Abstract
The dorsal raphe nucleus contains one of the largest groups of serotonergic neurons in the mammalian brain and is the main site of origin of the serotonergic projection to the cerebral cortex. Early electrophysiological studies suggested that serotonergic neurons in this cell group formed a homogeneous cell class. More recent studies however have reported heterogeneity among the core anatomical and electrophysiological properties of these neurons, thus raising the possibility that serotonergic neurons of this cell group may form two or more distinct cell classes. In this Viewpoint, we review these findings and suggest ways to look at cellular heterogeneity among serotonergic neurons.
Keywords: Serotonin, serotonergic neurons, DRN, dorsal raphe
One of the most emphatic aspects of mammalian serotonergic neurons is their extensive connectivity. This is remarkable given that serotonin is synthesized by a relatively small number of cells, numbering in the low thousands, yet these neurons project to essentially the entire neuroaxis.1 This organization has led to the view that serotonin plays a general modulatory role in the control of central nervous system activity. Underlying this unifying concept, however, is the long-standing idea that serotonin neurons are heterogeneous and can be subdivided into functional classes. This view emerged at the earliest stages of our exploration of the serotonergic system when Dahlström and Fuxe2 reported that serotonergic neurons form discrete, anatomically defined cell groups .
Nearly 50 years later, it is clear there is considerable specialization among serotonergic neurons.3 Different serotonin cell groups exhibit divergent afferent and efferent connectivity,1 and the use of intersectional genetics is allowing for the identification of specialized physiological roles for specific groups of serotonin neurons.4,5 But is each serotonergic cell group composed of a single homogeneous population of neurons? Or are different serotonergic cell groups themselves amalgamations of two or more functionally distinct neuronal populations? This is the question of serotonin neuron subtypes that we explore in this Viewpoint.
While the question of differentiating serotonin neuron subtypes appears remarkably straightforward at the conceptual level, its resolution is not simple. Difficulties arise in determining what constitutes a valid neuronal subtype. When faced with this question, we tend to be either “lumpers” or “splitters”. So how do we determine if we have more than one subtype of neurons? The classification of cortical GABAergic interneurons offers an example worth considering. The diversity of cortical interneurons was first surmised on anatomical grounds and was later strengthened by the discovery of molecular markers and distinctive electrophysiological characteristics. A consensus classification began to emerge with the realization that these features cosegregated to define specific cell classes. This classification was strengthened by cell-fating studies that provided a compelling mechanistic accounting for the broad outline of a classification system. As a result, different interneuron subtypes are now thought to correspond to different neuronal lineages, each executing a particular genetic program, which then determines the properties of that particular interneuron subtype. Recent work has extended these ideas to show that the basal functional properties of each cell are further refined by neuronal activity.6,7
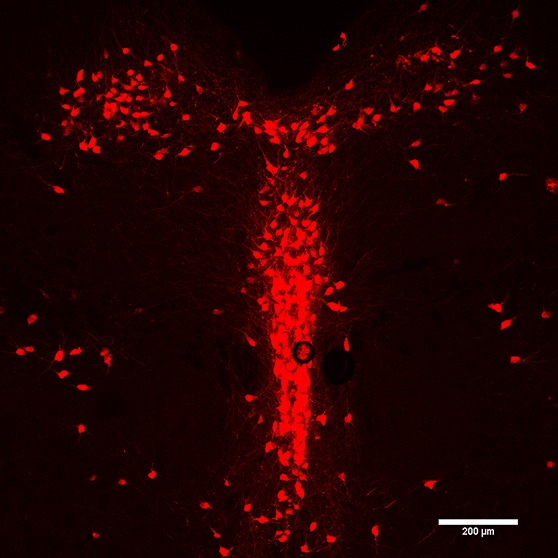
How do these ideas apply to the dorsal raphe nucleus (DRN)? This cell group is composed of serotonergic neurons as well as nonserotonergic neurons.8 Early anatomical studies established that DRN serotonin neurons could be divided into at least three morphologically distinct classes. Class I and II cells corresponded to spiny neurons exhibiting fusiform and large multipolar cell bodies, respectively.9,10 Class III cells corresponded to aspiny neurons with small oval/piriform cell bodies.11 Early immunohistochemical studies also suggested that there was considerable heterogeneity in terms of neuropeptide expression in the DRN (Figure 1), including coexpression of serotonin with substance P (SP),12 thyrotropin releasing hormone (TRH),13 galanin,14 or corticotropin releasing hormone (CRF).15 However, it is important to note that recent studies in mice have failed to replicate these findings,16 suggesting caution in interpreting these early results.
Figure 1.
Tryptophan hydroxylase immunohistochemical localization of serotonergic neurons in the mouse DRN.
Despite this apparent anatomical diversity, early in vivo and in vitro electrophysiological studies showed that DRN serotonergic neurons exhibited remarkably consistent electrophysiological and pharmacological characteristics. These cells were shown to exhibit slow, spontaneous activity in vivo, to fire broad action potentials followed by prominent, slow afterhyperpolarization, and to be hyperpolarized and inhibited by serotonin agonists that we now recognize as acting at 5-HT1A receptors.17−20 These characteristics made it possible to identify serotonergic neurons in vitro and in vivo, and over time, they came to be viewed as necessary and sufficient for serotonergic identity. This probably contributed to the idea that serotonergic neuron in the DRN constituted a homogeneous cell population.
More recent work, however, questioned the notion that serotonergic neurons form a homogeneous class in terms of electrophysiological properties. One line of investigation suggested that the application of the criteria outlined above may be unduly restrictive and, thus, subpopulations of serotonergic neurons could be overlooked.21−25 These studies provided evidence that some serotonergic neurons may not fire as regularly as dictated by the canonical classification. In addition, serotonergic neurons in different subareas of the DRN have been reported to exhibit somewhat different electrophysiological properties and 5-HT1A receptor sensitivity.26 A recent study also reported on the existence of a subpopulation of serotonin neurons in the DRN lacking 5-HT1A receptors (Fernandez et al., this issue). These studies hint at the possibility of different functional classes of serotonergic neurons. Consistent with such a possibility, several studies have examined the firing of presumed serotonergic neurons across the sleep–wake cycle or in response to a variety of stimuli.24,27,28 These studies report considerable heterogeneity among physiologically identified serotonergic neurons, again supporting the idea of heterogeneity within the DRN. Thus, anatomical and electrophysiological studies converge in supporting the idea of neuronal heterogeneity in the DRN. But is the evidence sufficient to conclude that the DRN contains different subtypes of serotonergic neurons? Alternately, could reported variability in electrophysiological properties simply reflect variability within a single cellular lineage or a single cell type?
A key issue is whether different features suggesting multiple serotonin DRN neuronal subtypes cosegregate to define specific cell types. For example, do morphologically identified serotonergic cell types correspond to electrophysiologically identified cell types? Unfortunately, this is currently unknown. One potential limitation is that classical electrophysiological identification criteria may not only fail to detect all serotonergic neurons of the DRN but they may also misidentify a subpopulation of nonserotonergic neurons.29−31 This makes it difficult to interpret studies relying solely on electrophysiological identification of serotonergic neurons. To circumvent this problem, some studies have used juxtacellular labeling of the recorded neurons followed by post hoc identification. This is by necessity a low throughput approach, yet results to date support the idea that serotonergic neurons may exhibit greater heterogeneity in terms of their electrophysiological properties than allowed by the classical criteria.24,28 However, whether electrophysiologically defined serotonergic “cell types” correspond to specific cell types defined based on morphology or molecular markers remains an open question. The recent development of optogenetic approaches enabling the unambiguous identification of genetically defined neurons during electrophysiological recordings in vivo32 is likely to allow for rapid progress in the regard.
Cell fating can provide insights into the origin of cellular heterogeneity. Serotonergic neurons arise from Nkx2.2+ progenitors in the ventral hindbrain in two distinct cell clusters separated by rhombomere 4.33 The anterior cell group is derived from rhombomeres 1–3 and gives rise to the B4–B9 serotonergic cell groups, while the posterior cell group is derived from rhombomeres 5 and 6 and develops into the B1–B3 serotonergic cell groups. Whole genome profiling has convincingly shown that serotonergic neurons arising from the anterior and posterior groups reflect implementation of substantially different genetic programs.34 Thus, serotonin neurons emerging from the anterior versus posterior cell clusters need to be regarded as two distinct classes of serotonergic neurons.
Should the serotonergic neurons making up the B4–B9 cell groups be further subdivided? In an elegant study, Jensen et al.4 used intersectional and subtractive genetic cell fating strategies to map how rhombomere-defined serotonin progenitor cells give rise to different serotonergic cell groups. This study showed that the DRN arises in toto from rhombomere 1, thus failing to provide evidence, at least at this level, for heterogeneity in the cell lineage giving rise to this nucleus. In contrast, serotonergic neurons of the median raphe nucleus (MRN) were shown to arise from rhombomeres 1, 2, and 3, and thus may represent functionally different lineages. Interestingly, previous studies have reported differences between the neuronal properties of the DRN and MRN.31 It is tempting to speculate that the MRN neuronal population sampled in this study may have corresponded to rhombomere 2–3 derived neurons. The availability of mice carrying intersectionally tagged neurons from these cell populations should greatly facilitate further exploration of this issue.
Several transcription factors, including LMX1b and Pet-1, have been shown to play an important role in terminal neuronal differentiation giving rise to the serotonergic neuronal phenotype.35,36 LMX1b is thought to act upstream of Pet-1, and deletion of LMX1b results in complete loss of serotonergic neurons.37 In contrast, deletion of Pet-1 results in partial loss of serotonin neurons (∼80%) in both the anterior and posterior cell groups.38 A careful analysis of the surviving serotonergic neurons has shown that they selectively project to a small subset of areas, while other areas remain devoid of serotonergic innervation.39 In the case of the DRN, the projection to the basolateral amygdala appeared to remain quantitatively intact after deletion of Pet-1, while the projection to the anterior cingulate cortex was nearly completely eliminated. These findings have been interpreted to indicate the existence of a distinct subpopulation of serotonergic neurons whose development is not regulated by Pet-1. While this interpretation offers a plausible explanation for the “sparing” of specific projections, this same study found significant changes in the axonal morphology and synaptic ultrastructure of the surviving synapses. Thus, it is not clear that the neurons present after Pet1-deletion necessarily correspond to a specific subpopulation of serotonergic neurons. For example, serotonin neuron survival after Pet-1 deletion could be random but the innervation of specific targets be sequentially determined. This could account for the persistent innervation of specific targets. Alternatively, it is possible that a subpopulation of serotonergic neurons survives and then distributes itself ectopically among the different serotonergic nuclei. This would explain the survival of a relatively constant fraction of neurons in the different serotonergic cell groups.38 Identification of specific molecular markers for the Pet-1 deletion-resistant cell population will be required to distinguish between these possibilities.
In summary, converging evidence derived from a range of technical approaches leaves little doubt that the column of serotonergic cell bodies extending from the B1 to the B9 cell groups can be subdivided into at least two and probably more lineages giving rise to functionally distinct classes of serotonin neurons. Furthermore, recent cell fating studies strongly suggest that some serotonergic nuclei, especially those of the MRN may be heterogeneous and composed of two or more lineages.
As electrophysiologists working on the DRN, our main interest has been the possibility that the serotonergic neurons in this cell group might be classified into distinct subtypes. While there is abundant evidence for functional heterogeneity among DRN cells, it is important to distinguish between differences reflecting separate lineages, and hence genetic programs, and differences resulting from the insertion of neurons into specific neuronal networks. In our view, the evidence to date does not unambiguously support the idea that the serotonergic neurons of the DRN represent more than a single cell lineage. This view may change as we learn more about serotonin neuronal specification, especially during its later steps. However, at this point, we see no clear evidence for more than a single lineage giving rise to the serotonergic neurons of the DRN. Therefore, siding with the “lumpers”, we argue that the reported functional differences in serotonergic neurons of the DRN appear more likely to reflect differences in history and network connectivity rather than the presence of multiple cell types in the DRN.
Acknowledgments
We would like to thank Dr. Sheryl G. Beck for helpful discussions during the preparation of this viewpoint.
Work in the authors’ laboratories is supported by NIH grants MH43985 and MH 078009.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
References
- Halliday G., Harding A., and Paxinos G. (1995) Serotonin and Tachykinin systems. In The rat nervous system (Paxinos G., Ed.), 2nd ed., pp 929–974, Academic Press, San Diego. [Google Scholar]
- Dahlstrom A.; Fuxe K. (1965) Evidence for the existence of monoamine-containing neurons in the central nervous system. I. demonstration of monoamines in the cell bodies of brain stem neurons. Acta Physiol. Scand. 62(suppl 232), 1–55. [PubMed] [Google Scholar]
- Gaspar P.; Lillesaar C. (2012) Probing the diversity of serotonin neurons. Philos. Trans. R. Soc. London, Ser. B 367, 2382–2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen P.; Farago A. F.; Awatramani R. B.; Scott M. M.; Deneris E. S.; Dymecki S. M. (2008) Redefining the serotonergic system by genetic lineage. Nat. Neurosci. 11, 417–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray R. S.; Corcoran A. E.; Brust R. D.; Kim J. C.; Richerson G. B.; Nattie E.; Dymecki S. M. (2011) Impaired respiratory and body temperature control upon acute serotonergic neuron inhibition. Science 333, 637–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Marco Garcia N. V.; Karayannis T.; Fishell G. (2011) Neuronal activity is required for the development of specific cortical interneuron subtypes. Nature 472, 351–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karayannis T.; De Marco Garcia N. V.; Fishell G. J. (2012) Functional adaptation of cortical interneurons to attenuated activity is subtype-specific. Front. Neural Circuits 6, 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelsen K. A.; Schmitz C.; Steinbusch H. W. (2007) The dorsal raphe nucleus--from silver stainings to a role in depression. Brain Res. Rev. 55, 329–342. [DOI] [PubMed] [Google Scholar]
- Diaz-Cintra S.; Cintra L.; Kemper T.; Resnick O.; Morgane P. J. (1981) The effects of protein deprivation on the nucleus raphe dorsalis: a morphometric Golgi study in rats of three age groups. Brain Res. 221, 243–255. [DOI] [PubMed] [Google Scholar]
- Descarries L.; Watkins K. C.; Garcia S.; Beaudet A. (1982) The serotonin neurons in nucleus raphe dorsalis of adult rat: a light and electron microscope radioautographic study. J. Comp. Neurol. 207, 239–254. [DOI] [PubMed] [Google Scholar]
- Steinbusch H. W.; Nieuwenhuys R.; Verhofstad A. A.; Van der Kooy D. (1981) The nucleus raphe dorsalis of the rat and its projection upon the caudatoputamen. A combined cytoarchitectonic, immunohistochemical and retrograde transport study. J. Physiol. 77, 157–174. [PubMed] [Google Scholar]
- Chan-Palay V.; Jonsson G.; Palay S. L. (1978) Serotonin and substance P coexist i, neurons of the rat’s central nervous system. Proc. Natl. Acad. Sci. U.S.A. 75, 1582–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachidian P.; Poulat P.; Marlier L.; Privat A. (1991) Immunohistochemical evidence for the coexistence of substance P, thyrotropin-releasing hormone, GABA, methionine-enkephalin, and leucin-enkephalin in the serotonergic neurons of the caudal raphe nuclei: a dual labeling in the rat. J. Neurosci. Res. 30, 521–530. [DOI] [PubMed] [Google Scholar]
- Xu Z. Q.; Zhang X.; Pieribone V. A.; Grillner S.; Hokfelt T. (1998) Galanin-5-hydroxytryptamine interactions: electrophysiological, immunohistochemical and in situ hybridization studies on rat dorsal raphe neurons with a note on galanin R1 and R2 receptors. Neuroscience 87, 79–94. [DOI] [PubMed] [Google Scholar]
- Commons K. G.; Connolley K. R.; Valentino R. J. (2003) A neurochemically distinct dorsal raphe-limbic circuit with a potential role in affective disorders. Neuropsychopharmacology 28, 206–215. [DOI] [PubMed] [Google Scholar]
- Fu W.; Le Maitre E.; Fabre V.; Bernard J. F.; David Xu, Z. Q.; Hokfelt T. (2010) Chemical neuroanatomy of the dorsal raphe nucleus and adjacent structures of the mouse brain. J. Comp. Neurol. 518, 3464–3494. [DOI] [PubMed] [Google Scholar]
- Aghajanian G. K.; Foote W. E.; Sheard M. H. (1968) Lysergic acid diethylamide: sensitive neuronal units in the midbrain raphe. Science 161, 706–708. [DOI] [PubMed] [Google Scholar]
- Aghajanian G. K.; Vandermaelen C. P. (1982) Intracellular identification of central noradrenergic and serotonergic neurons by a new double labeling procedure. J. Neurosci. 2, 1786–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghajanian G. K.; Vandermaelen C. P. (1982) Intracellular recordings from serotonergic dorsal raphe neurons: pacemaker potentials and the effect of LSD. Brain Res. 238, 463–469. [DOI] [PubMed] [Google Scholar]
- Li Y. Q.; Li H.; Kaneko T.; Mizuno N. (2001) Morphological features and electrophysiological properties of serotonergic and non-serotonergic projection neurons in the dorsal raphe nucleus. An intracellular recording and labeling study in rat brain slices. Brain Res. 900, 110–118. [DOI] [PubMed] [Google Scholar]
- Hajos M.; Gartside S. E.; Villa A. E.; Sharp T. (1995) Evidence for a repetitive (burst) firing pattern in a sub-population of 5-hydroxytryptamine neurons in the dorsal and median raphe nuclei of the rat. Neuroscience 69, 189–197. [DOI] [PubMed] [Google Scholar]
- Hajos M.; Sharp T.; Newberry N. R. (1996) Intracellular recordings from burst-firing presumed serotonergic neurones in the rat dorsal raphe nucleus in vivo. Brain Res. 737, 308–312. [DOI] [PubMed] [Google Scholar]
- Ranade S. P.; Mainen Z. F. (2009) Transient firing of dorsal raphe neurons encodes diverse and specific sensory, motor, and reward events. J. Neurophysiol. 102, 3026–3037. [DOI] [PubMed] [Google Scholar]
- Schweimer J. V.; Mallet N.; Sharp T.; Ungless M. A. (2011) Spike-timing relationship of neurochemically-identified dorsal raphe neurons during cortical slow oscillations. Neuroscience 196, 115–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajos M.; Allers K. A.; Jennings K.; Sharp T.; Charette G.; Sik A.; Kocsis B. (2007) Neurochemical identification of stereotypic burst-firing neurons in the rat dorsal raphe nucleus using juxtacellular labelling methods. Eur. J. Neurosci. 25, 119–126. [DOI] [PubMed] [Google Scholar]
- Calizo L. H.; Akanwa A.; Ma X.; Pan Y. Z.; Lemos J. C.; Craige C.; Heemstra L. A.; Beck S. G. (2011) Raphe serotonin neurons are not homogenous: electrophysiological, morphological and neurochemical evidence. Neuropharmacology 61, 524–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbain N.; Creamer K.; Debonnel G. (2006) Electrophysiological diversity of the dorsal raphe cells across the sleep-wake cycle of the rat. J. Physiol. 573, 679–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocsis B.; Varga V.; Dahan L.; Sik A. (2006) Serotonergic neuron diversity: identification of raphe neurons with discharges time-locked to the hippocampal theta rhythm. Proc. Natl. Acad. Sci. U.S.A. 103, 1059–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allers K. A.; Sharp T. (2003) Neurochemical and anatomical identification of fast- and slow-firing neurones in the rat dorsal raphe nucleus using juxtacellular labelling methods in vivo. Neuroscience 122, 193–204. [DOI] [PubMed] [Google Scholar]
- Schweimer J. V.; Ungless M. A. (2010) Phasic responses in dorsal raphe serotonin neurons to noxious stimuli. Neuroscience 171, 1209–1215. [DOI] [PubMed] [Google Scholar]
- Beck S. G.; Pan Y. Z.; Akanwa A. C.; Kirby L. G. (2004) Median and dorsal raphe neurons are not electrophysiologically identical. J. Neurophysiol. 91, 994–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Y.; Haesler S.; Vong L.; Lowell B. B.; Uchida N. (2012) Neuron-type-specific signals for reward and punishment in the ventral tegmental area. Nature 482, 85–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattyn A.; Vallstedt A.; Dias J. M.; Samad O. A.; Krumlauf R.; Rijli F. M.; Brunet J. F.; Ericson J. (2003) Coordinated temporal and spatial control of motor neuron and serotonergic neuron generation from a common pool of CNS progenitors. Genes Dev. 17, 729–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wylie C. J.; Hendricks T. J.; Zhang B.; Wang L.; Lu P.; Leahy P.; Fox S.; Maeno H.; Deneris E. S. (2010) Distinct transcriptomes define rostral and caudal serotonin neurons. J. Neurosci. 30, 670–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flames N.; Hobert O. (2011) Transcriptional control of the terminal fate of monoaminergic neurons. Annu. Rev. Neurosci. 34, 153–184. [DOI] [PubMed] [Google Scholar]
- Cordes S. P. (2005) Molecular genetics of the early development of hindbrain serotonergic neurons. Clin. Genet. 68, 487–494. [DOI] [PubMed] [Google Scholar]
- Ding Y. Q.; Marklund U.; Yuan W.; Yin J.; Wegman L.; Ericson J.; Deneris E.; Johnson R. L.; Chen Z. F. (2003) Lmx1b is essential for the development of serotonergic neurons. Nat. Neurosci. 6, 933–938. [DOI] [PubMed] [Google Scholar]
- Hendricks T. J.; Fyodorov D. V.; Wegman L. J.; Lelutiu N. B.; Pehek E. A.; Yamamoto B.; Silver J.; Weeber E. J.; Sweatt J. D.; Deneris E. S. (2003) Pet-1 ETS gene plays a critical role in 5-HT neuron development and is required for normal anxiety-like and aggressive behavior. Neuron 37, 233–247. [DOI] [PubMed] [Google Scholar]
- Kiyasova V.; Fernandez S. P.; Laine J.; Stankovski L.; Muzerelle A.; Doly S.; Gaspar P. (2011) A genetically defined morphologically and functionally unique subset of 5-HT neurons in the mouse raphe nuclei. J. Neurosci. 31, 2756–2768. [DOI] [PMC free article] [PubMed] [Google Scholar]


