Abstract
The finding of dieldrin (88 ng/g), DDE (52 ng/g), and heptachlor epoxide (19 ng/g) in earthworms from experimental plots after a single moderate application (9 kg/ha) 45 years earlier attests to the remarkable persistence of these compounds in soil and their continued uptake by soil organisms. Half-lives (with 95 % confidence intervals) in earthworms, estimated from exponential decay equations, were as follows: dieldrin 4.9 (4.3–5.7) years, DDE 5.3 (4.7–6.1) years, and heptachlor epoxide 4.3 (3.8–4.9) years. These half-lives were not significantly different from those estimated after 20 years. Concentration factors (dry weight earthworm tissue/dry weight soil) were initially high and decreased mainly during the first 11 years after application. By the end of the study, average concentration factors were 1.5 (dieldrin), 4.0 (DDE), and 1.8 (heptachlor epoxide), respectively.
Keywords: Organochlorine, Insecticide, Soil aging, Bioconcentration
Introduction
Woodwell et al. (1971) wrote that learning how persistent pesticides move into food chains from the environment was necessary to understanding hazards of persistent pesticides to wildlife. Earthworms are excellent bioindicators of soil quality, are useful in monitoring the accumulation of chemicals in the environment (Fruend et al. 2011), and provide a pathway through which insecticides are made available to wildlife. Barker (1958) showed how moderate applications of DDT to elm trees led to accumulation of DDT in earthworms and the subsequent death of robins preying on those earthworms. In a previous study, we had measured concentrations of dieldrin, DDE, and heptachlor epoxide in earthworms for 20 years after a single application of insecticides to field plots. Recently, earthworms and soils were analyzed after 45 years, allowing us to determine if degradation rates remained constant over those years as well as to evaluate possible changes in bioavailability with time. Both of these topics are useful to risk assessors. Although most uses of these insecticides were cancelled in the 1970s, they remain biologically available to wild birds over widespread areas (Harris et al. 2000) and are still the cause of fish advisories (New Department of Health 2012). They were the contaminants of concern at a minimum of 312 active EPA superfund sites 49 in 2011 (Search Superfund Site Information 2012). Assessors in the US Environmental Protection Agency analyze earthworms and soils for organochlorines at contaminated sites to guide their decision on the need for remediation (Tetra Tech 2009).
Stokes et al. (2006) stressed the importance of understanding the sorption of contaminants to soil organic matter and its effect on bioavailability, especially to organisms that biodegrade contaminants. Of several definitions of bioavailability in use (Stokes et al. 2006), we are using the term in the sense of a concentration factor, the pesticide concentration measured in earthworms (as determined by Soxhlet extraction) divided by the pesticide concentration in soil. Although the definition is simple, the concentration factor depends on where an earthworm feeds and where a pesticide is located in a soil profile (Wheatley and Hardman 1968), as well as on the bioaccessibility of an insecticide in soil, the fraction of that bioaccessible insecticide that is absorbed by an earthworm, and the kinetics of that insecticide in earthworms.
Concentration factors depend partially on sorption of an insecticide to soil, which begins soon after application. As the relative amount of insecticide that can be extracted by a chemical procedure decreases, it is said to be “bound” to soil (Gevao et al. 2000) and bioavailability decreases. Fuhremann and Lichtenstein (1978) demonstrated that Lumbricus terrestris accumulated mainly unbound, but some bound methyl parathion, residues from soil and concluded that binding in soil was reversible. Verma and Pillai (1991) showed similar results with DDT and also concluded that earthworms were able to crush soil particles as they fed, making the DDT available (Verma and Pillai 1991).
In addition to binding, there is an additional, overlapping process of soil “aging,” in which fewer pesticide molecules are bioaccessible to organisms, as the molecules become “sequestered,” and the pesticides gradually lose their biological activity and become more stable (Gevao et al. 2000; Barraclough et al. 2005). Alexander (2000) argued that because regulatory agencies do not take aging into account, toxic exposures are being over estimated in assessments and he cited his laboratory studies on earthworms in aged soils in support of his argument (Morrison et al. 2000; Kelsey et al. 2005). In contrast, Gaw et al. (2012), studying the accumulation of DDE in Aporrectodea caliginosa in aged horticultural soils under laboratory conditions, concluded that DDE remains biologically available to earthworms and to animals that prey on earthworms. Our field study documents the actual decrease in insecticide residues over 45 years. Our objectives are to estimate precisely the half-lives of dieldrin, DDE, and heptachlor epoxide, and to show how concentration factors change with time.
Materials and Methods
Study Site
In 1966, aluminum flashing had been trenched into a field on the Patuxent Wildlife Research Center, Laurel, MD, to form 18 square plots, 10 m on a side. Soils at the site were classified as Elsinboro loams, which are nearly level, deep, well-drained soils that formed on terraces and contain mica from crystalline rocks. In May, wettable powder formulations of dieldrin, DDT, and heptachlor were applied by backpack sprayer, one insecticide per plot, two replicates per treatment, at rates of 0.6, 2.2, or 9.0 kg active ingredients per ha (Beyer and Gish 1980). Vegetation in the plots gradually changed from orchard grass (Dactylis glomerata) to a mixture of orchard grass and mowed herbs and grasses. Gish and Hughes (1982) described seasonal changes in residues in soils and earthworms over the first 2 years. Our study addresses long-term changes based on sampling in late spring, starting a year after application. Soils at the site had previously been sampled and analyzed for 11 years and the earthworms for 20 years after application. In April 2011, earthworms were dug with a spade from the six highest treatment plots and then refrigerated at about 8 °C on paper for 3 days while they purged most of the soil from their intestines. Composite soil samples, of eight cores, were collected with a stainless steel corer to a depth of 7.5 cm from each of the plots. Earthworms were rinsed, frozen, and mailed with the soil samples in chemically clean jars to the Columbia Environmental Research Center. The earthworms comprised mainly A. turgida (Eisen), with some L. rubellus Hoffmeister. Earlier collections also comprised mainly A. turgida, with some A. trapezoids (Duges), Allolobophora chlorotica (Savigny), and L. terrestris Linnaeus (Beyer and Gish 1980). Nomenclature and keys follow Reynolds (1977). The pooled samples of mixed species of earthworms weighed from 13 to 20 g.
Chemical Methods
The soils were equilibrated at room temperature and the cores were sieved (No. 10) to remove detritus and were then manually blended. The earthworms were segmented into small pieces while frozen, macerated to a paste, and finally blended. When not in use, the soil and earthworm samples were stored at −20 °C. Approximately 10 g (wet weight) of homogenized soil or 5 g (wet weight) of homogenized earthworms were dehydrated by the addition of anhydrous sodium sulfate (Na2SO4) and allowed to react for at least 4 h. At the time of sample weighing, moisture was determined gravimetrically in duplicate on each matrix. About 1 g of soil or tissue was heated to ≥110 °C on a Multi-Temp-Blok (Lab-Line Instruments, Inc., Melrose Park, IL) until no further change in mass was observed (8 h).
The following quality control (QC) samples were incorporated into the sample set: Procedural Blanks (PB)—an anhydrous-Na2SO4 blank extraction (laboratory background check), “clean” soil and earthworm Matrix Blanks (MB-S and MB-W) (potential matrix interferences), soil and earthworm Matrix Spikes (MS-S and MS-W)—clean matrices fortified with OCPs (organochlorine pesticides) for recoveries and matrix effects, and triplicate analyses of selected soil and earthworm samples to estimate method reproducibility. The dehydrated samples were blended to a free-flowing powder, transferred to Whatman high purity 43 × 123-mm glass microfiber thimbles (Whatman International, Ltd., Maidstone, England) and fortified with procedural recovery standards (PRSs) to monitor sample preparation method recoveries: PCB-29 (2,4,5-trichlorobiphenyl), PCB-155 (2,2′,4,4′,6,6′-hexachlorobiphenyl), PCB-204 (2,2′,3,4,4′,5,6,6′-octachlorobiphenyl), d8-labeled p,p′-DDD. To evaluate the OCP method recoveries, the matrix spike QC samples (MS-S and MS-W) were fortified at 8 ng/g (soil) and 16 ng/g (worms) with an OCP standard mixture, obtained commercially (AccuStandard, New Haven, CT), containing 29 individual OCPs.
The fortified, dehydrated samples were Soxhlet extracted, using 300 mL dichloromethane (DCM; OPTIMA grade, Fisher Scientific, St. Louis, MO), and overnight at a cycle rate of 3–4 cycles per hour. Following extraction, the Soxhlet tubes and condensers were rinsed, the extracts rotary evaporated to about 3 mL (Buchi Rotovapor RE120, Buchi Corp., New Castle, DE), and the concentrated extracts were transferred to glass 15 mL screw-capped culture tubes with a final volume of 10 mL. Dichloromethane-extractable lipids were determined using 2 % of the extracts by heating at 105 °C on a Multi-Temp-Blok for 1 h. The remaining sample was nitrogen evaporated to about 1 mL (Meyer N-EVAP, Organomation Associates, Inc., Berlin, MA).
The sample extracts were processed through LP-SEC, which is capable of greater sample lipid loadings and is used to remove the majority of the interfering, co-extracted lipoidal materials. The raw extracts were fractionated at the rate of ≤500 mg lipid per LP-SEC cycle using DCM as the mobile phase, at a flow rate of 4 mL/min, and a 70-cm SX-3 Bio-Bead (Bio-Rad Laboratories, Inc., Hercules, CA) stationary phase column. The LP-SEC was calibrated for the collection of the appropriate target analytes fraction (c-PCB and OCPs). The collected fractions were concentrated in volume to about 1 mL by rotary and nitrogen evaporation, prior to further cleanup by high-performance size exclusion chromatography (HP-SEC).
The extractions were then processed using an HP-SEC technique, which has a greater chromatographic resolution and is used to remove any remaining residual lipoidal materials. The HP-SEC was calibrated for the collection of the appropriate target analyte fraction (c-PCB and OCPs) using DCM as the mobile phase, at a flow rate of 4 mL/min, and a Phenogel 10 300 × 21.2 mm 100 Å (Phenomenex, Inc., Torrance, CA) stationary phase column. The collected fractions were concentrated in volume to about 1 mL, by rotary and nitrogen evaporation, and solvent exchanged to hexanes prior to further cleanup by silica gel-60 and octadecyl silica (SGODS) fractionation.
Any residual polar interferences were removed from the samples and the c-PCBs and OCPs were fractionated by SGODS adsorption chromatography. The extracts were fractionated using two layered octadecyl silica and activated silica gel 60 gravity flow columns eluted with mobile phases which increased in solvent polarity. Most PCBs (≥90%) and several OCPs are slightly retained on the silica gel matrix bed, eluting in the first silica gel fraction (SG-1) using hexanes as the mobile phase. The remaining OCPs and several of the low-chlorinated PCBs (≤10 %) are more strongly retained and elute in the second silica gel fraction (SG-2) using hexane:methyl t-butyl ether (55:45 v:v) as the mobile phase. Any remaining biogenic materials are typically more highly retained than the target analytes and remained adhered to the silica gel matrix after the target analyte fractions were collected. The silica gel batch was calibrated to ensure appropriate separations of the analytes of interest prior to use. The collected fractions were concentrated in volume to 2 mL by rotary and nitrogen evaporation and then solvent exchanged to iso-octane, prior to instrumental analysis.
Following adjustment of the fractions to 2 mL, two instrumental internal standards, PCB-30 (2,4,6-trichlorobiphenyl) and PCB-207 (2,2′,3,3′,4,4′,5,6,6′-nonachlorobiphenyl), were added to each at 40 ng/mL. Individual organochlorine pesticides were measured in both the SG-1 and SG-2 fractions by gas chromatography–electron capture detection (GC/ECD). Analyses were performed using an Agilent 6890 gas chromatograph with cool on-column capillary injection systems and Agilent model 7683B autosamplers (Agilent Technologies, Inc., Santa Clara, CA). For all analyses, 3-m sections of 0.53 mm i.d. uncoated and deactivated capillary retention gaps were attached to each analytical column by Press-Tight® unions (Restek Corp, Bellefonte, CA). The analytical columns were Agilent J&W 60 m × 0.25 mm × 0.25 μm DB-1 and DB-5 capillary columns. The H2-carrier gas was pressure regulated at 25 psi. The temperature program for the OCP analysis was: initial temperature 60 °C, immediately ramped to 150 °C at 15 °C/min, then ramped to 240 °C at 1 °C/min, and finally ramped to 330 °C at 10 °C/min, and held for 15 min. The ECD temperature was 330 °C with nitrogen as the makeup gas at a flow rate of 25 mL/min.
The dual column method accurately identifies and quantifies OCP peaks from both columns, as peak identification—confirmatory method, based upon known standards. The GC/ECD data were collected, archived in digital form, and processed using a PerkinElmer chromatography data system, which included the model 970 interface and version 6.2 of TotalChrom workstation chromatography software. Six levels of OC pesticide standards (29 components) were used for calibration, with each pesticide at concentrations ranging from 0.1 to 80 ng/mL. The limits of quantification (LOQ) for individual OCPs were based on the lowest standard concentration included in the instrument calibration curve (0.1 ng/mL) converted to units of ng/g based on average sample dry weights and final sample volumes (0.03 ng/g soil and 0.21 ng/g earthworms). Procedural background, from the preparatory method, was also subtracted from the individual analyte concentrations to account for background OCP content, where applicable.
Organic matter content of the six soil samples was estimated from weight loss-on-ignition at 400 °C for 4 h.
Statistical Methods
Insecticide concentrations in earthworms from current and previously published (Beyer and Krynitsky 1989) studies were fitted to a two variable exponential decay equation (SigmaPlot® 9.0, Systat Software, Inc., Point Richard, CA), which has been shown to be appropriate when describing long-term changes in insecticide concentrations (Edwards 1966). Let a be a constant equal to the theoretical insecticide concentration at year 0, b be a constant describing the rate of degradation, and x the time, in years, then the concentration (mg/kg, dry weight) in soil or earthworms, or y, equals ae−bx. The half-life (ln2/−b), or time elapsed for the concentration to be reduced by half, is independent of the concentration and is useful in describing insecticide persistence. Because variances of concentrations were proportional to their values and to be consistent with previous methods (Beyer and Krynitsky 1989), we fitted the concentrations of dieldrin, DDE, and heptachlor epoxide to the linear form (its derivative) of this equation.
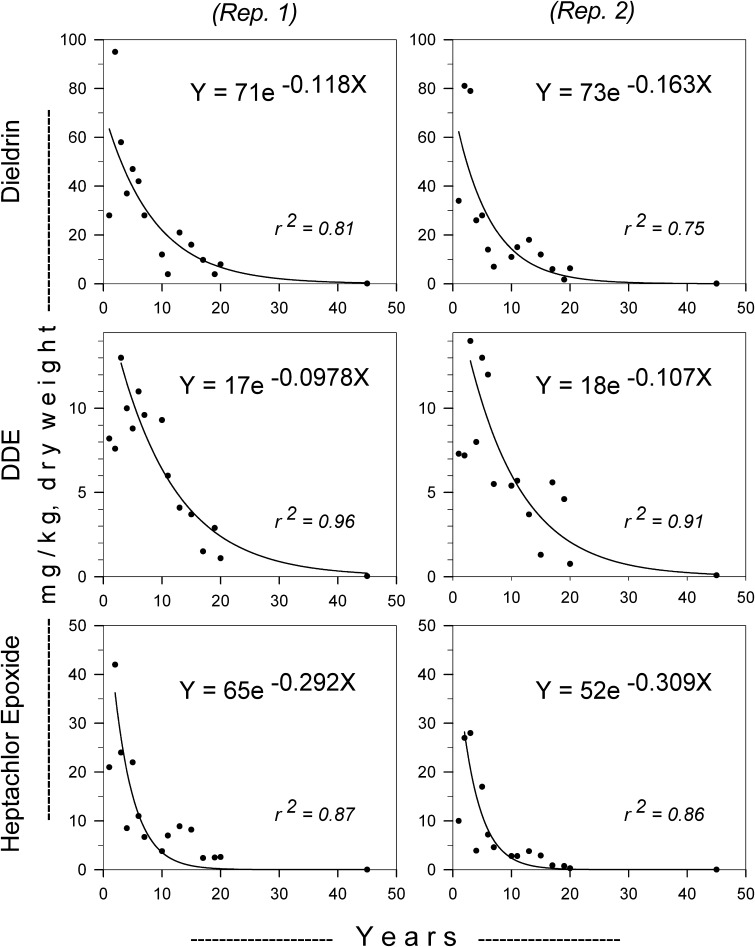
DDE and heptachlor epoxide were metabolites of the insecticides applied and were present at low concentrations in soil at the beginning of the study; consequently, consistent with earlier calculations (Beyer and Krynitsky 1989), we included data beginning with year 2 for heptachlor epoxide and beginning with year 3 for DDE (Fig. 1), when these metabolites had become more abundant than the parent compound in soil [note, however, that the estimated initial concentrations (the constant a) for these two metabolites refer to year 0]. Average concentration factors from paired replicate plots were estimated as geometric means, consistent with previous estimates.
Fig. 1.
Concentrations (mg/kg, dry weight) of dieldrin, DDE, and heptachlor epoxide detected in earthworms from replicate plots treated once with dieldrin, DDT, or heptachlor at 9.0 kg/ha. All adjusted r2 values exceeded 0.75 and all probability values (type 1 error) associated with estimates of the slope and intercept were below 0.05. Exponential decay equations shown were calculated from the linear form of the equations
Results and Discussion
The concentrations of the applied insecticides or their metabolites detected in soils and earthworms 45 years after application were low but, above their respective LOQ (Table 1). The principal toxic chemicals of interest detected in earthworms were dieldrin in plots treated with dieldrin, DDE in plots treated with DDT, and heptachlor epoxide in plots treated with heptachlor. Geometric mean concentrations (dry weight) in earthworms were 88 ng/g of dieldrin, 52 ng/g of DDE, and 19 ng/g of heptachlor epoxide. Cross contamination of insecticides in soil between plots was minor, estimated as 0.7 % for soil samples. A higher value of cross contamination of 4.0 % among earthworm samples suggests that some earthworms moved between plots. Weights of extractable lipids as a fraction of earthworm dry weights were from 6 to 9 % (dieldrin 1—8.7 %, dieldrin 2—9.0 %, DDE 1—9.2 %, DDE 2—7.8 %, heptachlor 1—7.3 %, Heptachlor 2—6.1 %).
Table 1.
Insecticide and metabolite concentrations detected in earthworms and soil and concentration factors, 45 years after treatment (9.0 kg/h) at the Patuxent Wildlife Research Center
| Plot | Replicate 1 (ng/g, dry weight) | Replicate 2 (ng/g, dry weight) | Average concentration factor (unitless) | ||
|---|---|---|---|---|---|
| Soil | Worm | Soil | Worm | ||
| Dieldrin plots | |||||
| Dieldrin | 60 | 110 | 56 | 68 | 1.5 |
| Aldrin | 0.23 | 0.34 | 0.19 | 0.22 | |
| DDT plots | |||||
| o,p-DDT | 0.91 | 0.31 | 0.41 | 0.78 | |
| p,p′-DDT | 7.1 | 5.7 | 3.0 | 15 | |
| p,p′-DDE | 18 | 31 | 9.5 | 85 | 4.0 |
| p,p′-DDD | 0.11 | <LOQ [0.21] | 0.09 | <LOQ [0.21] | |
| Σ-DDTs | 26 | 37 | 13 | 100 | |
| Heptachlor plots | |||||
| Heptachlor | 0.28 | <LOQ [0.21] | 0.17 | <LOQ [0.21] | |
| Heptachlor epoxide | 12 | 20 | 9.2 | 18 | 1.8 |
| c-Chlordane | 0.64 | 2.2 | 0.51 | 1.0 | |
| t-Chlordane | 2.0 | 0.31 | 1.04 | <LOQ [0.21] | |
| Oxychlordane | 2.9 | 14 | 2.3 | 10 | |
| c-Nonachlor | 1.5 | 3.6 | 0.91 | 1.3 | |
| Trans-nonachlor | 10 | 61 | 6.8 | 29 | |
LOQ limit of quantification
Although individual points were scattered about the curves in Fig. 1, exponential decay curves approximated the underlying trend reasonably well over 45 years. The adjusted r2s were 0.80 (dieldrin 1), 0.75 (dieldrin 2), 0.96 (DDE 1), 0.91 (DDE 2), 0.87 (heptachlor epoxide 1), and 0.86 (heptachlor epoxide 2). Dieldrin’s half-life (with 95 % confidence limits) in earthworms was estimated at 4.9 (4.3–5.7) years, DDE’s at 5.3 (4.7–6.1) years, and heptachlor epoxide’s at 4.3 (3.8–4.9) years. Estimates of half-lives of the three compounds over 45 years averaged 8 % lower than estimates based on the first 20 years of data (Table 2), a difference well within confidence limits. Our study sites were on well-drained Elsinboro loam, in a humid, temperate semi-continental climate (Kirby et al. 1967). The insecticides would have been less persistent if they had been applied to wetter, warmer, or cultivated soils (Edwards 1973) and they would have been more persistent if applied to soils (Edwards 1973) containing more than the 3–5 % organic matter we measured in 1977 (Beyer and Gish 1980) and the 4–6% organic matter in 2011.
Table 2.
Half-lives (with 95 % confidence limits) and concentration factors in earthworms from sites experimentally treated with organochlorine insecticides at the Patuxent Wildlife Research Center
| Dieldrin | DDE | Heptachlor epoxide | |
|---|---|---|---|
| Half-time in earthworms over first 20 yearsa | 5.4 (4.2–7.8) | 5.7 (4.5–8.0) | 4.3 (3.5–5.6) |
| Half-time in earthworms over 45 years | 4.9 (4.3–5.7) | 5.3 (4.7–6.1) | 4.3 (3.8–4.9) |
| Concentration factor after 1 year (unitless)b | 15 | 16 | 13 |
| Concentration factor after 11 year (unitless)b | 3.9 | 6.0 | 8.4 |
| Concentration factor after 45 year (unitless)c | 1.5 | 4.0 | 1.8 |
Immediately after spraying, insecticides coat vegetation and soil, leading to high concentration factors in earthworms. Our data in Fig. 1, however, start about a year after application when, presumably, weathering and decomposition of the vegetation had moved most of the insecticides into the upper mineral soil. Eleven years after application, the concentration factor of dieldrin had decreased from 15 to 3.9, that of DDE from 16 to 6, and that of heptachlor epoxide from 13 to 8.4 (Table 2). The reduction was presumably due to sorption, which reduced bioavailability. Bioavailability stabilized, however, or decreased very slightly per year after 11 years. At the end of the study, concentration factors were: dieldrin—1.5 years, DDE—4.0 years, and heptachlor epoxide—1.8 years. Based on data in Beyer and Gish (1980) and in Table 1, the concentration factor of DDE decreased by only a third in 34 years while soil concentrations of DDE decreased by more than 98 %.
Alternatively, we can evaluate long-term changes in bioavailability of insecticides to earthworms by comparing concentration factors from recent studies to concentration factors during the years when insecticides were being applied. In an extensive survey of pesticide concentrations detected in earthworms and soils from 67 agricultural fields in 1965, Gish and Hughes (Gish 1970) calculated mean concentration factors (dry weight) in earthworms as follows: dieldrin—9.9, DDE—7.4, and heptachlor epoxide—3.0. Edwards and Thompson (1973), writing at a time when insecticides had recently been applied, generalized that concentration factors of organochlorine pesticides were typically about 5, although it should be noted that they included values based on wet weight as well as on dry weight of earthworms. Concentration factors measured in recent studies are in the same range. As part of a study examining the bioaccumulation of DDE in robins inhabiting orchards in Canada, Harris et al. (2000) reported average DDE concentration factors (dry weight) of 3.77–5.21 at their three sites. Earthworms from parks in Beijing, China, had a median DDT (sum of all compounds) concentration factor (dry weight) of 7.47 (Li et al. 2010). Earthworms from fields historically contaminated on the Beltsville Agricultural Research Center in Maryland, USA, had geometric mean concentration factors (dry weight) of 8.9 (DDE), based on 9 plots, and 6.5 (dieldrin), based on 6 plots (Tetra Tech 2009). These recent studies may be based on different sampling depths and different species of earthworms but, together, they suggest that concentration factors of earthworms recently collected from the field are about the same as reported by Gish (1970). If soil aging were having a significant effect on concentrations of organochlorine insecticides in the earthworms studied, we would have expected a noticeable decrease in the concentration factors of the insecticides, applied as much as 40 years earlier.
In closing, the finding of dieldrin, DDE, and heptachlor epoxide in earthworms after a single moderate application 45 years earlier attests to the remarkable persistence of these compounds in soil and their continued uptake by soil organisms. Our data demonstrate that decreases in concentrations of these compounds in earthworms can be accurately estimated over 45 years with exponential decay equations. Our previous estimates of half-lives in earthworms from 20 years of data were reasonably close to estimates based on the entire period. Concentration factors of persistent insecticides decrease rapidly after application due to both ecological and chemical (sorption) reasons and then they remain approximately constant. Concentration factors in our plots decreased slowly, if at all, from 11 years on. It would be misleading to use concentration factors measured soon after application in a risk assessment of a site contaminated many years ago. We found no evidence, however, that soil aging, distinct from sorption, was rendering the insecticides unavailable to earthworms in the field over the long term.
Acknowledgment
This study was supported by funding from the Patuxent Wildlife Research Center (US Geological Survey).
Biographies
W. Nelson Beyer
is a research biologist who studies the uptake of environmental contaminants into food chains and the associated hazards to wildlife for the US Geological Survey.
Robert W. Gale
is the leader of the Chemical Fate and Dynamics Section at the Columbia Environmental Research Center in the US Geological Survey. He develops analytical methods for analyzing contaminants such as organochlorines and brominated flame retardants in fish.
Contributor Information
W. Nelson Beyer, Email: nbeyer@usgs.gov.
Robert W. Gale, Email: rgale@usgs.gov
References
- Alexander M. Aging, bioavailability, and overestimation of risk from environmental pollutants. Environmental Science and Technology. 2000;34:4259–4265. doi: 10.1021/es001069+. [DOI] [Google Scholar]
- Barker RJ. Notes on some ecological effects of DDT sprayed on elms. The Journal of Wildlife Management. 1958;22:269–274. doi: 10.2307/3796459. [DOI] [Google Scholar]
- Barraclough D, Kearney T, Croxford A. Bound residues: Environmental solution or future problem? Environmental Pollution. 2005;133:85–90. doi: 10.1016/j.envpol.2004.04.016. [DOI] [PubMed] [Google Scholar]
- Beyer W, Gish C. Persistence in earthworms and potential hazards to birds of soil applied DDT, dieldrin and heptachlor. Journal of Applied Ecology. 1980;17:295–307. doi: 10.2307/2402326. [DOI] [Google Scholar]
- Beyer W, Krynitsky A. Long-term persistence of dieldrin, DDT, and heptachlor epoxide in earthworms. AMBIO. 1989;18:271–273. [Google Scholar]
- Edwards C. Insecticide residues in soils. Residue Reviews. 1966;13:132. doi: 10.1007/978-1-4615-8407-0_4. [DOI] [Google Scholar]
- Edwards C. Pesticide residues in soil and water. In: Edwards C, editor. Environmental pollution by pesticides. New York: Plenum Press; 1973. pp. 409–458. [Google Scholar]
- Edwards C, Thompson A. Pesticides and the soil fauna. Residue Reviews. 1973;45:1–79. doi: 10.1007/978-1-4615-8493-3_1. [DOI] [PubMed] [Google Scholar]
- Fruend H, Graefe U, Tischer S. Earthworms as bioindicators of soil quality. In: Karaca A, editor. Biology of earthworms. Berlin: Springer; 2011. pp. 261–278. [Google Scholar]
- Fuhremann TW, Lichtenstein EP. Release of soil-bound methyl [14C] parathion residues and their uptake by earthworms and oat plants. Journal of Agricultural and Food Chemistry. 1978;26:605–610. doi: 10.1021/jf60217a011. [DOI] [Google Scholar]
- Gaw S, Northcott G, Kim N, Wilkins A, Jensen J. Comparison of earthworm and chemical assays of the bioavailability of aged 1,1-dichloro-2,2-bis(p-chlorophenyl)ethylene, 1,1,1-trichloro-2,2-bis(p-chlorophenyl)ethane, and heavy metals in orchard soils. Environmental Toxicology and Chemistry. 2012;31:1306–1316. doi: 10.1002/etc.1817. [DOI] [PubMed] [Google Scholar]
- Gevao B, Semple KT, Jones KC. Bound pesticide residues in soils: A review. Environmental Pollution. 2000;108:3–14. doi: 10.1016/S0269-7491(99)00197-9. [DOI] [PubMed] [Google Scholar]
- Gish CD. Organochlorine insecticide residues in soils and soil invertebrates from agricultural lands. Pesticides Monitoring Journal. 1970;3:241. [PubMed] [Google Scholar]
- Gish, C.D., and D.L. Hughes. 1982. Residues of DDT, dieldrin, and heptachlor in earthworms during two years following application. Special scientific report—wildlife/United States. Fish and Wildlife Service.
- Harris M, Wilson L, Elliott J, Bishop C, Tomlin A, Henning K. Transfer of DDT and metabolites from fruit orchard soils to American robins (Turdus migratorius) twenty years after agricultural use of DDT in Canada. Archives of Environmental Contamination and Toxicology. 2000;39:205–220. doi: 10.1007/s002440010098. [DOI] [PubMed] [Google Scholar]
- Kelsey J, Colino A, White J. Effect of species differences, pollutant concentration, and residence time in soil on the bioaccumulation of 2,2-bis(p-chlorophenyl)-1,1-dichloroethylene by three earthworm species. Environmental Toxicology and Chemistry. 2005;24:703–708. doi: 10.1897/04-293R.1. [DOI] [PubMed] [Google Scholar]
- Kirby, R.M., E. Matthews, and M. Bailey. 1967. Soil survey, Prince Georges County, Maryland, US Soil Conservation Service, Maryland Agricultural Experiment Station.
- Li XH, Wang XZ, Wang W, Jiang XN, Xu XB. Profiles of Organochlorine pesticides in earthworms from urban leisure areas of Beijing, China. Bulletin of Environmental Contamination and Toxicology. 2010;84:473–476. doi: 10.1007/s00128-010-9968-1. [DOI] [PubMed] [Google Scholar]
- Morrison DE, Robertson BK, Alexander M. Bioavailability to earthworms of aged DDT, DDE, DDD, and dieldrin in soil. Environmental Science and Technology. 2000;34:709–713. doi: 10.1021/es9909879. [DOI] [Google Scholar]
- New Department of Health. 2012. Retrieved July 18, 2012, fromhttp://www.health.state.ny.us/environmental/outdoors/fish/health_advisories/.
- Reynolds JW. The earthworms (Lumbricidae and Sparganophilidae) of Ontario. Toronto, ON: Royal Ontario Museum; 1977. [Google Scholar]
- Search Superfund Site Information. 2012. Retrieved Oct 12, 2011, from http://cumulis.epa.gov/supercpad/cursites/srchsites.cfm.
- Stokes JD, Paton G, Semple KT. Behaviour and assessment of bioavailability of organic contaminants in soil: Relevance for risk assessment and remediation. Soil Use and Management. 2006;21:475–486. doi: 10.1079/SUM2005347. [DOI] [Google Scholar]
- Tetra Tech. 2009. Bioavailability Study Report for BARC 4-Building 033 Washdown Area and BARC 19—Trenches Behind Building 029. http://ars.usda.dandp.com/barcsuperfund/search_results.php. Accessed 18 July 2012.
- Verma A, Pillai M. Bioavailability of soil-bound residues of DDT and HCH to earthworms. Current Science. 1991;61:840–843. [Google Scholar]
- Wheatley G, Hardman J. Organochlorine insecticide residues in earthworms from arable soils. Journal of the Science of Food and Agriculture. 1968;19:219–225. doi: 10.1002/jsfa.2740190410. [DOI] [Google Scholar]
- Woodwell GM, Craig PP, Johnson HA. DDT in the biosphere: Where does it go? Science. 1971;174:1101. doi: 10.1126/science.174.4014.1101. [DOI] [PubMed] [Google Scholar]