion.
Nikon Labophat microscope with 40× objective was used to count mitotic figures and points were given as follows: 1 point, 0 to 5 mitosis per high power field; 2 points, 6 to 10 mitosis per high power field; 3 points >11 mitosis per high power field. Modified Scarff-Bloom-Richardson grading was used and grade 1 to 3 were assigned according to differentiation of tumor. Estrogen, progesterone receptor and HER-2-Neu status were ascertained by staining the paraffin sections using monoclonal antibodies to ER, PR, and HER 2neu respectively. Adipose tissue invasion was graded with focal involvement as 1+ and extensive involvement as 2+ grading.
Statistical Analysis
The quantitative variables were analyzed by non parametric Mann Whitney U test for two groups or by Kruskal Wallis test for more than two groups. Wilcoxan signed rank test was used to determine the statistical significance on different points of time within the same group. The categorical variables were analyzed by using Pearson Chi-Square test/Fischer’s exact test in case when expected cell count was less than five. The Spearman rank correlation was determined to examine the correlation between two quantitative variables. P value ≤ 0.05 (2 tailed) was considered to be statistically significant. The data was analyzed by using SPPS statistical software version 16.0.
Results
The demographic profile of the study population is shown in Table 1. Majority of the patients were in the age group of 41–50 years and were postmenopausal. The most frequent stage of presentation was T3N1M0. The serum level of IL-6 increased as the disease progressed from T3N1M0 to T4dN2M0 (41.4 ± 31.9 vs. 164.0 ± 31.1 pg/ml respectively) (Table 2).
Table 1.
Clinical profile of the study population
| Variable | Number of patients | Percentage (%) |
|---|---|---|
| Age (years) | ||
| 30–40 | 7 | 23.33 |
| 41–50 | 14 | 46.67 |
| 51–60 | 8 | 26.67 |
| >60 | 1 | 3.33 |
| TNM staging | ||
| T3N1M0 | 20 | 66.67 |
| T4bN0M0 | 5 | 16.67 |
| T4bN1M0 | 2 | 6.67 |
| T4bN2M0 | 2 | 6.67 |
| T4dN2M0 | 1 | 3.33 |
| Menstrual status | ||
| Premenopausal | 13 | 43.34 |
| Postmenopausal | 17 | 56.67 |
Table 2.
Distribution of IL-6 levels in the cases classified according to stage of the disease
| TNM stage | Number (%age) | IL-6 (Mean) |
|---|---|---|
| Baseline (pg/ml) | ||
| T3N1M0 | 20 (66.67) | 41.4 ± 31.9 |
| T4bN0M0 | 5 (16.67) | 115.7 ± 26.9 |
| T4bN1M0 | 2 (6.67) | 124.3 ± 22.1 |
| T4bN2M0 | 2 (6.67) | 164.0 ± 31.1 |
| T4dN2M0 | 1 (3.33) | 200.0 |
| P value* | 0.001 |
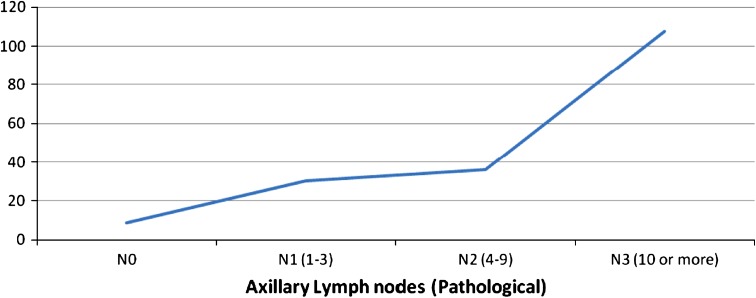
Figure 1 illustrates the variation of IL-6 levels with increasing lymph node involvement. The figure clearly depicts the increasing trend of IL-6 with advancing LN involvement with IL-6 concentration of 30.166 ± 31.392 pg/ml in N1 and 107.650 ± 52.091 pg/ml in N3 respectively, the difference being statistically significant.
Fig. 1.
IL-6 levels in different stages of lymph node involvement
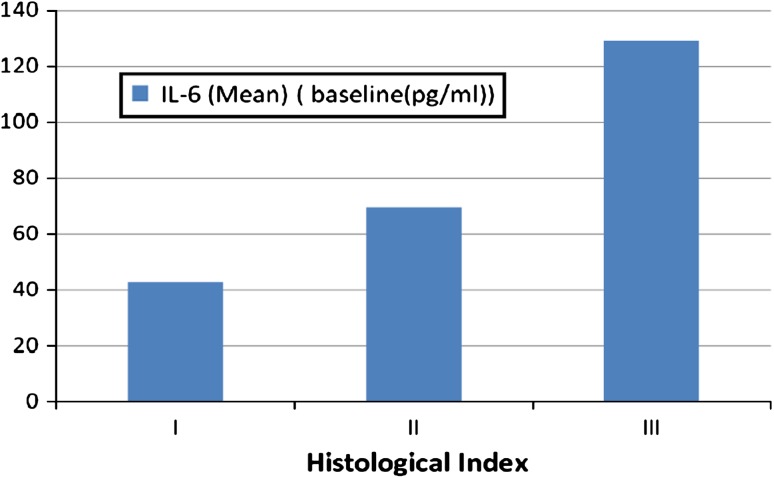
Table 3 shows the variation in serum IL-6 level with receptor status. The difference between mean values of IL-6 according to ER status, PR status and her-2/neu was not significant (P value 0.063, 0.786, 0.239 respectively). Figure 2 summarizes changes in IL-6 levels according to histological grade. The difference between mean values of IL-6 according to histologic grade was significant (P value 0.005) with IL-6 levels being 42.8 ± 32.2 pg/ml in grade I and 128.9 ± 61.0 in grade III respectively.
Table 3.
Variation in serum IL-6 level with receptor status
| Receptor status | Number of subjects (n) | IL-6 level (pg/ml) | P value |
|---|---|---|---|
| Estrogen receptor status | |||
| ER+ | 9 | 103.2 ± 63.6 | 0.063 |
| ER− | 21 | 59.7 ± 48.3 | |
| Progesterone receptor status | |||
| PR+ | 9 | 79.9 ± 73.3 | 0.786 |
| PR− | 21 | 69.7 ± 48.7 | |
| Her-2 neu status | |||
| Her-2 neu+ | 21 | 77.2 ± 55.1 | 0.239 |
| Her-2 neu− | 9 | 62.5 ± 60.1 | |
Fig. 2.
Figure showing the trend of baseline levels of IL-6 with histological grade
Table 4 highlights the correlation of serum IL-6 levels with adipose tissue invasion and mitotic index of the tumor. Serum IL-6 levels were significantly higher in the tumors exhibiting marked adipose tissue invasion (P = 0.001). a similar trend was observed during the assessment of IL-6 with increasing mitotic index. The level was 102.0 ± 68.3 pg/ml and 55.8 ± 40.5 pg/ml in tumors with mitotic index 2 and 1 respectively.
Table 4.
Variation of IL-6 levels with adipose tissue invasion and mitotic index of the tumor
| Variable | Number of subjects (n) | IL-6 level (pg/ml) | P value |
|---|---|---|---|
| Adipose tissue invasion | |||
| Absent | 11 (36.67) | 32.314 ± 27.230 | 0.001 |
| 1+ | 10 (33.33) | 70.380 ± 51.227 | |
| 2+ | 9 (30) | 124.889 ± 46.816 | |
| Mitotic index | |||
| 1 point | 19 (63.33) | 55.855 ± 40.514 | 0.037 |
| 2 points | 11 (36.67) | 102.000 ± 68.323 | |
Discussion
Breast cancer is the second most common cancer after cervix, accounting for 19 % of the total cancer burden in women in India. The incidence rate in Asian women has been rising and is associated with a shift towards a more westernized lifestyle. The Bombay Cancer registry has also recorded a consistent rise in the incidence of breast cancer, which has now overtaken cervical cancer, to be the leading cancer in women of Mumbai [10].
Substantial progress has been made in our understanding of the epidemiology, clinical course and basic biology of breast cancer. There are various predictive factors which provide information about potential patient outcome in the absence of systemic therapy. As systemic therapy does have substantial side effects it would therefore be prudent to be able to optimally select patients who are most likely to benefit from such therapy. Predictive factors may select patients most likely to recur without neoadjuvant or adjuvant therapy and therefore potentially benefit from therapy. Emerging molecular markers are being investigated for breast cancer prognosis assessment and prediction of response to chemotherapy including selection of best possible treatment modality.
Although the mechanism of action is not yet clearly elucidated, there are many studies which suggest that the raised serum IL-6 level observed in cancer patients is associated with the advanced stage of cancer as well as with the poor prognosis of the patients [8, 9]. Various studies have shown that the disease usually takes aggressive course where the IL-6 level is found to be elevated thereby indicating possibility of it being used as prognostic marker [9]. It is accepted that IL-6 promotes tumoral growth by upregulating antiapoptotic and angiogenic proteins in tumor cells [4–6]. Levels of IL-6 are reported to be considerably high in cancer patients compared to healthy control subjects [7, 8].
In the present study, 30 patients of breast cancer were included. All these patients had histologically proven disease. Our purpose was to find a correlation of serum levels of IL-6 with tumor burden and its relationship with other variables such as histological characteristics, lymph node status and immunohistochemistry markers (ER, PR and Her-2neu).
Our study showed that there is progressive increase in IL-6 levels as the stage of disease progresses. This increase is found to be statistically significant. Our results are in accordance with study done by Kozlowski et.al, which assessed the concentration of IL-6 in blood serum of breast cancer patients to determine whether it correlates with the disease progression [8]. Another study by Nishimura et al. [11] analyzed the significance of IL-6 in advanced or recurrent breast cancer, with serum IL-6 to be significantly higher in recurrent cases (6.5 ± 7.8 pg/ml), especially in those with visceral metastasis, than in non-recurrent cases (1.96 ± 1.38 pg/ml). Jablonska et al. [12] found serum levels of IL-6 in breast cancer patients in stage II to be higher (16.7 ± 8.7) than in the control (9.81 ± 3.96 pg/ml), furthermore, breast cancer patients in stage III/IV had increase serum levels of IL-6 (29.4 ± 12.9) compared with stage II, and they concluded that changes in values of certain cytokines could have a diagnostic and prognostic role in cancer disease.
In our study we found statistically significant relationship between IL-6 levels and adipose tissue invasion by the tumor cells. The levels were found to increase according to increasing severity of invasion of adipose tissue by tumor cells again confirming utility of these markers as prognostic factors. Bębenek et al. [13] found that the perilymphatic fat infiltration significantly shortened the overall survivals in breast cancer patients. We also found statistically significant relationship between IL-6 levels and mitotic index of tumor suggesting a plausible role of IL-6 in determination of tumor morphology. There was no statistically significant correlation between serum baseline levels of IL-6 and ER/PR/Her 2 neu status in our study. Lv et al. [14] have reported significant differences in levels of serum IL-6 between different Her 2 groups in their study conducted in Chinese patients.
We found significant relationship between IL-6 levels and number of lymph nodes involved pathologically. As axillary lymph node involvement is most important prognostic factor [15], this relationship inspires confidence in suggesting IL-6 levels as prognostic factors further. So serum IL-6 levels may be used in those patients who are node negative for an individualized assessment and use of systemic therapy.
According to a comprehensive review on the role of Il-6 in carcinogenesis, by Knüpfer and Preiss [6], serum IL-6 emerged as a negative prognosticator in breast cancer patients. Our data from Indian subset of patients also supports the same by virtue of increasing Il-6 titers with advanced stage, adipose tissue invasion, mitotic index and axillary lymph node involvement in breast cancer patients.
References
- 1.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 2.Porta C, Larghi P, Rimoldi M, Totaro MG, Allavena P, Mantovani A, et al. Cellular and molecular pathways linking inflammation and cancer. Immunobiology. 2009;214(9–10):761–777. doi: 10.1016/j.imbio.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 3.Valero V, Buzdar AU, Hortobagyi GN. Locally advanced breast cancer. Oncologist. 1996;1:8–17. [PubMed] [Google Scholar]
- 4.Wang Y, Li L, Guo X, Jin X, Sun W, Zhang X, et al. Interleukin-6 signaling regulates anchorage-independent growth, proliferation, adhesion and invasion in human ovarian cancer cells. Cytokine. Accessed 15 May 2012 (Epub ahead of print). [DOI] [PubMed]
- 5.Rokavec M, Wu W, Luo JL. IL6-mediated suppression of miR-200c directs constitutive activation of inflammatory signaling circuit driving transformation and tumorigenesis. Mol Cell. 2012;45(6):777–789. doi: 10.1016/j.molcel.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knüpfer H, Preiss R. Significance of interleukin-6 (IL-6) in breast cancer (review) Breast Cancer Res Treat. 2007;102(2):129–135. doi: 10.1007/s10549-006-9328-3. [DOI] [PubMed] [Google Scholar]
- 7.Jiang XP, Yang CC, Elliott RL, Head JF. Reduction in serum IL-6 after vaccination of breast cancer patients with tumor-associated antigens is related to estrogen receptor status. Cytokine. 2000;12:458–465. doi: 10.1006/cyto.1999.0591. [DOI] [PubMed] [Google Scholar]
- 8.Kozlowski L, Zakrzewska I, Tokajuk P, Wojtukiewicz MZ. Concentration of interlukin-6 (IL-6), interlukin-8(IL-8) and interlukin-10 (IL-10) in blood serum of breast cancer patients. Rocz Akad Med Bialymst. 2003;48:82–84. [PubMed] [Google Scholar]
- 9.Bozcuk H, Uslu G, Samur M, Yildiz M, Ozben T, Ozdoğan M, et al. Tumor necrosis factor-alpha, interlukin-6, and fasting serum insulin correlate with clinical outcome in metastatic breast cancer patients treated with chemotherapy. Cytokine. 2004;27:58–65. doi: 10.1016/j.cyto.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Consolidated Report of the Population Based Cancer Registries Incidence and Distribution of Cancer: 1990–96. New Delhi: ICMR; 2001.
- 11.Nishimura R, Nagao K, Miyayama H, Matsuda M, Baba K, Matsuoka Y, et al. An analysis of serum interlukin-6 levels to predict benefits of medroxyprogesterone acetate in advanced or recurrent breast cancer. Oncology. 2000;59(2):166–173. doi: 10.1159/000012155. [DOI] [PubMed] [Google Scholar]
- 12.Jablonska E, Kiluk M, Markiewicz W, Piotrowski L, Grabowska Z, Jablonski J. TNF-alpha, IL-6 and their soluble receptor serum levels and secretion by neutrophils in cancer patients. Arch Immunol Ther Exp (Warsz) 2001;49(1):63–69. [PubMed] [Google Scholar]
- 13.Bębenek M, Duś D, Koźlak J. Fas expression in primary breast cancer is related to neoplastic infiltration of perilymphatic fat. Adv Med Sci. 2008;53(1):49–53. doi: 10.2478/v10039-008-0015-y. [DOI] [PubMed] [Google Scholar]
- 14.Lv M, Xiaoping X, Cai H, Li D, Wang J, Fu X, Yu F, Sun M, Lv Z. Cytokines as prognstic tool in breast carcinoma. Front Biosci. 2011;1(17):2515–2526. doi: 10.2741/3869. [DOI] [PubMed] [Google Scholar]
- 15.Carter CL, Allen C, Henson DE. Relation of tumor size, lymph node status, and survival in 24,740 breast cancer cases. Cancer. 1989;63(1):181–187. doi: 10.1002/1097-0142(19890101)63:1<181::AID-CNCR2820630129>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]