Abstract
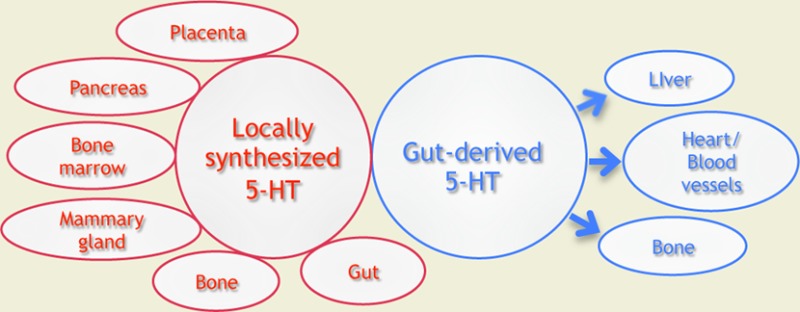
Since its identification, 75 years ago, the monoamine serotonin (5-HT) has attracted considerable attention toward its role as a neurotransmitter in the central nervous system. Yet, increasing evidence, from a growing number of research groups, substantiates the fact that 5-HT regulates important nonneuronal functions. Peripheral 5-HT, synthesized by the enzyme tryptophan hydroxyase (Tph) in intestinal cells, was assumed to be distributed throughout the entire body by blood platelets and to behave as a pleiotropic hormone. A decade ago, generation of a mouse model devoid of peripheral 5-HT lead to the discovery of a second isoform of the enzyme Tph and also suggested that 5-HT might act as a local regulator in various organs. The objective of this review is to highlight the newly discovered functions played by the monoamine using the Tph1 KO murine model and to outline current findings that led to the discovery of complete serotonergic systems in unexpected organs. Within an organ, both the presence of local Tph enzymatic activity and serotonergic components are of particular importance as they support the view that 5-HT meets the criteria to be qualified as a monoamine with a paracrine/autocrine function.
Keywords: Serotonin, serotonergic network, tryptophan hydroxylase 1, mouse model
Dichotomy of 5-HT Synthesis Systems
In 1937, Erspamer and Asero identified an amine from enterochromaffin cells of the gastrointestinal (GI) epithelium which made intestines contract and named it enteramine.1 Years later, in 1948, Rapport and colleagues discovered a vasoconstrictor substance in blood, and, as it was a serum agent affecting vascular tone, named it serotonin (5-hydroxytryptamine, 5-HT).2 It took 4 more years to recognize that enteramine and 5-HT were in fact the same molecule, and soon after, Twarog and Page identified 5-HT in the brain of mammals.1,3 This finding led to the recognition of 5-HT as an important neurotransmitter and stimulated interest in the role of the amine in regulating brain functions, even though only a small percentage of the body’s 5-HT (∼5%) can be found in the brain while most of its content (∼95%) is found in peripheral tissues.4
5-HT is synthesized by a 2-step enzymatic pathway in which tryptophan is first converted into 5-hydroxy-tryptophan (5-HTP) by the enzyme tryptophan hydroxylase (Tph).5 The intermediate product (5-HTP) is next converted to 5-HT by a ubiquitous aromatic l-amino acid decarboxylase (AADC). Tph catalyzes the rate-limiting step in the biosynthesis of 5-HT and, until the discovery of the second isoform, Tph enzymatic activity and 5-HT synthesis were reported to be mainly expressed in enterochromaffin cells of the gut, neurons of the raphe, the myenteric plexus of the gut, and pinealocytes of the pineal gland.6−8 Furthermore, the classical view indicated that circulating 5-HT synthesized by enterochromaffin cells of the gut was actively incorporated into platelets and distributed throughout the body like a hormone. It was also assumed that a single gene encoded the Tph enzyme, albeit 5-HT was implicated in a variety of physiological processes ranging from cardiovascular regulation, thermoregulation, to the sleep-wake cycle, appetite, aggression, sexual behavior, and learning.9 Yet, in 2003, Tph KO mice were generated and they expressed normal levels of brain 5-HT, showed no significant differences in 5-HT-related behaviors, but had nearly no peripheral 5-HT.10,11 Once assumed to be a single gene product, Tph was revealed to exist in two isoforms derived from two different genes that share high homology in their amino acid content both in mouse and human.10 Ten years later, a number of studies have characterized the spatial and temporal distribution of Tph1 and Tph2 in rodents and human using RT-PCR, in situ hybridization, and immnohistochemistry with specific mRNA probes and antibodies against both isoforms. The newly discovered Tph2 is mostly restricted to neuronal cells while Tph1 (formely known as Tph) is broadly expressed in nonneuronal tissues. Throughout development, and in adult life, there is no appreciable overlap in the expression of the two isoforms as well as no compensatory activation of Tph1 or Tph2 expression.11−17
We describe below some unexpected roles played by 5-HT in peripheral tissues that were discovered using the Tph1 KO mouse model. We also illustrate how the model helped to better understand previously recognized peripheral functions of 5-HT. Thorough analysis of this model led to the discovery of complete serotonergic systems in unexpected locations. Within an organ, the presence of such a complete network is of particular importance because it emphasizes the link between local 5-HT synthesis and a precise function within the organ, rather than considering 5-HT as a pleiotropic hormone synthesized in the gut and distributed throughout the entire organism. We will not present the data concerning the cardiovascular system, as an excellent review was published recently.18
Early Source of 5-HT: Maternal, Embryonic, Placental Origins?
Presence of 5-HT, its receptors, and the serotonin transporter (SERT) in preimplantation embryos and the ability of 5-HT-specific pharmacological agents to interfere with development suggested that 5-HT functions at the earliest stages of development.19−22 However, the source of 5-HT acting on those targets has eluded identification until very recently. In 1999, before the discovery of a second Tph isoform, a report showed that 5-HT was present in embryonic stem cells and that a Tph protein was detected in mouse zygotes soon after fertilization, suggesting that the embryo itself has the capacity to synthesize 5-HT.23 Still, in 2005, another report showed that Tph1 was not expressed by the embryo and concluded that embryonic 5-HT is not synthesized by the embryo itself, but captured from the surrounding milieu by SERT, expressed and functional throughout preimplantation development.20 The apparent discrepancy in these results was reconciled when Basu and colleagues demonstrated that a Tph2 mRNA is present in oocytes and 2-cell embryos, indicating that the Tph protein detected by Walther and Bader was the Tph2 isoform.24 Unpublished results form our lab (Amireault et al., submitted) confirm the emergence of a growing Tph2 enzymatic activity from the two-cell embryo to the blastocyst stage, showing that a nonneuronal cell type, the totipotent blastomere, can synthesize 5-HT. However, local levels of 5-HT in the reproductive tract of the mother originate from Tph1 since very low levels of 5-HT are detected in the oviduct and uterus of Tph1 KO mothers in the 3 days following fertilization (Figure 1A). Interestingly, the Tph1-derived 5-HT level rises shortly after fertilization in both these tissues implying that preimplantation embryos not only synthesize 5-HT, but also bathe in maternal 5-HT (Figure 1A). Yet, the source of this 5-HT remains to be identified since no Tph enzymatic activity was detected in oviduct or uterus (Figure 1B). Taken together, these results reveal a complex interplay and possible redundancy of mechanisms between the Tph2-derived embryonic source and the Tph1-derived maternal source of 5-HT, to ensure early embryonic development. It is likely that Tph1-derived 5-HT from maternal tissues combines with Tph2-derived 5-HT from the embryo to regulate cleavage divisions and may be involved in implantation as proposed recently.25
Figure 1.
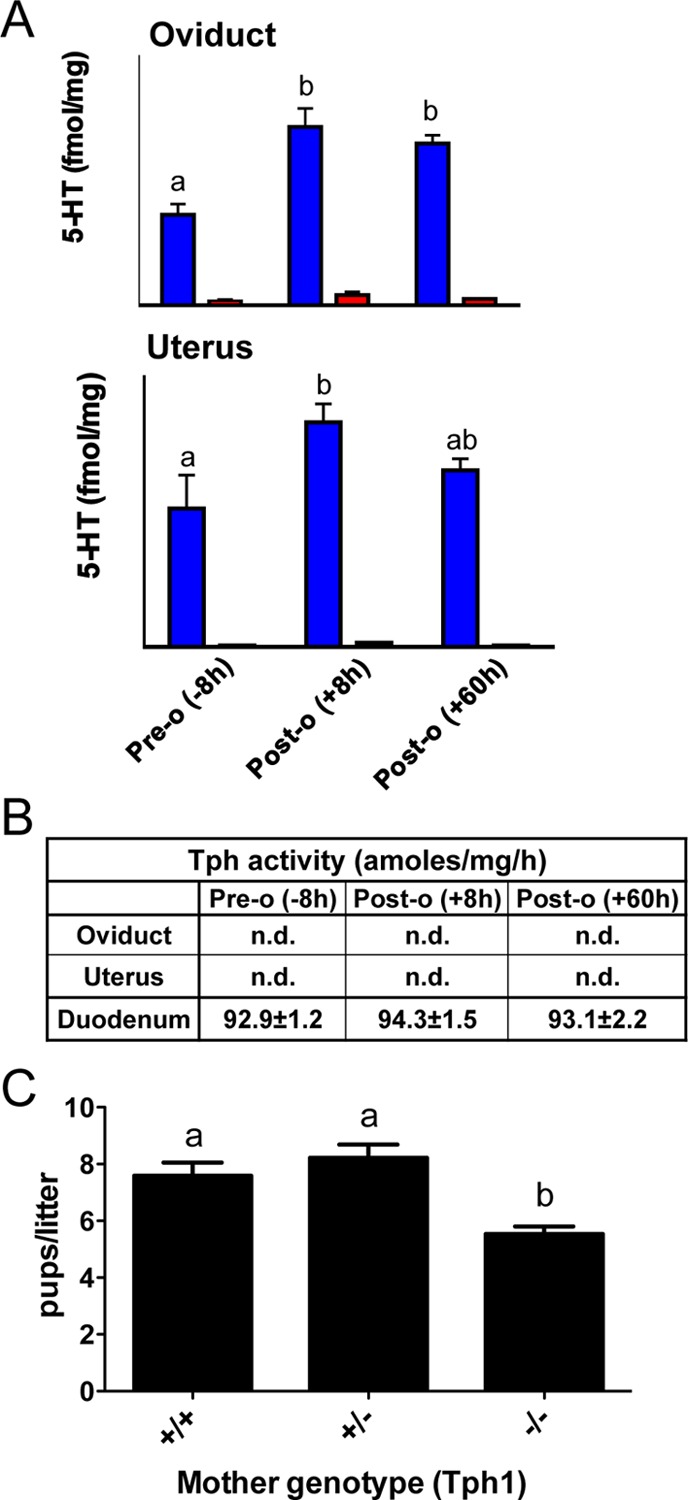
(A) Presence of 5-HT but absence of Tph activity in the reproductive tract of female mice. The 5-HT content of the oviduct (upper panel) and the uterus (lower panel) was evaluated by HPLC in superovulated WT(blue bar; n = 5) or Tph1 KO mice (red bar; n = 5). The 5-HT content of both the oviduct and the uterus increases after ovulation (8 h postovulation), when compared to tissues harvested before ovulation (8 h preovulation), and is greatly reduced in the reproductive tract of Tph1 KO mice. (B) Tph activity is not detected in the oviduct and uterus of superovulated mice at any stage tested. Tph activity was determined by a radioenzymatic assay. Briefly, tissue homogenate was added to a reaction mixture containing 0.05 mM tryptophan, 50 mM Hepes (pH 7.6), 5 mM DTT, 0.01 mM Fe(NH4)2(SO4)2, 0.5 mM 6-MPH4, 0.1 mg/mL catalase, and [3H]-tryptophan (1 mCi/reaction). Unreacted tryptophan and the product 5-HTP were adsorbed with charcoal in 1 M HCl at the end of the incubation. The samples were then centrifuged, the supernatant added to scintillation fluid, and the radioactivity was measured by a liquid scintillation counter. (C) Genetic crosses were performed in which WT males were mated with WT (+/+; n = 12), heterozygous (+/–; n = 9), or Tph1 KO (−/–; n = 13) females and revealed a reduction in the number of pups per litter born from Tph1 KO mothers. In (A) and (C), the letters denote values that are statistically different using a one-way ANOVA followed by a Tukey’s multiple comparison test. P < 0.05 was considered significant.
In comparison, during postimplantation stages of development, Tph1-derived 5-HT was rather hypothesized to function in the regulation of morphogenesis. Yavaronne et al hypothesized that a maternal source of 5-HT was critical for normal murine development during the postimplantation stages that precede appearance of serotonergic neurons.26 Using the Tph1 KO mouse, Côté et al. showed that 5-HT-deficient mothers give birth to pups that are significantly smaller in size than the offspring of heterozygous or wild-type (WT) mice.27 Our recent data demonstrate a reduction in the number of neonates from Tph1 KO mothers implying that embryos are lost in utero (Figure 1C). From these results, Côté and co-workers hypothesized that the defective development was due to a lack of circulating maternal 5-HT in the blood.27 Bonnin and colleagues recently challenged this result. Using Pet1-null mice, in which most dorsal raphe neurons lack 5-HT, and SERT-null mice, lacking 5-HT in the blood, they demonstrated that the exogenous source of 5-HT in the hindbrain is not of maternal origin but originates from the placenta.28 They also showed that maternal tryptophan was the source of placental 5-HT and both mouse and human placenta express Tph1 in the trophoblastic cell layer. Lastly, Bonnin and co-workers argue that 5-HT synthesized by Tph1 in the murine placenta behaves like a hormone, and is critical in fetal brain development to influence behavior later in life.28,29 Aside from this proposal, it is also likely that placental 5-HT has a local role during pregnancy as different groups reported the presence of 5-HT, SERT, and 5-HT receptors in the placenta and trophoblast cell lines, showing that a complete serotonergic network is present.26,30−35 In particular, 5-HT may well play a local role in the placenta through the involvement of SERT and the 5-HT2A receptor in the villous trophoblast and the fetal capillary endothelium of normal term placental tissue.31
Serotonin Network in the Mammary Gland: Role in Homeostasis and Involution
Matsuda et al. found that mouse mammary glands stimulated by prolactin express Tph1 mRNA, which is elevated during pregnancy and lactation.36 Local 5-HT production in the mammary gland is involved in a negative feedback loop limiting milk production and modulating epithelial homeostasis.37,38 Experiments using the Tph1 KO mouse model confirmed these results since 5-HT-deficient mice are hyperesponsive to prolactin and are resistant to milk-stasis involution.36−39 Accordingly, 5-HT is detected in the mammary epithelium, accumulates in the interstitial fluid surrounding it, and activates 5-HT7 receptors to induce a rise in cAMP, leading to a downregulation of a number of milk proteins and opening of tight junctions.37,40 SERT is also expressed in the tissue and modulates 5-HT signaling since its inhibition leads to an involution-like state.37,39,40 Taken together, these results reveal a complete serotonergic network in the mammary gland and this local paracrine/autocrine system does not depend on an indirect source of 5-HT such as circulating platelets.
Tph1-Derived Serotonin in the Gut: What Does It Really Do?
Enterochromaffin cells are the most well characterized subset of GI endocrine cells, are dispersed throughout the GI mucosa and are the main source of 5-HT in the gut.41 As most of the body’s 5-HT is synthesized and stored in the intestine, along with the presence of many different 5-HT receptors within the intestinal wall, it has been widely accepted that 5-HT has a major role in regulating GI function (reviewed in (41)). But, within the gut, presence of both neuronal (Tph2) and nonneuronal (Tph1) sources of 5-HT along with the overlapping distribution of specific 5-HT receptor subtypes have made difficult to identify its precise function. Li and colleagues studied Tph1–/–, Tph2–/– or double KOs to identify the role of neuronal vs nonneuronal 5-HT in the gut.42 Their results were quite surprising and revealed that peripheral 5-HT (Tph1-derived 5-HT: ∼95% of gut 5-HT content) has only a very minor role in regulation of GI motility in the mouse, while neuronal (Tph2 derived 5-HT: ∼5% of gut 5-HT content) has a much more considerable role than previously believed. Tph1 KO mice did not differ from controls in GI functions measured (gastric emptying, total intestinal transit, and colonic motility.42 In comparison, Tph2 KO animals had major changes in each function and the double KOs were indistinguishable from Tph2-null mice. The results of Li et al. indicate that Tph1-derived 5-HT is trivial for normal GI motility. However, since Tph1 is responsible for more than 90% of 5-HT synthesis in the gut, this result was clearly unexpected and as presented below, Tph1-derived 5-HT may be necessary following a pathophysiological insult such as inflammation.
Abundant studies suggest a role for Tph1-derived 5-HT in the pathogenesis of GI diseases by influencing pro-inflammatory mediator production and immune modulation (reviewed in ref (43)). First, serotonergenic receptors have been identified on various immune cells such as type B and T lymphocytes, monocytes, macrophage, and dentritic cells.44,45 Second, León-Ponte and colleagues proposed that Tph1-derived 5-HT synthesized by T cells may act as an autocrine factor and demonstrated a fundamental role for 5-HT as an intrinsic cofactor in T-cell activation and function.46 To address the role of 5-HT in immune activation and regulation of gut inflammation, Ghia et al. studied Tph1 KO mice and hypothesized that 5-HT activates and promotes survival of immune cells.47 Using different models of colitis, they observed a delayed onset and decreased severity of clinical disease together with lower macroscopic and histological damage scores in Tph1–/– mice and mice treated with parachlorophenylalanine (PCPA; an inhibitor of Tph activity), as compared to WT mice after induction of colitis. Along with a down-regulation of macrophage infiltration, they observed a decreased production of pro-inflammatory cytokines. Additional studies by Margolis et al., have shown that inhibition of 5-HT synthesis using the specific inhibitor of Tph1 (LP-533401), reduces the severity of trinitrobenzene sulfonic acid-induced colitis in mice.48 This finding further supports the previous observation made using Tph1 KO mice that 5-HT is a critical molecule in the pathogenesis of colitis.
Altogether, the analysis of Tph1–/– mutant mice provided crucial information for the understanding of the GI dual serotonergic system and revealed a precise role for Tph1-derived 5-HT in the pathogenesis of GI disease while the motility of the GI tract depends on Tph2-derived 5-HT. Yet again, based on the fact that most of the body’s 5-HT is produced by Tph1, secreted in the intestine, stored in platelets and is the source of all circulating 5-HT, one can propose that Tph1-derived 5-HT has other remaining functions yet to be described.
Emerging role for 5-HT in the Pancreas and Its Implication in Diabetes
Pancreatic β-cells produce and release insulin and make up the majority of cells found in islets of Langerhans of the pancreas. Colocalization of 5-HT and insulin in secretory β-granules of pancreatic β-cells, along with the fact that they are coreleased when pancreatic islets are stimulated,49 led scientists to design experiments to define the role played by 5-HT in the pancreas.
In 2009, in an attempt to elucidate physiological factors important for driving β-cell-specific expansion during pregnancy, Rieck et al. identified nearly 2000 genes that were differentially expressed in mice islets on E14.5 of gestation when compared with non pregnant controls and, surprisingly, Tph1 mRNA was increased by almost 20-fold.50 In mammals, the onset of pregnancy causes a female to double the number of insulin-producing islet cells in her pancreas as more insulin is needed to support growth and energy homeostasis of the mother.51 Thus, this increase in the level of Tph1 mRNA at this crucial stage of pancreatic development suggested a role for 5-HT in the expansion of β-cells.
In line with the previous results, two independent research groups demonstrated that, during pregnancy, lactogenic signaling promotes Tph1 expression in β-cells and an increase in 5-HT synthesis and storage in islets.52,53 In β-cells and in pregnant mice, the expression of the 5-HT2B increased significantly from E6 through E15 to stimulate β-cell proliferation while 5-HT1D expression increased at the end of gestation and postpartum to generate an inhibitory signal capable of reducing β-cell proliferation and β-cell mass.53 In their study, the authors provided evidence for a paracrine/autocrine role played by 5-HT (secreted by islet cells) that primarily impact local 5-HT concentrations. Further support for this local 5-HT synthesis in β-cells of the pancreas came from the work of Paulmann and colleagues. They studied Tph1-deficient mice to demonstrate that inhibition of 5-HT production causes the β-cells to stop proliferating and lead to diabetes in adult mice.54 The authors showed that, under normal conditions, 5-HT controls insulin release through the hormone prolactin which activates mRNA for tph1 in β-cells.54 Next, 5-HT stimulates serotonergic receptors (5-HT1A) on β-cells and induces their proliferation, generating the increase in insulin. In addition to the receptor signaling pathway, the authors proposed serotonylation as the underlying molecular mechanism that regulates secretion of storage granules from β-cells.
The Role of Serotonin in Bone Biology
Bone remodeling is a delicate balance between bone resorption by osteoclasts and bone formation by osteoblasts. Close cooperation between these two cell types along with the action of several molecules and cytokines are needed to achieve proper rates of growth and differentiation required for physiological processes.55 5-HT might be such a molecule as both in mouse and human, alterations in 5-HT levels and signaling have been shown to regulate bone remodelling.56,57 In addition, in vitro studies also reported the existence of 5-HT receptors and a functional SERT in primary bone cells or in bone cell lines.56,58−62
Results from Gerard Karsenty’s group using loss- and gain-of-function mutations in mouse (Tph1, 5-HT1B, LRP5) argue that “gut-derived serotonin” is a powerful inhibitor of osteoblast proliferation and bone formation without any effect on bone resorption.63−65 More precisely, circulating 5-HT stored in platelets reduced osteoblast proliferation through the activation of 5-HT1B receptors present on osteoblasts. They showed that 5-HT synthesized in the gut by Tph1 is regulated by the LDL receptor-related protein 5 (LRP5) gene. Lrp5 encodes a cell-surface molecule assumed to be a coreceptor for Wnt proteins.66 Individuals with the Osteoporosis-Pseudoglioma syndrome, a low bone mass disorder, have loss-of-function mutations in the LRP5 gene while heterozygous missense mutations in the gene have been observed in individuals with dominantly inherited high bone mass.67 The authors hypothesized that the role of LRP5 in bone is not owed to the expression of this gene in cells of the osteoblast lineage but is dependent on 5-HT synthesis by the gut, which is regulated by LRP5. In comparison, Cui et al. inactivated the LRP5 gene specifically in the intestine, and observed no significant effect on bone mass and thereby concluded that, in mice, LRP5 functions via the canonical Wnt pathway in osteocytes to regulate bone mass rather than regulating bone mass indirectly via the gut as proposed by Karsenty and colleagues. Cui and co-workers also used mice with a global Tph1 knockout and observed no change in bone density at 4 months of age. Recently, Chabbi-Achengli et al., using mice with a constitutive Tph1 gene inactivation, provided evidence that a local serotoninergic system expressed in osteoclasts regulates bone remodeling.68 The authors observed an elevated bone density in young Tph1–/– animals that returns to normal at maturity68 in agreement with the above-mentioned report of Cui et al. studying mice with a global Tph1 KO. They also showed that Tph1-derived 5-HT produced by osteoclasts in the bone could act locally on both osteoclasts and osteoblasts.68 In vivo experiments demonstrated that WT bone marrow cells transplanted in Tph1–/– mice retard the deficit in bone resorption, suggesting that an intrinsic osteoclast defect is responsible for the abnormal bone phenotype.
The issue of 5-HT and bone biology is still controversial. Whether osteoporosis could be prevented in mice with a drug that blocks production of “gut-derived serotonin” or whether “gut-derived serotonin” has no effect on bone or whether osteoclasts are able to synthetize 5-HT that acts locally to induce osteoclast precursors differentiation awaits further studies. Possible explanations for the contradicting results include the mouse model used and the age of the animal in the different experimental setups. For instance, the work by Karsenty and colleagues could not and did not observe the same results as the one published by Chabbi-Achengli and colleagues as the former analyzed only specific invalidation of Tph1 in the gut. However, part of the results described in the study by Cui et al., showing that mice with a global Tph1 KO had no change in bone density at 4 months of age, are in agreement with the data of Chabbi-Achengli et al., that observed, in Tph1 mutant mice, an increase in bone mass that is resolved at maturity.
Role of Serotonin in Liver Regeneration
Partial hepatectomy has been widely used as an experimental model in order to gain a deeper understanding of the mechanisms underlying liver regeneration. The number of platelets (filled with 5-HT) strongly correlates with hepatocyte proliferative capacity.69 It has also been demonstrated that 5-HT, through activation of a 5-HT2 receptor subtype, could act as a potent hepatocyte mitogen and induce DNA synthesis in primary cultures of rat hepatocytes.70,71
Lesurtel et al. showed in mice that, 2 days after hepatectomy, a 3- to 4-fold up-regulation of 2A and 2B receptor expression is observed.72 Also, hepatocyte proliferation is reduced when a 5-HT2 receptor antagonist is used, suggesting that both receptor subtypes mediate 5-HT-dependent regeneration. To test directly the function of 5-HT in liver regeneration, partial hepatectomy was performed on Tph1–/– and WT control mice. In hepatectomized Tph1–/– mice, all markers of hepatocyte proliferation were reduced suggesting that a molecular action of 5-HT is involved in the induction of hepatoctye proliferation after a major loss of hepatic tissue.69,72 In contrast, studies in SERT-deficient rats revealed that the regenerative process is not influenced when the platelet 5-HT level is greatly reduced.73 The authors proposed that liver regeneration is not dependent on the release of 5-HT from platelets but rather on residual levels of 5-HT (1–5%) in blood serum. These results demand cautious interpretation and, even though they clearly demonstrate the implication of 5-HT in liver regeneration, they leave open the question of the source of 5-HT responsible for liver regeneration. For example, plasma-derived 5-HT might play a role in liver regeneration that is overshadowed by abundant platelets-derived 5-HT. On the other hand, part of the 5-HT required for liver regeneration could originate from local 5-HT synthesis in hepatocytes that would have been inactivated in the study using the Tph1 KO,72 but not in the one using the SERT KO.73 In any event, additional studies will be needed to identify the source(s) of 5-HT synthesis involved in this important physiological function.
Serotonin and Red Blood Cell Production and Survival
Little is known regarding the mechanism of action of 5-HT during hematopoiesis. 5-HT was hypothesized long ago to have a strong erythropoietic effect in mice through the stimulation of the 5-HT2 receptor.74,75 Published data also suggested that a serotonergic network exists in murine hematopoietic organs and a number of reports argue in favor of a possible effect of 5-HT on hematopoiesis. For example, 5-HT has been shown to stimulate megakaryocyte colony formation whereas 5-HT2 receptor antagonists block the mitogenic effect of 5-HT on megakaryocytopoiesis.76 More recently, it has been reported that in early stages of megakaryopoiesis, 5-HT binds to 5-HT2B receptors on megakaryocytes to promote their proliferation and differentiation.77 A more detailed study demonstrated that 5-HT significantly enhance the expansion of CD34+ cells to early stem/progenitors and multilineage committed progenitors.78 5-HT alone or in addition to fibroblast growth factor, platelet-derived growth factor, or vascular endothelial growth factor stimulated bone marrow colony-forming unit fibroblast formation.78
We revealed a pivotal role for 5-HT in erythropoiesis and red blood cell survival using the Tph1–/– KO mouse.79 Our data established that 5-HT regulates in vivo erythropoiesis as 5-HT–deficient animals present a phenotype of macrocytic anemia due to an ineffective erythropoiesis. In the bone marrow, absence of 5-HT down-regulates the rate of red blood cell production since erythroid precursors progression toward terminal differentiation is hampered. In vitro experiments with isolated Tph1–/– erythroid precursors showed they have a decreased proliferative capacity, that is restored by addition of 5-HT, suggesting they possess the capacity to synthesize 5-HT.79 Further evidence to support a direct role for 5-HT in erythroid cells came from binding experiments demonstrating the presence of 5-HT2A and 5-HT2B receptors and SERT on erythroid precursors. On the basis of our data, although the definitive presence of Tph1 has yet to be proven, we propose that a local paracrine/autocrine serotonergic network exists in erythroid cells. Contribution of this potential local source of 5-HT and identification of other cell types expressing Tph1 in other hemapoietic lineages should be an intense field of research in the coming years.
Serotonylation
5-HT is abundant in the blood and particularly in the dense granules of the platelets. It was believed that 5-HT helps in the coagulation process by activating 5-HT receptors expressed by platelets. However, Walther et al., using the Tph1 KO mouse, revealed a receptor-independent signaling pathway by post-translational modification of intracellular proteins.80 This process was named serotonylation and is proposed to be mediated by the enzyme transglutaminase that creates glutamyl-amide bonds by covalently attaching 5-HT to glutamine residues of intracellular proteins. Such serotonylation of small GTPases mediates exocytosis of platelet alpha granules,80 insulin release from pancreatic beta cells,54 proliferation and migration of pulmonary artery smooth muscle cells81 and contraction of vascular smooth muscle cells.82
Conclusion
A hormone is defined as a chemical released by a cell in one part of the body that contains chemical signals that affect distant cells in other parts of the organism. Given the classical view that circulating 5-HT synthesized by the intestinal cells was actively incorporated and stored into platelets and then distributed throughout the entire body, 5-HT was regarded as a pleiotropic hormone. The discovery, 10 years ago, of a second isoform of the enzyme Tph and the generation of mice devoided of peripheral 5-HT challenged that view. Table 1 presents the current findings uncovered by the analysis of the Tph1 KO mouse model. Perhaps one of the most interesting findings is the presence of 5-HT production in new cell types with paracrine/autocrine effects within the organ that does not depend on an indirect source of 5-HT such as circulating platelets. In addition, as outlined in this review, analysis of the Tph1 KO mouse model provided a better understanding of previously recognized functions and helped in the identification of novel roles of peripheral 5-HT. With regard to the newly identified sources and functions of Tph1-derived 5-HT, one important aspect to be considered is a potential interplay of superimposed actions of 5-HT. For instance, during pregnancy and embryonic development, one should not overlook the fact that the overall state of the Tph1–/– female (anemia, diabetes, bone remodeling defect, etc.) and any kind of growth restriction or gestational phenotypes might well be responsible for the embryonic phenotype. In summary, the presence of a number of serotonergic systems disseminated throughout the body makes the study of 5-HT-related physiological functions complex. The testing of new hypotheses and analysis of additional animal models should extend our comprehension of 5-HT-related roles and lead to a better uderstanding of the hormonal and/or autocrine/paracrine nature of Tph1-derived 5-HT.
Table 1. Hormonal and/or Paracrine/Autocrine Nature of Tph1-Derived 5-HT through Analysis of the Tph1 KO Mouse Model Lead to the Discovery of Complete Serotonergic Systems (synthesis, uptake, and receptors) in Unexpected Locationsa.
| source of 5-HT |
|||
|---|---|---|---|
| organ/tissue | local synthesis | gut-derived | suggested function |
| embryo | Tph2 | survival factor24 | |
| placenta | Tph1 | hindbrain development28 | |
| mammary gland | Tph1 | involution and homeostasis36,37 | |
| bone marrow | Tph1 | erythroblast differentiation/proliferation79 | |
| gut (enterochromaffin cells) | Tph1 | immune modulation46,47 | |
| gut (myenteric neurons) | Tph2 | intestinal motility42 | |
| pancreas | Tph1 | β-cells proliferation52−54 | |
| bone | Tph1 | osteoclasts differentiation68 | |
| Tph1 | osteoblasts differentiation63,64 | ||
| liver | Tph1 | hepatocytes proliferation72 | |
| heart/blood vessels | Tph1 | Tph1 | vessels contractility, cardiac myocytes proliferation18 |
Within an organ, the presence of such a complete network highlights the link between local 5-HT synthesis and a precise function. We did not present the data concerning the cardiovascular system, as an excellent review was published recently.18
Author Contributions
Pascal Amireault, Francine Côté designed, performed experiments and analyzed data. Pascal Amireault, David Sibon, and Francine Côté wrote the paper.
The authors declare no competing financial interest.
References
- Erspamer V.; Asero B. (1952) Identification of enteramine, the specific hormone of the enterochromaffin cell system, as 5-hydroxytryptamine. Nature 169, 800–801. [DOI] [PubMed] [Google Scholar]
- Rapport M. M.; Green A. A.; Page I. H. (1948) Crystalline Serotonin. Science 108, 329–330. [DOI] [PubMed] [Google Scholar]
- Twarog B. M.; Page I. H. (1953) Serotonin content of some mammalian tissues and urine and a method for its determination. Am. J. Physiol. 175, 157–161. [DOI] [PubMed] [Google Scholar]
- Gershon M. D. (1999) Review article: roles played by 5-hydroxytryptamine in the physiology of the bowel. Aliment Pharmacol. Ther. 13(Suppl 2), 15–30. [PubMed] [Google Scholar]
- Fitzpatrick P. F. (2003) Mechanism of aromatic amino acid hydroxylation. Biochemistry 42, 14083–14091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legay C.; Faudon M.; Hery F.; Ternaux J. P. (1983) 5-HT metabolism in the intestinal wall of the rat-I. The mucosa. Neurochem. Int. 5, 721–727. [DOI] [PubMed] [Google Scholar]
- Legay C.; Faudon M.; Hery F.; Ternaux J. P. (1983) 5-HT metabolism in the intestinal wall of the rat-II. The nerves plexuses-interactions between 5-HT containing cells. Neurochem. Int. 5, 571–577. [DOI] [PubMed] [Google Scholar]
- Lovenberg W.; Jequier E.; Sjoerdsma A. (1967) Tryptophan hydroxylation: measurement in pineal gland, brainstem, and carcinoid tumor. Science 155, 217–219. [DOI] [PubMed] [Google Scholar]
- Berger M.; Gray J. A.; Roth B. L. (2009) The expanded biology of serotonin. Annu. Rev. Med. 60, 355–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther D. J.; Peter J. U.; Bashammakh S.; Hortnagl H.; Voits M.; Fink H.; Bader M. (2003) Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science 299, 76. [DOI] [PubMed] [Google Scholar]
- Cote F.; Thevenot E.; Fligny C.; Fromes Y.; Darmon M.; Ripoche M. A.; Bayard E.; Hanoun N.; Saurini F.; Lechat P.; Dandolo L.; Hamon M.; Mallet J.; Vodjdani G. (2003) Disruption of the nonneuronal tph1 gene demonstrates the importance of peripheral serotonin in cardiac function. Proc. Natl. Acad. Sci. U.S.A. 100, 13525–13530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutknecht L.; Kriegebaum C.; Waider J.; Schmitt A.; Lesch K. P. (2009) Spatio-temporal expression of tryptophan hydroxylase isoforms in murine and human brain: convergent data from Tph2 knockout mice. Eur. Neuropsychopharmacol. 19, 266–282. [DOI] [PubMed] [Google Scholar]
- Sakowski S. A.; Geddes T. J.; Thomas D. M.; Levi E.; Hatfield J. S.; Kuhn D. M. (2006) Differential tissue distribution of tryptophan hydroxylase isoforms 1 and 2 as revealed with monospecific antibodies. Brain Res. 1085, 11–18. [DOI] [PubMed] [Google Scholar]
- Patel P. D.; Pontrello C.; Burke S. (2004) Robust and tissue-specific expression of TPH2 versus TPH1 in rat raphe and pineal gland. Biol. Psychiatry 55, 428–433. [DOI] [PubMed] [Google Scholar]
- Zill P.; Buttner A.; Eisenmenger W.; Muller J.; Moller H. J.; Bondy B. (2009) Predominant expression of tryptophan hydroxylase 1 mRNA in the pituitary: a postmortem study in human brain. Neuroscience 159, 1274–1282. [DOI] [PubMed] [Google Scholar]
- Zill P.; Buttner A.; Eisenmenger W.; Moller H. J.; Ackenheil M.; Bondy B. (2007) Analysis of tryptophan hydroxylase I and II mRNA expression in the human brain: a post-mortem study. J. Psychiatr. Res. 41, 168–173. [DOI] [PubMed] [Google Scholar]
- Zhang X.; Beaulieu J. M.; Sotnikova T. D.; Gainetdinov R. R.; Caron M. G. (2004) Tryptophan hydroxylase-2 controls brain serotonin synthesis. Science 305, 217. [DOI] [PubMed] [Google Scholar]
- Watts S. W.; Morrison S. F.; Davis R. P.; Barman S. M. (2012) Serotonin and blood pressure regulation. Pharmacol. Rev. 64, 359–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amireault P.; Dube F. (2005) Intracellular cAMP and calcium signaling by serotonin in mouse cumulus-oocyte complexes. Mol. Pharmacol. 68, 1678–1687. [DOI] [PubMed] [Google Scholar]
- Amireault P.; Dube F. (2005) Serotonin and its antidepressant-sensitive transport in mouse cumulus-oocyte complexes and early embryos. Biol. Reprod. 73, 358–365. [DOI] [PubMed] [Google Scholar]
- Il’kova G.; Rehak P.; Vesela J.; Cikos S.; Fabian D.; Czikkova S.; Koppel J. (2004) Serotonin localization and its functional significance during mouse preimplantation embryo development. Zygote 12, 205–213. [DOI] [PubMed] [Google Scholar]
- Vesela J.; Rehak P.; Mihalik J.; Czikkova S.; Pokorny J.; Koppel J. (2003) Expression of serotonin receptors in mouse oocytes and preimplantation embryos. Physiol. Res. 52, 223–228. [PubMed] [Google Scholar]
- Walther D. J.; Bader M. (1999) Serotonin synthesis in murine embryonic stem cells. Brain Res. Mol. Brain Res. 68, 55–63. [DOI] [PubMed] [Google Scholar]
- Basu B.; Desai R.; Balaji J.; Chaerkady R.; Sriram V.; Maiti S.; Panicker M. M. (2008) Serotonin in pre-implantation mouse embryos is localized to the mitochondria and can modulate mitochondrial potential. Reproduction 135, 657–669. [DOI] [PubMed] [Google Scholar]
- Doherty L. F.; Kwon H. E.; Taylor H. S. (2011) Regulation of tryptophan 2,3-dioxygenase by HOXA10 enhances embryo viability through serotonin signaling. Am. J. Physiol. Endocrinol. Metab. E86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yavarone M. S.; Shuey D. L.; Sadler T. W.; Lauder J. M. (1993) Serotonin uptake in the ectoplacental cone and placenta of the mouse. Placenta 14, 149–161. [DOI] [PubMed] [Google Scholar]
- Cote F.; Fligny C.; Bayard E.; Launay J. M.; Gershon M. D.; Mallet J.; Vodjdani G. (2007) Maternal serotonin is crucial for murine embryonic development. Proc. Natl. Acad. Sci. U.S.A. 104, 329–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnin A.; Goeden N.; Chen K.; Wilson M. L.; King J.; Shih J. C.; Blakely R. D.; Deneris E. S.; Levitt P. (2011) A transient placental source of serotonin for the fetal forebrain. Nature 472, 347–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnin A.; Levitt P. (2011) Fetal, maternal, and placental sources of serotonin and new implications for developmental programming of the brain. Neuroscience 197, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W. Q.; Zhang C. L.; Di X. Y.; Zhang R. Q. (1998) Studies on the localization of 5-hydroxytryptamine and its receptors in human placenta. Placenta 19, 655–661. [DOI] [PubMed] [Google Scholar]
- Viau M.; Lafond J.; Vaillancourt C. (2009) Expression of placental serotonin transporter and 5-HT 2A receptor in normal and gestational diabetes mellitus pregnancies. Reprod. Biomed. Online 19, 207–215. [DOI] [PubMed] [Google Scholar]
- Sonier B.; Lavigne C.; Arseneault M.; Ouellette R.; Vaillancourt C. (2005) Expression of the 5-HT2A serotoninergic receptor in human placenta and choriocarcinoma cells: mitogenic implications of serotonin. Placenta 26, 484–490. [DOI] [PubMed] [Google Scholar]
- Balkovetz D. F.; Tiruppathi C.; Leibach F. H.; Mahesh V. B.; Ganapathy V. (1989) Evidence for an imipramine-sensitive serotonin transporter in human placental brush-border membranes. J. Biol. Chem. 264, 2195–2198. [PubMed] [Google Scholar]
- Klempan T.; Hudon-Thibeault A. A.; Oufkir T.; Vaillancourt C.; Sanderson J. T. (2011) Stimulation of serotonergic 5-HT2A receptor signaling increases placental aromatase (CYP19) activity and expression in BeWo and JEG-3 human choriocarcinoma cells. Placenta 32, 651–656. [DOI] [PubMed] [Google Scholar]
- Oufkir T.; Vaillancourt C. (2011) Phosphorylation of JAK2 by serotonin 5-HT (2A) receptor activates both STAT3 and ERK1/2 pathways and increases growth of JEG-3 human placental choriocarcinoma cell. Placenta 32, 1033–1040. [DOI] [PubMed] [Google Scholar]
- Matsuda M.; Imaoka T.; Vomachka A. J.; Gudelsky G. A.; Hou Z.; Mistry M.; Bailey J. P.; Nieport K. M.; Walther D. J.; Bader M.; Horseman N. D. (2004) Serotonin regulates mammary gland development via an autocrine-paracrine loop. Dev. Cell. 6, 193–203. [DOI] [PubMed] [Google Scholar]
- Stull M. A.; Pai V.; Vomachka A. J.; Marshall A. M.; Jacob G. A.; Horseman N. D. (2007) Mammary gland homeostasis employs serotonergic regulation of epithelial tight junctions. Proc. Natl. Acad. Sci. U.S.A. 104, 16708–16713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai V. P.; Horseman N. D. (2011) Multiple cellular responses to serotonin contribute to epithelial homeostasis. PLoS One 6, e17028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall A. M.; Nommsen-Rivers L. A.; Hernandez L. L.; Dewey K. G.; Chantry C. J.; Gregerson K. A.; Horseman N. D. (2010) Serotonin transport and metabolism in the mammary gland modulates secretory activation and involution. J. Clin. Endocrinol. Metab. 95, 837–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai V. P.; Horseman N. D. (2008) Biphasic regulation of mammary epithelial resistance by serotonin through activation of multiple pathways. J. Biol. Chem. 283, 30901–30910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon M. D.; Tack J. (2007) The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology 132, 397–414. [DOI] [PubMed] [Google Scholar]
- Li Z.; Chalazonitis A.; Huang Y. Y.; Mann J. J.; Margolis K. G.; Yang Q. M.; Kim D. O.; Cote F.; Mallet J.; Gershon M. D. (2011) Essential roles of enteric neuronal serotonin in gastrointestinal motility and the development/survival of enteric dopaminergic neurons. J. Neurosci. 31, 8998–9009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manocha M.; Khan W. I. (2012) Serotonin and GI disorders: an update on clinical and experimental studies. Clin. Transl. Gastroenterol. 3, e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell P. J.; Wang X.; Leon-Ponte M.; Griffiths C.; Pingle S. C.; Ahern G. P. (2006) A novel form of immune signaling revealed by transmission of the inflammatory mediator serotonin between dendritic cells and T cells. Blood 107, 1010–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahern G. P. (2011) 5-HT and the immune system. Curr. Opin. Pharmacol. 11, 29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- León-Ponte M.; Ahern G. P.; O’Connell P. J. (2007) Serotonin provides an accessory signal to enhance T-cell activation by signaling through the 5-HT7 receptor. Blood 109, 3139–3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghia J. E.; Li N.; Wang H.; Collins M.; Deng Y.; El-Sharkawy R. T.; Cote F.; Mallet J.; Khan W. I. (2009) Serotonin has a key role in pathogenesis of experimental colitis. Gastroenterology 137, 1649–1660. [DOI] [PubMed] [Google Scholar]
- Margolis K. G.; Stevanovic K. D.; Yang Q. M.; Li Z.; Mazo R.; Gershon M. D. (2011) An inhibitor of tryptophanhydroxylase successfully ameliorates TNBS-induced colitis. Gastroenterology 140, S478. [Google Scholar]
- Richmond J. E.; Codignola A.; Cooke I. M.; Sher E. (1996) Calcium- and barium-dependent exocytosis from the rat insulinoma cell line RINm5F assayed using membrane capacitance measurements and serotonin release. Pflugers Arch. 432, 258–269. [DOI] [PubMed] [Google Scholar]
- Rieck S.; White P.; Schug J.; Fox A. J.; Smirnova O.; Gao N.; Gupta R. K.; Wang Z. V.; Scherer P. E.; Keller M. P.; Attie A. D.; Kaestner K. H. (2009) The transcriptional response of the islet to pregnancy in mice. Mol. Endocrinol. 23, 1702–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwens L.; Rooman I. (2005) Regulation of pancreatic beta-cell mass. Physiol. Rev. 85, 1255–1270. [DOI] [PubMed] [Google Scholar]
- Schraenen A.; Lemaire K.; de Faudeur G.; Hendrickx N.; Granvik M.; Van Lommel L.; Mallet J.; Vodjdani G.; Gilon P.; Binart N.; in’t Veld P.; Schuit F. (2010) Placental lactogens induce serotonin biosynthesis in a subset of mouse beta cells during pregnancy. Diabetologia 53, 2589–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.; Toyofuku Y.; Lynn F. C.; Chak E.; Uchida T.; Mizukami H.; Fujitani Y.; Kawamori R.; Miyatsuka T.; Kosaka Y.; Yang K.; Honig G.; van der Hart M.; Kishimoto N.; Wang J.; Yagihashi S.; Tecott L. H.; Watada H.; German M. S. (2010) Serotonin regulates pancreatic beta cell mass during pregnancy. Nat. Med. 16, 804–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulmann N.; Grohmann M.; Voigt J. P.; Bert B.; Vowinckel J.; Bader M.; Skelin M.; Jevsek M.; Fink H.; Rupnik M.; Walther D. J. (2009) Intracellular serotonin modulates insulin secretion from pancreatic beta-cells by protein serotonylation. PLoS Biol. 7, e1000229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teitelbaum S. L.; Ross F. P. (2003) Genetic regulation of osteoclast development and function. Nat. Rev. Genet. 4, 638–649. [DOI] [PubMed] [Google Scholar]
- Battaglino R.; Fu J.; Spate U.; Ersoy U.; Joe M.; Sedaghat L.; Stashenko P. (2004) Serotonin regulates osteoclast differentiation through its transporter. J. Bone Miner. Res. 19, 1420–1431. [DOI] [PubMed] [Google Scholar]
- Warden S. J.; Robling A. G.; Sanders M. S.; Bliziotes M. M.; Turner C. H. (2005) Inhibition of the serotonin (5-hydroxytryptamine) transporter reduces bone accrual during growth. Endocrinology 146, 685–693. [DOI] [PubMed] [Google Scholar]
- Collet C.; Schiltz C.; Geoffroy V.; Maroteaux L.; Launay J. M.; de Vernejoul M. C. (2008) The serotonin 5-HT2B receptor controls bone mass via osteoblast recruitment and proliferation. FASEB J. 22, 418–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliziotes M. M.; Eshleman A. J.; Zhang X. W.; Wiren K. M. (2001) Neurotransmitter action in osteoblasts: expression of a functional system for serotonin receptor activation and reuptake. Bone 29, 477–486. [DOI] [PubMed] [Google Scholar]
- Westbroek I.; van der Plas A.; de Rooij K. E.; Klein-Nulend J.; Nijweide P. J. (2001) Expression of serotonin receptors in bone. J. Biol. Chem. 276, 28961–28968. [DOI] [PubMed] [Google Scholar]
- Warden S. J.; Nelson I. R.; Fuchs R. K.; Bliziotes M. M.; Turner C. H. (2008) Serotonin (5-hydroxytryptamine) transporter inhibition causes bone loss in adult mice independently of estrogen deficiency. Menopause 15, 1176–1183. [DOI] [PubMed] [Google Scholar]
- Bliziotes M.; Eshleman A.; Burt-Pichat B.; Zhang X. W.; Hashimoto J.; Wiren K.; Chenu C. (2006) Serotonin transporter and receptor expression in osteocytic MLO-Y4 cells. Bone 39, 1313–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav V. K.; Ryu J. H.; Suda N.; Tanaka K. F.; Gingrich J. A.; Schutz G.; Glorieux F. H.; Chiang C. Y.; Zajac J. D.; Insogna K. L.; Mann J. J.; Hen R.; Ducy P.; Karsenty G. (2008) Lrp5 controls bone formation by inhibiting serotonin synthesis in the duodenum. Cell 135, 825–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav V. K.; Balaji S.; Suresh P. S.; Liu X. S.; Lu X.; Li Z.; Guo X. E.; Mann J. J.; Balapure A. K.; Gershon M. D.; Medhamurthy R.; Vidal M.; Karsenty G.; Ducy P. (2010) Pharmacological inhibition of gut-derived serotonin synthesis is a potential bone anabolic treatment for osteoporosis. Nat. Med. 16, 308–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav V. K.; Arantes H. P.; Barros E. R.; Lazaretti-Castro M.; Ducy P. (2010) Genetic analysis of Lrp5 function in osteoblast progenitors. Calcif. Tissue Int. 86, 382–388. [DOI] [PubMed] [Google Scholar]
- Tamai K.; Zeng X.; Liu C.; Zhang X.; Harada Y.; Chang Z.; He X. (2004) A mechanism for Wnt coreceptor activation. Mol. Cell 13, 149–156. [DOI] [PubMed] [Google Scholar]
- Boyden L. M.; Mao J.; Belsky J.; Mitzner L.; Farhi A.; Mitnick M. A.; Wu D.; Insogna K.; Lifton R. P. (2002) High bone density due to a mutation in LDL-receptor-related protein 5. N. Engl. J. Med. 346, 1513–1521. [DOI] [PubMed] [Google Scholar]
- Chabbi-Achengli Y.; Coudert A. E.; Callebert J.; Geoffroy V.; Cote F.; Collet C.; de Vernejoul M. C. (2012) Decreased osteoclastogenesis in serotonin-deficient mice. Proc. Natl. Acad. Sci. U.S.A. 109, 2567–2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata S.; Ohkohchi N.; Matsuo R.; Ikeda O.; Myronovych A.; Hoshi R. (2007) Platelets promote liver regeneration in early period after hepatectomy in mice. World J. Surg. 31, 808–816. [DOI] [PubMed] [Google Scholar]
- Balasubramanian S.; Paulose C. S. (1998) Induction of DNA synthesis in primary cultures of rat hepatocytes by serotonin: possible involvement of serotonin S2 receptor. Hepatology 27, 62–66. [DOI] [PubMed] [Google Scholar]
- Papadimas G. K.; Tzirogiannis K. N.; Panoutsopoulos G. I.; Demonakou M. D.; Skaltsas S. D.; Hereti R. I.; Papadopoulou-Daifoti Z.; Mykoniatis M. G. (2006) Effect of serotonin receptor 2 blockage on liver regeneration after partial hepatectomy in the rat liver. Liver Int. 26, 352–361. [DOI] [PubMed] [Google Scholar]
- Lesurtel M.; Graf R.; Aleil B.; Walther D. J.; Tian Y.; Jochum W.; Gachet C.; Bader M.; Clavien P. A. (2006) Platelet-derived serotonin mediates liver regeneration. Science 312, 104–107. [DOI] [PubMed] [Google Scholar]
- Matondo R. B.; Punt C.; Homberg J.; Toussaint M. J.; Kisjes R.; Korporaal S. J.; Akkerman J. W.; Cuppen E.; de Bruin A. (2009) Deletion of the serotonin transporter in rats disturbs serotonin homeostasis without impairing liver regeneration. Am. J. Physiol. Gastrointest. Liver Physiol. 296, G963–968. [DOI] [PubMed] [Google Scholar]
- Lowy P. H.; Keighley G.; Cohen N. S. (1970) Stimulation by serotonin of erythropoietin-dependent erythropoiesis in mice. Br. J. Hamaetol. 19, 711–718. [DOI] [PubMed] [Google Scholar]
- Noveck R. J.; Fisher J. W. (1971) Erythropoietic effects of 5-hydroxytryptamine. Proc. Soc. Exp. Biol. Med. 138, 103–107. [DOI] [PubMed] [Google Scholar]
- Yang M.; Srikiatkhachorn A.; Anthony M.; Chong B. H. (1996) Serotonin stimulates megakaryocytopoiesis via the 5-HT2 receptor. Blood Coagul. Fibrinolysis 7, 127–133. [DOI] [PubMed] [Google Scholar]
- Liu Y. S.; Yang M. (2006) The effect of 5-hydroxtryptamine on the regulation of megakaryocytopoiesis. Hematology 11, 53–56. [DOI] [PubMed] [Google Scholar]
- Yang M.; Li K.; Ng P. C.; Chuen C. K.; Lau T. K.; Cheng Y. S.; Liu Y. S.; Li C. K.; Yuen P. M.; James A. E.; Lee S. M.; Fok T. F. (2007) Promoting effects of serotonin on hematopoiesis: ex vivo expansion of cord blood CD34+ stem/progenitor cells, proliferation of bone marrow stromal cells, and antiapoptosis. Stem Cells 25, 1800–1806. [DOI] [PubMed] [Google Scholar]
- Amireault P.; Hatia S.; Bayard E.; Bernex F.; Collet C.; Callebert J.; Launay J. M.; Hermine O.; Schneider E.; Mallet J.; Dy M.; Cote F. (2011) Ineffective erythropoiesis with reduced red blood cell survival in serotonin-deficient mice. Proc. Natl. Acad. Sci. U.S.A. 108, 13141–13146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther D. J.; Peter J. U.; Winter S.; Holtje M.; Paulmann N.; Grohmann M.; Vowinckel J.; Alamo-Bethencourt V.; Wilhelm C. S.; Ahnert-Hilger G.; Bader M. (2003) Serotonylation of small GTPases is a signal transduction pathway that triggers platelet alpha-granule release. Cell 115, 851–862. [DOI] [PubMed] [Google Scholar]
- Guilluy C.; Eddahibi S.; Agard C.; Guignabert C.; Izikki M.; Tu L.; Savale L.; Humbert M.; Fadel E.; Adnot S.; Loirand G.; Pacaud P. (2009) RhoA and Rho kinase activation in human pulmonary hypertension: role of 5-HT signaling. Am. J. Respir. Crit. Care Med. 179, 1151–1158. [DOI] [PubMed] [Google Scholar]
- Watts S. W.; Priestley J. R.; Thompson J. M. (2009) Serotonylation of vascular proteins important to contraction. PLoS One 4, e5682. [DOI] [PMC free article] [PubMed] [Google Scholar]


