Abstract
Vitamin D is recognized to serve a wide range of biological functions. The presence of vitamin D receptors on different tissues explains it’s diversity of actions. Reduced levels of vitamin D is associated with insulin resistance and increased diabetes risk. The study included 50 normal healthy individuals and 49 type 2 diabetes subjects. Fasting blood glucose, total cholesterol, triglycerides, HDLc, fasting insulin, parathyroid hormone, calcium, albumin and Homeostasis model for assessment of insulin resistance (HOMAIR) were measured in all the study participants. Type 2 diabetes subjects were divided into group 1 with 25 hydroxy vitamin D (25(OH)D) ≤20 ng/ml and group 2 with 25(OH)D >20 ng/ml. By the results of this study, the mean 25(OH)D level was low (20.09 ng/ml) in type 2 diabetes compared to controls (23.89 ng/ml) and the p value was 0.02. The estimated insulin resistance by HOMAIR was more in group 1 than in group 2 of diabetes with p value of 0.037. The Pearson’s correlation-coefficient was negative for 25(OH)D and insulin in type 2 diabetes (r = −0.294), 25(OH)D was negatively correlated with HOMAIR in total subjects. Type 2 diabetes subjects had reduced levels of vitamin D than normal individuals. The insulin resistance was more in vitamin D deficiency state. Hence vitamin D has a role in glucose metabolism, deficiency can result in insulin resistance and diabetes.
Keywords: Diabetes, Insulin resistance, 25 hydroxy vitamin D
Introduction
Currently vitamin D is called as “sunshine Hormone”. Major amount of vitamin D is synthesized by the skin on exposure to UVB rays (290–310 nm) [1]. Vitamin D endocrine system plays an important role in calcium and mineral metabolism. It is now recognized that vitamin D serves a wide range of biological functions [2]. The skeletal effects of vitamin D occur via endocrine mechanism. The other biological effects of vitamin D like cell differentiation, immune regulation and prevention of neoplastic transformation are due to paracrine/autocrine mechanisms. These diverse effects of vitamin D are due to distribution of vitamin D receptors (VDR) on 30 different tissues [3].
There is a growing global concern about the deficiency of vitamin D [4].The best marker for vitamin D status is 25 hydroxy vitamin D (25(OH)D).Accumulating evidence suggests that vitamin D deficiency is associated with increased risk for diabetes [5–7]. The prevalence of diabetes is increasing world wide. The estimated increase in India is 58 %, from 51 million in 2010 to 87 million in 2030 [8, 9].Recent research on prevention of diabetes highlights the importance of modifiable risk factors, nutritional factors. One such factor is vitamin D, which is gaining lot of importance. Vitamin D deficiency is prevalent in India. In 1973, Hodgkin first published data regarding vitamin D deficiency [10]. Between 1995 and 2000, studies from north India proved the deficiency of vitamin D in our population [11, 12].Subsequently south Indian studies also confirmed it [13–16].
Hypovitaminosis is a risk factor for glucose intolerance and diabetes. Pittas et al. summarized the role of vitamin D on glucose metabolism [17, 18]. Cross sectional and case control studies suggest an inverse association between vitamin D status, glucose intolerance and type 2 diabetes [19, 20].This could be due the distribution of VDR on pancreatic beta cells, adipose tissue and skeletal muscle. Vitamin D status influences insulin secretion/insulin sensitivity. The effect of vitamin D on insulin secretion may be mediated by changes in intracellular calcium concentration in beta cells [21, 22].Vitamin D improves insulin sensitivity by its anti-inflammatory activity. Vitamin D attenuates the expression of proinflammatory cytokines involved in insulin resistance (IR) such as interleukins, IL-1, IL-6, TNF-α, also down regulates NF-Kβ (Nuclear factor) activity[23, 24]. Vitamin D deficiency impairs insulin sensitivity by increasing parathyroid hormone (PTH) levels [25, 26].
Data regarding association of 25(OH)D with insulin resistance in Asians is very limited. A study from Chinese population proved the inverse association between 25(OH)D and increased risk of insulin resistance, metabolic syndrome [27]. Thus we intended to study the association of vitamin D deficiency with insulin resistance in south Indians. The aim of our case-control study was (1) to compare the levels of 25(OH)D in normal and diabetes subjects, (2) to evaluate the association of 25(OH)D with markers of insulin resistance such as fasting insulin, Homeostasis model for assessment of insulin resistance (HOMAIR).
Materials and Methods
The study population included normal healthy individuals without diabetes, hypertension, cardiovascular, cerebrovascular disorders as controls, and type 2 diabetes subjects as cases. Both males and females of mean age 45 years were included. Normal subjects were the staff of the institution, newly diagnosed type 2 diabetes subjects were recruited from the department of internal medicine. After the study was approved by the institutional ethics committee, informed written consent was taken from all the study participants. Through a standard questionnaire, personal and family history was elicited. History regarding the intake of calcium and vitamin D supplementation was taken, those subjects on supplementation were excluded from the study. Subjects with renal, hepatic, cardio and cerebro vascular disorders were excluded from the study as vitamin D metabolism can be altered by these diseases. Thyroid/parathyroid, cushings disease subjects were also excluded as these disorders will alter insulin resistance status. Female subjects with poly cystic ovarian disease were also excluded. After all the inclusion and exclusion criteria were met, 50 normal healthy subjects and 49 type 2 diabetes subjects were included in the study population as controls and cases respectively. Type 2 diabetes subjects were divided into two groups—group 1 with 25(OH)D ≤20 ng/ml and group 2 with 25(OH)D >20 ng/ml.
Fasting blood samples were collected from all the subjects for biochemical analysis. Serum and plasma were separated and stored at −20 °C until analyzed as a batch. The fasting blood glucose (FBS), total cholesterol (TC), triglycerides (TGL), HDL-c, calcium, alkaline phosphatase (ALP), were measured by automated photometric method. Insulin and PTH were measured by automated chemiluminescence method 0.25(OH)D was measured by HPLC method. HOMAIR was calculated from fasting insulin and fasting glucose [28].
Statistical Analysis
SPSS 12.0 software (SPSS Inc; Chicago II USA) was used for statistical analysis. Continuous variables were expressed as mean ± SEM (standard error of mean). Student’s t test was used to assess the difference between the cases, controls and different groups in diabetes. Binary logistic regression analysis was performed with study group (based on 25(OH)D) as dependent variable and other significantly associated variables as independent variables, p value < 0.05 was considered significant. Pearson’s correlation coefficients were used to assess the association between the continuous variables.
Results
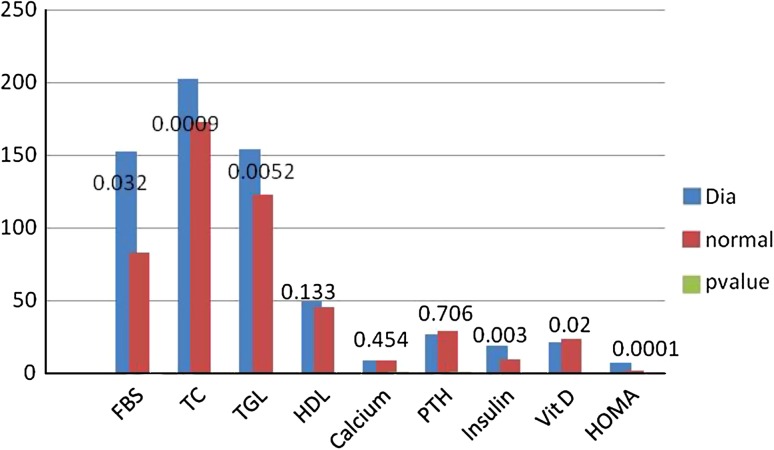
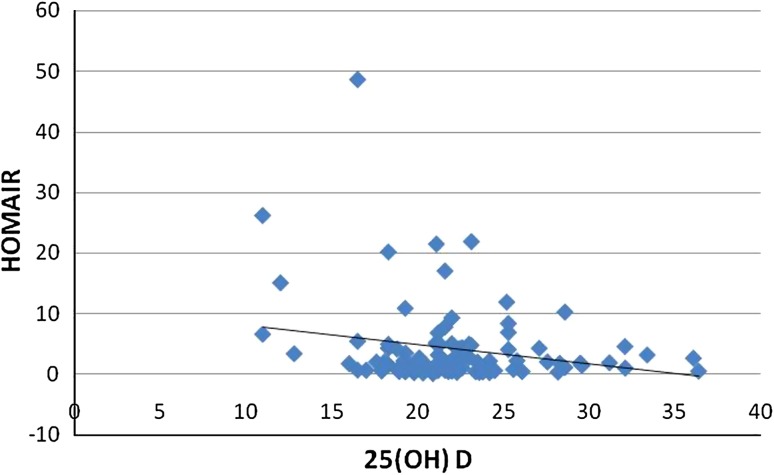
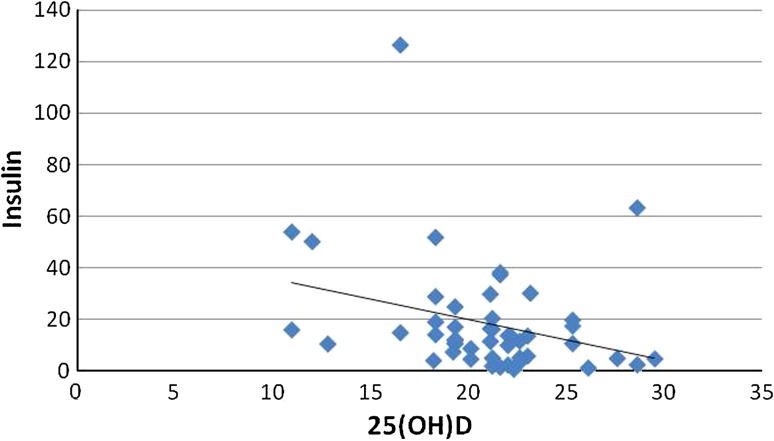
The results of the variables between normal and diabetics were expressed as a bar chart (Fig. 1). p value was significant for FBS(0.032), TC (0.0009), TGL (0.005), insulin (0.003), HOMAIR (0.0001), 25(OH)D (0.02). The analysis of the results between group 1 and group 2 of diabetics is given as mean ± SEM in Table 1; among all the parameters p value was significant for HOMAIR (p = 0.037), Insulin (p = 0.028). According to Fig. 2 there was a negative correlation between 25(OH)D and insulin resistance in total number of subjects, thus inverse association between 25(OH)D and insulin resistance was proved. Figure 3 shows the negative correlation of 25(OH)D and insulin in type 2 diabetes subjects.
Fig. 1.
Comparison of the parameters in normal and type 2 diabetes subjects
Table 1.
Comparison of parameters in group 1 and group 2 of type 2 diabetes
| Parameters | Group 1 n = 18 25(OH)D ≤20 ng/ml. Mean ± SEM | Group 2 n = 31 25(OH)D >20 ng/ml. Mean ± SEM | p Value |
|---|---|---|---|
| Age (Years) | 49.27 ± 2.56 | 52.12 ± 2.98 | 0.41 |
| Sex (Male/Female) | 9/9 | 18/13 | |
| Height (cm) | 162.5 ± 1.52 | 163.74 ± 1.26 | 0.54 |
| Weight (Kg) | 69.55 ± 3.09 | 66.7 ± 1.74 | 0.39 |
| Waist (cm) | 98.87 ± 1.62 | 98.53 ± 1.09 | 0.94 |
| FBS (mg/dl) | 155.77 ± 16.7 | 160.32 ± 10.27 | 0.27 |
| PPBS (mg/dl) | 233.88 ± 15.85 | 261.16 ± 13.31 | 0.20 |
| ALP (U/L) | 283.83 ± 7.50 | 296.06 ± 11.98 | 0.47 |
| TC (mg/dl) | 214.77 ± 9.35 | 194.80 ± 7.86 | 0.11 |
| HDL (mg/dl) | 51.11 ± 2.96 | 47.58 ± 2.35 | 0.36 |
| TGL (mg/dl) | 157.66 ± 13.24 | 151.51 ± 7.68 | 0.89 |
| Sr Insulin (mU/L) | 27.0 ± 3.85 | 13.83 ± 2.48 | 0.028* |
| HOMAIR | 9.12 ± 1.54 | 5.32 ± 1.02 | 0.037* |
| PTH (ng/L) | 26.21 ± 4.30 | 27.25 ± 5.60 | 0.66 |
| Cal (mg/dl) | 8.67 ± 0.19 | 8.56 ± 0.12 | 0.61 |
| Alb (g/dl) | 3.75 ± 0.07 | 3.87 ± 0.05 | 0.21 |
n Number of subjects
*p < 0.05 (significant)
Fig. 2.
Scatter diagram showing the negative correlation between 25(OH)D and HOMAIR
Fig. 3.
Scatter diagram showing the association of 25(OH) D and serum insulin in type 2 diabetes
Discussion
The results of the present case-control study explains that the mean 25(OH)D levels were low (20.07 ng/ml) in diabetes compared to controls (23.89 ng/ml). The levels of 25(OH)D >30 ng/ml is considered normal. The levels between 20 and 30 ng/ml are insufficiency and <20 ng/ml is defined as deficiency [29]. Hence vitamin D deficiency/insufficiency was observed in the study group. This observation was supported by the study from Goswami et al. [12].These findings explain that the insufficient vitamin D level is mainly due to the use of sun protective creams which decrease the penetration of UVB rays into the skin thus preventing the vitamin synthesis. The foods are not fortified with vitamin D and only a very few sea foods are rich sources, modern lifestyle habits limiting to indoor activity, are some of the reasons for deficient/insufficient vitamin D levels.
The recent data from Australian diabetes, obesity and lifestyle study suggests that the 25(OH)D levels were low in diabetes subjects and there was inverse association between 25(OH)D and type 2 diabetes risk in their population [30]. From our case-control study, we observed decreased 25(OH)D levels in type 2 diabetes. The effects of vitamin D on glucose metabolism are mainly due to the distribution of its receptors (VDR) on pancreatic β cells, skeletal muscle and adipose tissue. Presence of 1α hydroxylase in β cells, the presence of vitamin D response element in the human insulin receptor gene promoter also influence the insulin sensitivity. The calcitriol directly activates the transcription of human insulin receptor gene, activates peroxisome proliferator activator receptor δ (PPAR). Vitamin D stimulates the expression of insulin receptor and enhances insulin mediated glucose transport in vitro [31–34].Certain allelic variations in vitamin D receptor gene and vitamin D binding protein might influence glucose tolerance and insulin secretion. Thus contributing to the genetic risk for type 2 diabetes [35, 36].
According to Ely prospective study, the baseline 25(OH)D concentration was inversely associated with glucose, IR, metabolic syndrome risk at 10 year follow up [37, 38]. National health and nutrition examination survey III has also proposed the inverse association of 25(OH)D levels with HOMAIR [19]. The Asian study from China-suggested that reduced 25(OH)D was associated with metabolic syndrome and there was an inverse association of 25(OH)D with insulin resistance [27]. Consistent with the above findings, the present study also concludes that there was inverse association of 25(OH)D with HOMAIR and insulin. Insulin resistance was more with mean HOMAIR value of 9.12 in group 1 (25(OH)D <20 ng/ml) subjects compared to the mean HOMAIR of 5.32 in group 2. But the similar findings were not observed in normal subjects, which may be due several factors like small number of subjects, the data relating vitamin D with insulin resistance in normal subjects is very limiting in Asians. We require the large population based studies to prove the association. Asians should establish their own cut off values for serum vitamin D status, recommended dietary allowance of vitamin D. Now the revised dietary recommendations have to be implemented. In 2003 American academy of pediatrics committee on nutrition and section on breast feeding advocated 200 IU of vitamin D intake for children of all ages, but this will only prevent the rickets. But now the recommendation is 400 IU/day for children of all ages, and 600–800 IU for adults as per National Institute of Health guidelines [39]. Hence large population based and cross sectional studies are required in view of ongoing research on vitamin D effects on glucose metabolism.
Conclusion
Thus the present study concludes that the mean 25(OH)D levels in type 2 diabetes subjects is lower than normal individuals. The insulin resistance as measured by HOMAIR is higher in type 2 diabetes subjects with 25(OH)D <20 ng/ml than in subjects with >20 ng/ml. Low 25(OH)D levels in diabetic patients can contribute to higher IR. Large population studies are required to validate the findings.
References
- 1.Kochhupalli N. The physiology of vitamin D. Indian J Med Res. 2008;127:256–262. [PubMed] [Google Scholar]
- 2.Nagapal S, Na S, Rathnachalam R. Noncalcemic actions of vitamin D receptor ligands. Endocr Rev. 2005;26:662–667. doi: 10.1210/er.2004-0002. [DOI] [PubMed] [Google Scholar]
- 3.Zittermann A. Vitamin D in preventive medicine: are we ignoring the evidence? Br J Nutr. 2003;89(5):55–72. doi: 10.1079/BJN2003837. [DOI] [PubMed] [Google Scholar]
- 4.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 5.Takiishi T, Gysemans C, Bouillon R, Mathieu C. Vitamin D and diabetes. Rheum Dis Clin North Am. 2012;38(1):179–206. doi: 10.1016/j.rdc.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 6.Paul K, Maarit L, Catharina M, Tommi H, Jukka M, H Markku, et al. Serum vitamin D and subsequent occurrence of type 2 diabetes. Epidemiology. 2008;19(5):666–671. doi: 10.1097/EDE.0b013e318176b8ad. [DOI] [PubMed] [Google Scholar]
- 7.Pittas AG, Sun Q, Manson JE, Dawson-Hughes B, Hu FB. Plasma 25-hydroxy vitamin D concentration and risk of incident type 2 diabetes in women. Diabetes Care. 2010;33:2021–2023. doi: 10.2337/dc10-0790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramachandran A, Snehalatha C. Current scenario of diabetes in India. J Diabetes. 2009;1(1):18–28. doi: 10.1111/j.1753-0407.2008.00004.x. [DOI] [PubMed] [Google Scholar]
- 9.Mohan V, Sandeep S, Deepa R, Shah B, Vrghese C. Epidemiology of type 2 diabetes: Indian scenario. Indian J Med Res. 2007;125:217–230. [PubMed] [Google Scholar]
- 10.Hodgkin P, Hine PM, Kay GH, Lumb GA, Stanbury SW. Vitamin D deficiency in Asians at home and in Britain. Lancet. 1973;302:167–172. doi: 10.1016/S0140-6736(73)93004-3. [DOI] [PubMed] [Google Scholar]
- 11.Harinarayan CV, Gupta N, Kochupillai N. Vitamin D status in primary hyperparathyroidism in India. Clin Endocrinol. 1995;43:351–358. doi: 10.1111/j.1365-2265.1995.tb02043.x. [DOI] [PubMed] [Google Scholar]
- 12.Goswami R, Gupta N, Goswami D, Marwaha RK, Tandon N, Kochupillai N. Prevalence and significance of low 25-hydroxy vitamin D concentration in healthy subjects in Delhi. Am J Clin Nutr. 2000;72:472–475. doi: 10.1093/ajcn/72.2.472. [DOI] [PubMed] [Google Scholar]
- 13.Harinarayan CV, Ramalakshmi T, Prasad UV, Sudhakar D, Srinivasarao PVLN, Sarma KVS, et al. High prevalence of low dietary calcium, high phytate consumption, and vitamin D deficiency in healthy south Indians. Am J Clin Nutr. 2007;85:1062–1067. doi: 10.1093/ajcn/85.4.1062. [DOI] [PubMed] [Google Scholar]
- 14.Harinarayan CV. Prevalence of vitamin D insufficiency in postmenopausal south Indian women. Osteoporos Int. 2005;16:397–402. doi: 10.1007/s00198-004-1703-5. [DOI] [PubMed] [Google Scholar]
- 15.Harinarayan CV, Ramalakshmi T, Prasad UV, Sudhakar D. Vitamin D status in Andhra Pradesh: a population based study. Indian J Med Res. 2008;127:211–218. [PubMed] [Google Scholar]
- 16.Harinarayan CV, Ramalakshmi T, Venkata Prasad U. High prevalence of low dietary calcium and low vitamin D status in healthy South Indians. Asia Pac J Clin Nutr. 2004;13:359–364. [PubMed] [Google Scholar]
- 17.Chiu KC, Chu A, Go VLW, Saad MF. Hypovitaminosis D is associated with insulin resistance and β cell dysfunction. Am J Clin Nutr. 2004;79:820–825. doi: 10.1093/ajcn/79.5.820. [DOI] [PubMed] [Google Scholar]
- 18.Pittas AG, Lau J, Hu FB, Dawson-Hughes B. The role of vitamin D and calcium in type 2 diabetes. a systematic review and meta-analysis. J Clin Endocrinol Metab. 2007;92:2017–2029. doi: 10.1210/jc.2007-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.scragg R, Sowers M, Bell C. Serum 25-hydroxy vitamin D, diabetes and ethnicity in the third national health and nutrition examination survey. Diabetes Care. 2004;27:2813–2818. doi: 10.2337/diacare.27.12.2813. [DOI] [PubMed] [Google Scholar]
- 20.Ford ES, Ajani UA, McGuire LC, Liu S. Concentration of serum vitamin D and the metabolic syndrome among US adults. Diabetes Care. 2005;28:1228–1230. doi: 10.2337/diacare.28.5.1228. [DOI] [PubMed] [Google Scholar]
- 21.Bourlon PM, Billaudel B. Dussert. Influence of Vitami D3 deficiency and 1,25 dihydroxy D3 on denovo insulin biosynthesis in islets of the rat endocrine pancreas. J Endocrinol. 1999;160:87–95. doi: 10.1677/joe.0.1600087. [DOI] [PubMed] [Google Scholar]
- 22.Danescu LG, Levy S, Levy J. Vitamin D and diabetes mellitus. Endocrine. 2009;35:11–17. doi: 10.1007/s12020-008-9115-5. [DOI] [PubMed] [Google Scholar]
- 23.Giulietti A, Van Etten E, Overbergh L, Stoffels K, Bouillon R, Mathieu C. Monocytes from type 2 diabetic patients have a pro-inflammatory profile: 1,25-dihydroxy vitamin D3 works as anti inflammatory. Diabetes Res Clin Pract. 2007;77(1):47–57. doi: 10.1016/j.diabres.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 24.Cohen-Lahav M, Douvdevani A, Chaimovitz C, Shany S. The anti inflammatory activity of 1,25 dihydroxy vitamin D3 in macrophages. J Steroid Biochem Mol Biol. 2007;103:558–562. doi: 10.1016/j.jsbmb.2006.12.093. [DOI] [PubMed] [Google Scholar]
- 25.Saxe AW, Gibson G, Gingerich RL, Levy J. Parathyroid hormone decreases in vivo insulin effect on glucose utilization. Calcif Tissue Int. 1995;57:127–132. doi: 10.1007/BF00298433. [DOI] [PubMed] [Google Scholar]
- 26.Teegarden D, Donkin SS. Vitamin D: emerging new roles in insulin sensitivity. Nutr Res Rev. 2009;22:82–92. doi: 10.1017/S0954422409389301. [DOI] [PubMed] [Google Scholar]
- 27.Ling L, Zhijie Y, Pan A, Hu FB, Franco OH, Li H, et al. Plasma 25-hydroxy vitamin D concentration and metabolic syndrome among middle-aged and elderly Chinese individuals. Diabetes Care. 2009;32:1278–1283. doi: 10.2337/dc09-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bonora E, Formentini G, Calcaterra F, Lombardi S, Marini F, Zenari L, et al. HOMA estimated insulin resistance is an independent predictor of cardiovascular disease in type 2 diabetes subjects. Diabetes Care. 2002;25(7):135–141. doi: 10.2337/diacare.25.7.1135. [DOI] [PubMed] [Google Scholar]
- 29.Harinarayan CV, Joshi SR. Vitamin D status in India—its implications and remedial measures. J Assoc Physicians India. 2009;57:40–48. [PubMed] [Google Scholar]
- 30.Gagnon C, Lu ZX, Magliano DJ, Dunstan DW, Shaw JE, Zimmet PZ, et al. Serum 25-hydroxy vitamin D, calcium intake, and risk of type 2 diabetes after 5 years: results from a national, population-based prospective study (the Australian diabetes, obesity and lifestyle study) Diabetes Care. 2011;34:1133–1138. doi: 10.2337/dc10-2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bischoff HA, Borchers M, Gudat F, Duermueller U, Theiler R, et al. In situ detection of 1, 25 dihydroxy vitamin D receptor in human skeletal muscle tissue. Histochem J. 2001;33(1):19–24. doi: 10.1023/A:1017535728844. [DOI] [PubMed] [Google Scholar]
- 32.Alvarez JA, Ashraf A. Role of Vitamin D in Insulin secretion and insulin sensitivity for glucose homeostasis. Int J Endocrinol. 2010;2010:1–18. doi: 10.1155/2010/351385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Norman AW. Vitamin D receptor: new assignments for an already busy receptor. Endocrinology. 2006;147:5542–5548. doi: 10.1210/en.2006-0946. [DOI] [PubMed] [Google Scholar]
- 34.Li J, Byrne ME, Chang E, Jiang Y, Donkin SS, Buhman KK, et al. 1α,25 Dihydroxy vitamin D hydroxylase in adipocytes. J Steroid Biochem Mol Biol. 2008;112:122–126. doi: 10.1016/j.jsbmb.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ogunkolade BW, Boucher BJ, Prahl JM. Vitamin D receptor (VDR) mRNA and VDR protein levels in relation to vitamin D status, insulin secretory capacity, and VDR genotype in Bangladeshi Asians. Diabetes. 2002;51:2294–2300. doi: 10.2337/diabetes.51.7.2294. [DOI] [PubMed] [Google Scholar]
- 36.Calle C, Maestro B.Garcia–arencibia M. Genomic actions of 1,25-dihydroxy vitamin D3 on insulin receptor gene expression, insulin receptor number and insulin activity in kidney, liver and functions: VDRs, gene expression, and insulin secretion Endocrinology 19941341602–1610. 10.1210/en.134.4.16028137721 [DOI] [Google Scholar]
- 37.Nita G, Luan J, Cooper A, Boucher BJ, Wareham NJ. Baseline 25 hydroxy vitamin D is predictive of future glycemic status and Insulin resistance. The medical research council Ely prospective study—1990–2000. Diabetes. 2008;57(10):2619–2625. doi: 10.2337/db08-0593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pinelli NR, Jaber LA, Brown MB, Herman WH. Serum 25-hydroxy vitamin D and Insulin resistance, metabolic syndrome, and glucose intolerance among Arab Americans. Diabetes Care. 2010;33:1373–1375. doi: 10.2337/dc09-2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rathi N, Rathi A. Vitamin D and child health in the 21st century. Indian Pediatr. 2011;48:619–625. doi: 10.1007/s13312-011-0107-9. [DOI] [PubMed] [Google Scholar]