Abstract
Prepulse inhibition (PPI) is a model of sensorimotor gating, a sensory filtering mechanism which is disrupted in schizophrenia. Here, investigation of the role of the serotonin-1A (5-HT1A) receptor in the regulation of PPI in two mouse strains, C57Bl/6 and Balb/c, was used to address findings in the PPI literature on species and mouse strain differences that question the usefulness of PPI as a cross-species preclinical test. Although the full 5-HT1A receptor agonist, 8-OH-DPAT, induced markedly different strain-specific responses in PPI, other selective 5-HT1A receptor ligands with partial agonist or antagonist activity elicited similar effects across strains. Pretreatment with the serotonin precursor, 5-HTP, to increase serotonergic activity in the brain, unmasked a decrease in PPI caused by 8-OH-DPAT in C57Bl/6 mice. Pretreatment with the serotonin synthesis inhibitor, PCPA, to decrease serotonergic activity in the brain, unmasked an 8-OH-DPAT-induced increase in PPI in this strain. These studies show that the strain-dependent involvement of 5-HT1A receptors in PPI can be modulated by the type of 5-HT1A ligand used, or increasing or decreasing serotonin levels in the brain. These results help to clarify some of the mouse strain and species differences in PPI regulation and strengthen its usefulness as a cross-species measure of sensorimotor gating.
Keywords: Prepulse inhibition, serotonin, 5-HT1A receptors, strain differences, schizophrenia
Prepulse inhibition (PPI) is a widely used behavioral model of sensory gating, a filtering mechanism that is disrupted (i.e., reduced) in schizophrenia and other psychiatric and neurological illnesses.1,2 The principle of PPI of acoustic startle is that the startle response, which is elicited by a short loud sound pulse, can be inhibited if it is preceded by a low-intensity prepulse sound, which presumably initiates a short-term inhibitory response to prevent sensory flooding and to allow focused attention.3,4 While the startle response is mediated by a simple ponto-medullary brain circuit, regulation of PPI is more complex and involves a number of other brain regions, such as the superior colliculus and pedunculopontine tegmental nucleus.3 The set-point and activity of these circuits is modulated by numerous forebrain regions, including the nucleus accumbens and hippocampus.3,5 At the same time, multiple neurotransmitter systems in the brain have been implicated in the modulation of PPI, including receptors of the dopamine and serotonin systems.6,7
PPI is often referred to as a “cross-species” phenomenon, and this has been generally accepted as being one of its advantages. Indeed, methodology for PPI of acoustic startle is remarkably similar between humans and animals, except that eye-blink startle responses are most commonly used in humans versus whole-body startle in small animals. An attractive extension of this similarity has been that pharmacological PPI studies in experimental animals may have direct relevance to humans and, more specifically, psychiatric illness.6,8 However, it is becoming increasingly clear that the effects of some drugs to modify PPI appear to differ markedly between humans and rodents9 or even between rats and mice or between mouse strains. For example, while the disruption of PPI after treatment with the dopamine receptor agonist, apomorphine, has been widely used as a model of dopaminergic involvement, apomorphine appears to have no effect on PPI in some substrains of rats.10 Similarly, dopaminergic stimulation with amphetamine treatment had species and strain-specific effects on PPI: no effect in normal women, disrupted PPI in female Sprague–Dawley rats, and increased PPI in Long–Evans rats.11 Further, low doses of the NMDA receptor antagonist, ketamine, caused an increase of PPI in men,12 whereas ketamine disrupted PPI in rats and mice.13 Finally, the prototypical 5-HT1A receptor agonist, 8-hydroxy-dipropylaminotetralin (8-OH-DPAT) reduced PPI in rats but not in mice.14
Observations such as these and others6 can be seen as challenging the concept of PPI as being a reliable cross-species phenomenon and, consequently, its applicability to study mechanisms involved in psychiatric illness and drug development.2,15,16 We were interested to pursue this question and used the involvement of 5-HT1A receptors in PPI in two strains of mice, C57Bl/6 and Balb/c, as a model system. There is considerable evidence for a role for 5-HT1A receptors in psychiatric illnesses, such as schizophrenia, and the action of some antipsychotic drugs. For example, post-mortem studies have shown altered expression of 5-HT1A receptors in schizophrenia17,18 and some atypical antipsychotic drugs have high affinity for this receptor subtype.17,19 Previous studies by us and others have consistently shown that administration of 8-OH-DPAT robustly reduces PPI in Sprague–Dawley rats, an effect that was mediated by an interaction with 5-HT1A receptors and not 5-HT7 receptors, and which most likely involved downstream activation of dopaminergic activity.14,20 However, 8-OH-DPAT did not have significant effects on PPI in C57Bl/6 mice, the most commonly used background strain for knockout and transgenic modifications. In contrast, in Balb/c mice and 129sv mice, 8-OH-DPAT induced a marked increase in PPI.14,20,21 It should be noted that the increase of PPI after 8-OH-DPAT administration was not present in 5-HT1A receptor knockout mice on a 129sv background,22,23 indicating this effect was indeed mediated by 5-HT1A receptors. C57Bl/6 mice and Balb/c mice differ in a polymorphism in the tryptophan hydroxylase 2 (TPH-2) gene, resulting in lower serotonin synthesis in the brains of Balb/c mice compared to C57Bl/6 mice.24−26 This specific neurochemical difference could provide an explanation for differential drug effects seen in these mouse strains and, ultimately, could serve as an example of possible differences in central neurotransmitter activity explaining apparent species differences in the effects of drugs on PPI, including in humans.
The aim of the present pharmacological analysis was to investigate if there were conditions under which 8-OH-DPAT administration would result in a “ratlike” disruption of PPI in either C57Bl/6 mice, Balb/c mice, or both strains. Additionally, we aimed to investigate if, in C57Bl/6 mice, we would be able to elicit “Balb/c-like” PPI responses. Three specific experiments were carried out: first, we compared a series of 5-HT1A receptor ligands with differential intrinsic activities, ranging from the full agonist, 8-OH-DPAT, through a range of partial agonists, to the antagonist, WAY100,635. It was hypothesized that different serotonergic activity in C57Bl/6 versus Balb/c mice would result in some of these drugs being effective in the former and other drugs being effective in the latter strain. More specifically, because of higher serotonergic activity hypothesized to occur in C57Bl/6 mice, it was predicted that drugs with higher intrinsic activity would have little effect in this strain as opposed to Balb/c mice. Conversely, drugs with 5-HT1A receptor antagonist properties might have effects in C57Bl/6 but not in Balb/c mice where serotonergic activity is hypothesized to be lower due to the low-activity TPH-2 polymorphism. In the second experiment, we assessed whether increasing serotonergic activity by pretreatment of animals with the 5-HT precursor, 5-hydroxytryptophan (5-HTP),27,28 would alter the effects of 8-OH-DPAT on PPI. This pretreatment was predicted to alter the effect of 8-OH-DPAT in Balb/c mice to resemble that seen in C57Bl/6 mice. Conversely, in the third experiment, we investigated whether reduction of serotonergic activity by pretreatment with the serotonin synthesis inhibitor, parachlorophenylalanine (PCPA),29 would alter effects on PPI. This pretreatment was predicted to alter the effects of 8-OH-DPAT in C57Bl/6 mice to resemble those seen in Balb/c mice.
The results identify a number of possible mechanisms that could explain mouse strain differences in PPI regulation, at least those involving 5-HT1A receptor activation. In a broader context, the results support PPI as a behavioral test with cross-species applicability. Published or novel experimental PPI findings apparently contradicting this concept may be explained by differences in endogenous neurotransmitter activity, the types of drugs used, or receptor binding levels.
Results
Experiment 1: Effects of a Range of 5-HT1A Receptor Ligands with Differential Intrinsic Activities on PPI
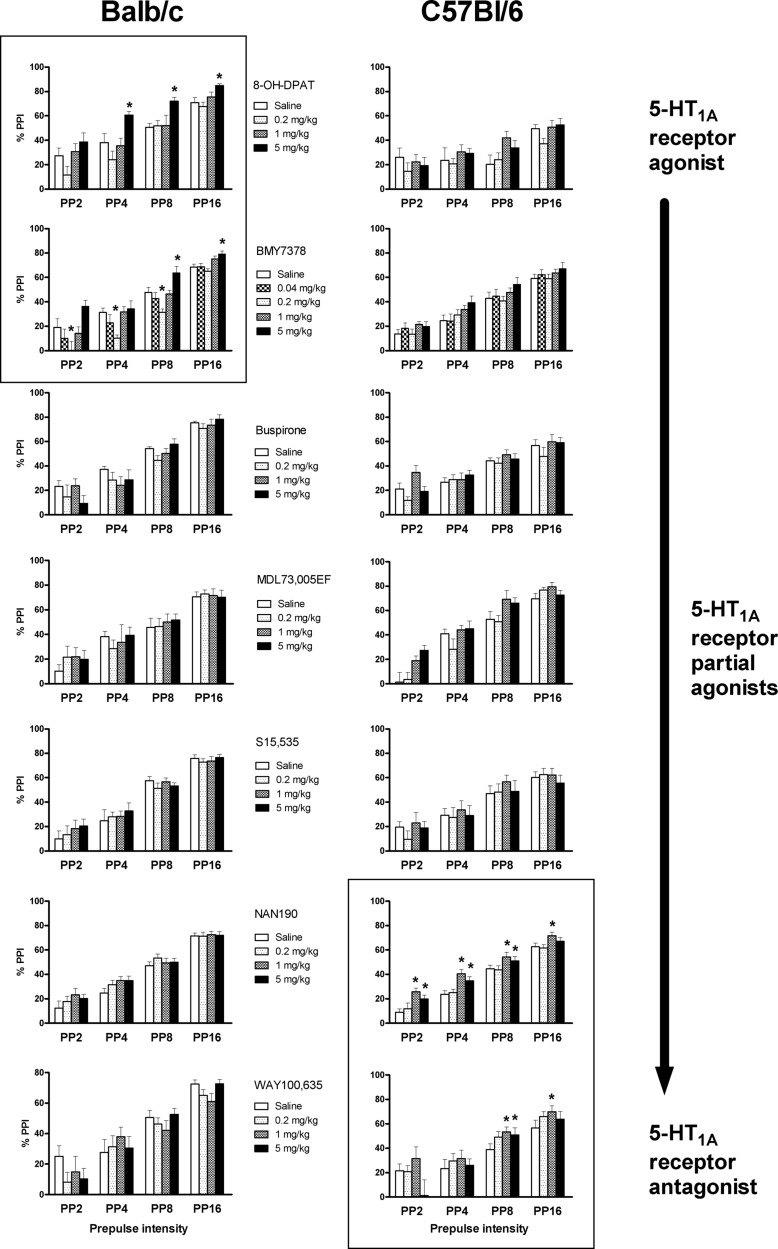
As per previous findings, administration of 8-OH-DPAT differentially affected PPI between Balb/c and C57Bl/6 mice (dose × strain interaction: F(3,39) = 3.0, P = 0.044). In Balb/c mice, 8-OH-DPAT increased PPI (F(3,21) = 8.5, P = 0.001). The PPI increase was significant for the 5 mg/kg dose at the PP4, PP8, and PP16 levels (Figure 1). There was no significant effect of 8-OH-DPAT on PPI in C57Bl/6 mice (Figure 1).
Figure 1.
Effects on prepulse inhibition of acoustic startle (PPI) of different doses of the 5-HT1A receptor agonist, 8-OH-DPAT, the 5-HT1A receptor partial agonists, buspirone, BMY7378, MDL73,005EF, NAN-190 and S15,535, and the 5-HT1A receptor antagonist, WAY100,635. Drugs were tested in Balb/c mice (left column of graphs) or C57Bl/6 mice (right column of graphs). Data are presented for each level of prepulse (2, 4, 8, or 16 dB over background noise). *P < 0.05 for differences with responses seen after saline treatment. For numbers of animals per group, see Table 1. Treatment with 8-OH-DPAT and BMY7378 caused an increase of PPI in Balb/c mice (top left box) but not C57Bl/6 mice. Treatment with WAY100,635 and NAN-190 caused an increase in PPI in C57Bl/6 mice (bottom right box) but not Balb/c mice.
Administration of 0.04 mg/kg of the partial agonist BMY7378 had no effect on PPI (Figure 1). In contrast, administration of 0.2 mg/kg BMY7378 caused a disruption of PPI which was dependent on mouse strain and prepulse intensity (dose × PP × strain interaction: F(4.0, P = 0.009; dose × strain interaction: F(1,43) = 13.1, P = 0.001). The effect of this dose was significant in Balb/c mice (main effect of dose: F(1,20) = 25.0, P < 0.001; dose × PP interaction: F(3,60) = 5.5, P = 0.002; P < 0.05 at PP2, PP4, and PP8) but not in C57Bl/6 (Figure 1). There was no effect of 1 mg/kg BMY7378. In contrast, the 5 mg/kg dose of BMY7378 increased PPI (main effect of dose: F(1,20) = 10.4, P = 0.001) and an interaction of dose × PP × strain (F(3,60) = 3.1, P = 0.032) suggested a different pattern across prepulse intensities between the strains. Indeed, in Balb/c mice, 5 mg/kg of BMY7378 increased PPI at the PP8 and PP16 levels but there was no significant effect at any specific prepulse intensity in C57Bl/6 mice (Figure 1).
Treatment with buspirone appeared to have an effect on PPI which was dependent on the prepulse intensity (dose × PP: F(9,198) = 2.5, P = 0.011). Further analysis showed that at PP2, but not any of the other prepulse intensities, there was a significant main effect of dose (F(3,66) = 4.7, P = 0.001). However, none of the individual buspirone doses had significant effects at this prepulse intensity in either strain (Figure 1).
Treatment with MDL73,005EF or S15,535 had no significant effect on PPI (Figure 1). NAN-190 had a differential effect on PPI in C57Bl/6 vs Balb/c mice (dose × strain interaction: F(3,132) = 3.1, P = 0.030). Analysis per strain revealed a significant effect of dose in C57Bl/6 mice (F(3,69) = 17.5, P < 0.001) but not Balb/c mice (Figure 1). Subsequent analysis at each dose showed significant increases in PPI in C57Bl/6 mice after treatment with 1 mg/kg (F(1,23) = 38.0, P < 0.001) or 5 mg/kg of NAN-190 (F(1,23) = 12.7, P < 0.002). This effect was seen at all prepulse intensities for the 1 mg/kg dose and at the PP2, PP4, and PP8 intensities for the 5 mg/kg dose (Figure 1).
After administration of the 5-HT1A antagonist, WAY100,635, an interaction of dose and prepulse intensity was found (F(9,126) = 2.3, P = 0.018). Further analysis at individual prepulse intensities revealed an interaction between dose and strain, which was significant at PP16 (F(3,42) = 4.6, P = 0.007) and was at the trend level at PP8 (F(3,42) = 2.7, P < 0.058). In Balb/c mice, none of the WAY100,635 doses caused significant effects on PPI at PP16. However, in C57Bl/6 mice, both the 1 and 5 mg/kg doses of WAY100,635 significantly increased PPI at PP8, while the 1 mg/kg dose increased PPI at PP16 (Figure 1).
Experiment 1: Effects of a Range of 5-HT1A Receptor Ligands with Differential Intrinsic Activity on Startle Responses
Administration of 8-OH-DPAT induced a slight, but significant decrease in average startle amplitudes (main effect of dose: F(3,42) = 3.1, P < 0.036). Analysis of the effects of individual doses showed significant reductions in startle amplitudes after treatment with 5 mg/kg of 8-OH-DPAT (F(1,14) = 8.8, P = 0.010), but not the lower doses. There was no interaction of the effect of 8-OH-DPAT with strain (Table 1).
Table 1. Average Startle Responses in Balb/c Mice and C57Bl/6 Mice after Treatment with Different 5-HT1A Receptor Ligandsa.
| Balb/c | C57Bl/6 | |
|---|---|---|
| 8-OH-DPAT | (n = 8) | (n = 8) |
| 0 (saline vehicle) | 470 ± 88 | 365 ± 43 |
| 0.2 mg/kg | 389 ± 68 | 299 ± 38 |
| 1 mg/kg | 296 ± 55 | 354 ± 40 |
| 5 mg/kg | 322 ± 63* | 279 ± 40 |
| BMY7378 | ||
| 0 (saline vehicle) | 243 ± 19 (n = 23) | 301 ± 27 (n = 24) |
| 0.04 mg/kg | 302 ± 47(n = 11) | 292 ± 36(n = 12) |
| 0.2 mg/kg | 188 ± 16(n = 23)* | 276 ± 25 (n = 24)* |
| 1 mg/kg | 205 ± 20 (n = 23)* | 260 ± 23(n = 24)* |
| 5 mg/kg | 163 ± 13(n = 12)* | 240 ± 24(n = 12) |
| buspirone | (n = 12) | (n = 12) |
| 0 (saline vehicle) | 372 ± 28 | 362 ± 30 |
| 0.2 mg/kg | 375 ± 42 | 368 ± 22 |
| 1 mg/kg | 270 ± 37 | 371 ± 38 |
| 5 mg/kg | 332 ± 46 | 325 ± 38 |
| MDL73,005EF | (n = 8) | (n = 7) |
| 0 (saline vehicle) | 307 ± 41 | 271 ± 49 |
| 0.2 mg/kg | 411 ± 71 | 239 ± 29 |
| 1 mg/kg | 293 ± 44 | 266 ± 72 |
| 5 mg/kg | 314 ± 57 | 188 ± 44 |
| S15,535 | (n = 12) | (n = 12) |
| 0 (saline vehicle) | 309 ± 22 | 317 ± 28 |
| 0.2 mg/kg | 310 ± 37 | 272 ± 44 |
| 1 mg/kg | 273 ± 27 | 258 ± 35* |
| 5 mg/kg | 278 ± 30 | 216 ± 37* |
| NAN-190 | (n = 23) | (n = 24) |
| 0 (saline vehicle) | 231 ± 21 | 266 ± 22 |
| 0.2 mg/kg | 189 ± 13* | 276 ± 22 |
| 1 mg/kg | 189 ± 14* | 239 ± 19 |
| 5 mg/kg | 156 ± 11* | 243 ± 24 |
| WAY100,635 | (n = 8) | (n = 8) |
| 0 (saline vehicle) | 311 ± 50 | 280 ± 28 |
| 0.2 mg/kg | 268 ± 37 | 374 ± 45 |
| 1 mg/kg | 245 ± 66 | 300 ± 35 |
| 5 mg/kg | 225 ± 34 | 291 ± 34 |
Data are mean arbitrary units ± SEMs for the numbers of animals indicated per strain. *P < 0.05 for differences with values obtained after saline treatment as shown by ANOVA of data from individual strains.
Administration of BMY7378 also caused a decrease in startle amplitudes at 0.2 mg/kg (F(1,45) = 20.9, P < 0.001), 1 mg/kg (F(1,45) = 10.8, P = 0.001), and 5 mg/kg (F(1,22) = 7.7, P = 0.011). There was no interaction with strain at any of these doses, suggesting a similar effect in Balb/c and C57Bl/6 mice (Table 1).
Administration of buspirone had no significant effect on startle amplitudes (Table 1). In contrast, treatment with MDL73,005EF dose-dependently affected startle amplitudes (F(3,42) = 21.4, P < 0.001). Further analysis at each dose only revealed a dose × strain interaction for the 0.2 mg/kg dose (F(1,14) = 5.0, P = 0.043). There were no significant effects when data from either Balb/c mice or C57Bl/6 mice were analyzed separately (Table 1).
Similar to most of the other compounds, administration of S15,535 reduced startle amplitudes (main effect of dose: F(3,66) = 5.3, P = 0.003), an effect that was significant for the 1 and 5 mg/kg doses (F(1,22) = 10.4 and 13.5; P = 0.004 and P = 0.001, respectively) (Table 1).
Administration of NAN-190 also dose-dependently reduced startle amplitudes (F(3,132) = 5.2, P = 0.002), and this was significant for the 1 and 5 mg/kg doses (F(1,44) = 5.7 and 10.8; P = 0.021 and P = 0.002, respectively, Table 1). There was no significant effect of treatment with the antagonist WAY100,635 on startle amplitudes (Table 1).
Experiment 2: Effect of 5-HTP Pretreatment on 8-OH-DPAT-Induced PPI Changes
There was no difference in PPI between the first and second 8-OH-DPAT session in the cohort of 8-OH-DPAT/vehicle/8-OH-DPAT-treated mice. However, a treatment × strain interaction (F(1,20) = 5.7, P = 0.027) reflected a tendency in C57Bl/6 mice for PPI to be slightly higher in the second PPI session than in the first session (F(1,11) = 7,8, P = 0.017) with no significant change occurring in Balb/c mice. The cause of this change in C57Bl/6 mice is unclear but could reflect habituation to repeated testing within the relatively short time frame of this protocol.
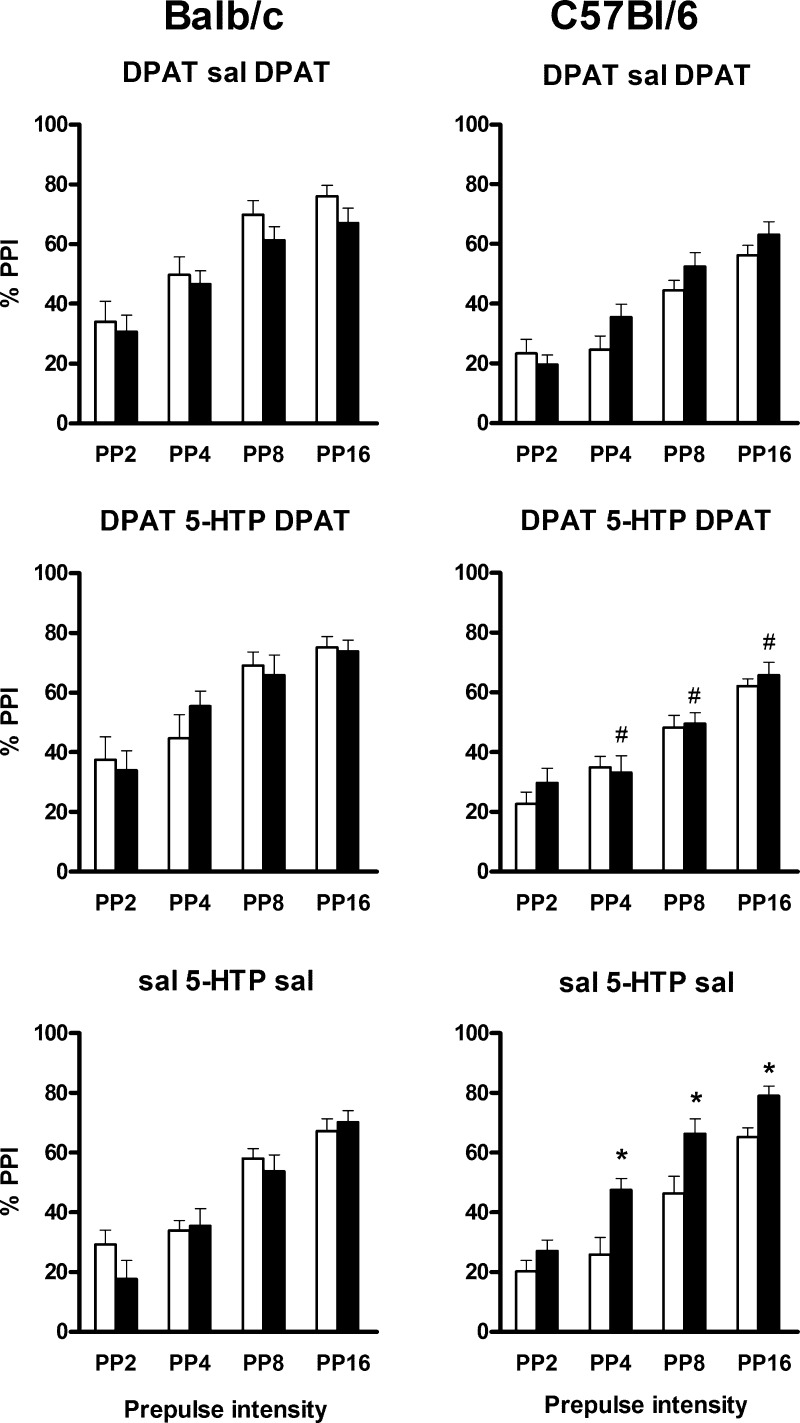
In the cohort of 8-OH-DPAT/5-HTP/8-OH-DPAT-treated mice, there was no significant difference in PPI tested before and after 5-HTP treatment. In contrast, in the third cohort of saline/5-HTP/saline-injected mice, there was a strain-dependent increase in PPI after 5-HTP pretreatment (treatment × strain interaction: F(1,22) = 10.9, P = 0.003). Further analysis revealed a highly significant increase in PPI in C57Bl/6 after 5-HTP treatment (F(1,11) = 23.7, P < 0.001) but no effect in Balb/c mice (Figure 2). To ascertain that this change from before versus after 5-HTP treatment was not simply the habituation observed in the first cohort, combined analysis of the first and third cohort revealed a significantly greater change in PPI from the first to the second PPI session after 5-HTP pretreatment compared to saline (F(1,22) = 7.2, P = 0.013).
Figure 2.
Effect of pretreatment with the serotonin precursor, 5-HTP, on the action of 5 mg/kg of 8-OH-DPAT on PPI in Balb/c mice (left panels) and C57Bl/6 mice (right panels). Data are presented for each level of prepulse (2, 4, 8, or 16 dB over baseline). Top panels show the effects of 8-OH-DPAT (DPAT) on PPI 1 day before (white bars) and 1 h after (black bars) treatment with saline (DPAT sal DPAT). Middle panels show the effects of 8-OH-DPAT (DPAT) on PPI 1 day before (white bars) and 1 h after (black bars) treatment with 5-HTP (DPAT 5-HTP DPAT). Bottom panels show baseline PPI after injection of saline 1 day before (white bars) and 1 h after (black bars) treatment with 5-HTP (sal 5-HTP sal). *P < 0.05 for differences from the first PPI session in the same animals. #P < 0.05 for differences between PPI after 8-OH-DPAT treatment in 5-HTP-treated mice and the corresponding control group (saline) PPI in 5-HTP-treated mice of the same strain. For numbers of animals per group, see Table 2. Injection of 8-OH-DPAT induced a decrease in PPI in C57Bl/6 mice treated with 5-HTP. There was no such effect without 5-HTP treatment or in Balb/c mice.
The aforementioned increase in PPI in C57Bl/6 mice in the third cohort after 5-HTP pretreatment was not seen in the second cohort. Because the latter animals were injected with 8-OH-DPAT, their second PPI session may in fact represent a disrupted PPI compared to the high PPI levels seen in the third cohort, which was injected with saline. Indeed, in C57Bl/6 mice from the second and third cohorts, both of which received 5-HTP treatment, comparison of data from the second PPI session revealed a significantly lower PPI in the mice injected with 8-OH-DPAT than those injected with saline (main effect of 8-OH-DPAT: F(1,22) = 4.8, P = 0.040; 8-OH-DPAT × PP interaction: F(3,66) = 4.1, P = 0.010; P < 0.05 at PP4, PP8, and PP16). Thus, in C57Bl/6 mice, the lack of effect of 8-OH-DPAT treatment on PPI (see Figure 1) was reversed into a marked 8-OH-DPAT-induced disruption of PPI after 5-HTP treatment (Figure 2).
Startle amplitudes were higher in the second PPI session compared to the first (F(1,20) = 7.1, P = 0.015), irrespective of whether mice received 8-OH-DPAT before and after vehicle treatment, 8-OH-DPAT before and after 5-HTP treatment, or saline before and after 5-HTP treatment (Table 2).
Table 2. Average Startle Amplitudes in Balb/c Mice and C57Bl/6 Mice after Treatment with Saline or 8-OH-DPAT with or without Treatment with Saline or 5-Hydroxytryptophan (5-HTP)a.
| Balb/c | C57Bl/6 | |
|---|---|---|
| 8-OH-DPAT–saline–8-OH-DPAT | ||
| before | 179 ± 38 | 217 ± 33 |
| after | 196 ± 46 | 237 ± 31 |
| 8-OH-DPAT–5-HTP–8-OH-DPAT | ||
| before | 179 ± 40 | 165 ± 27 |
| after | 240 ± 49 | 319 ± 59 |
| saline–5-HTP–saline | ||
| before | 163 ± 29 | 263 ± 38 |
| after | 180 ± 46 | 322 ± 29 |
Data are mean arbitrary units ± SEMs for n = 10–12 mice per group. For statistical comparisons, see text.
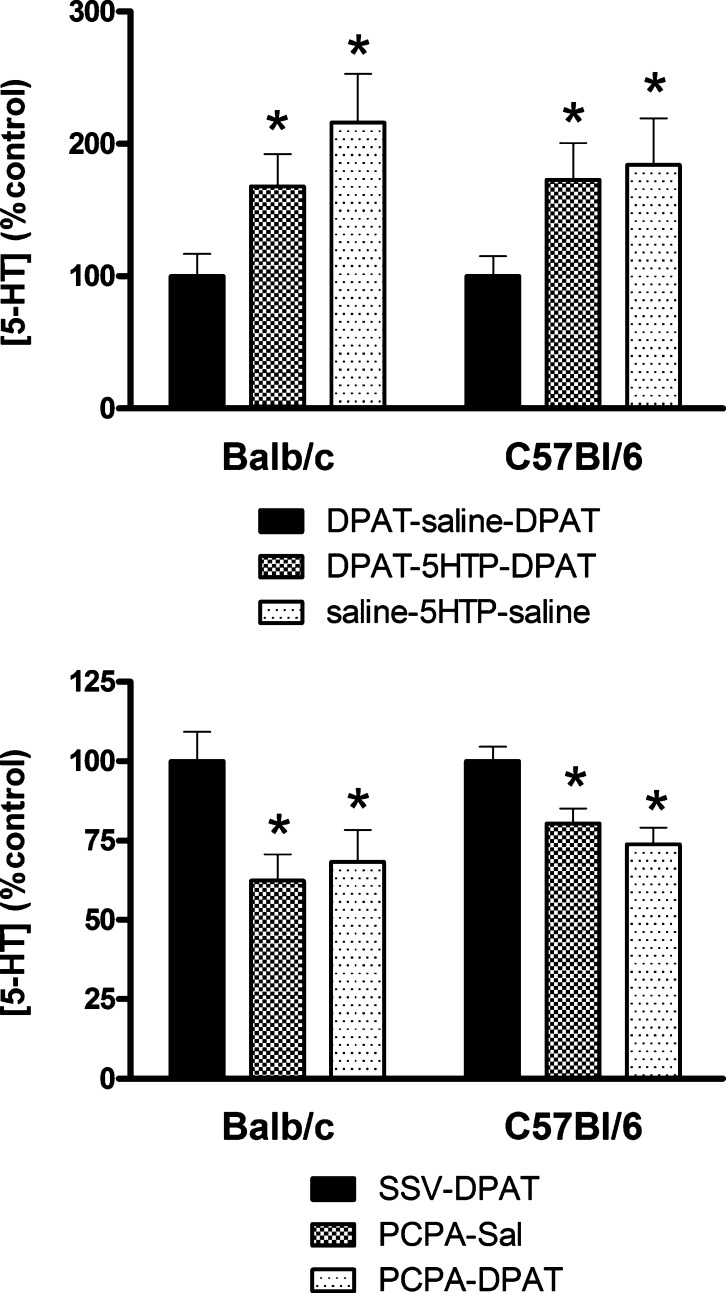
5-HTP treatment significantly increased serotonin levels in the hippocampus by 70–110% compared to control values (Figure 3). There were no differences in the extent of this effect between Balb/c and C57Bl/6 mice. There were also no differences between the magnitude of the 5-HTP effect between mice subsequently tested with 8-OH-DPAT or saline (Figure 3).
Figure 3.
Effect of the serotonin precursor, 5-HTP (top panels), or the serotonin synthesis inhibitor, PCPA (bottom panels) on 5-HT levels in the hippocampus of Balb/c mice and C57Bl/6 mice. *P < 0.05 for difference with saline- or SSV vehicle-treated controls. Treatment with 5-HTP induced a significant increase, whereas PCPA induced a significant decrease in 5-HT levels.
Experiment 3: Effect of PCPA Pretreatment on 8-OH-DPAT-Induced PPI Changes
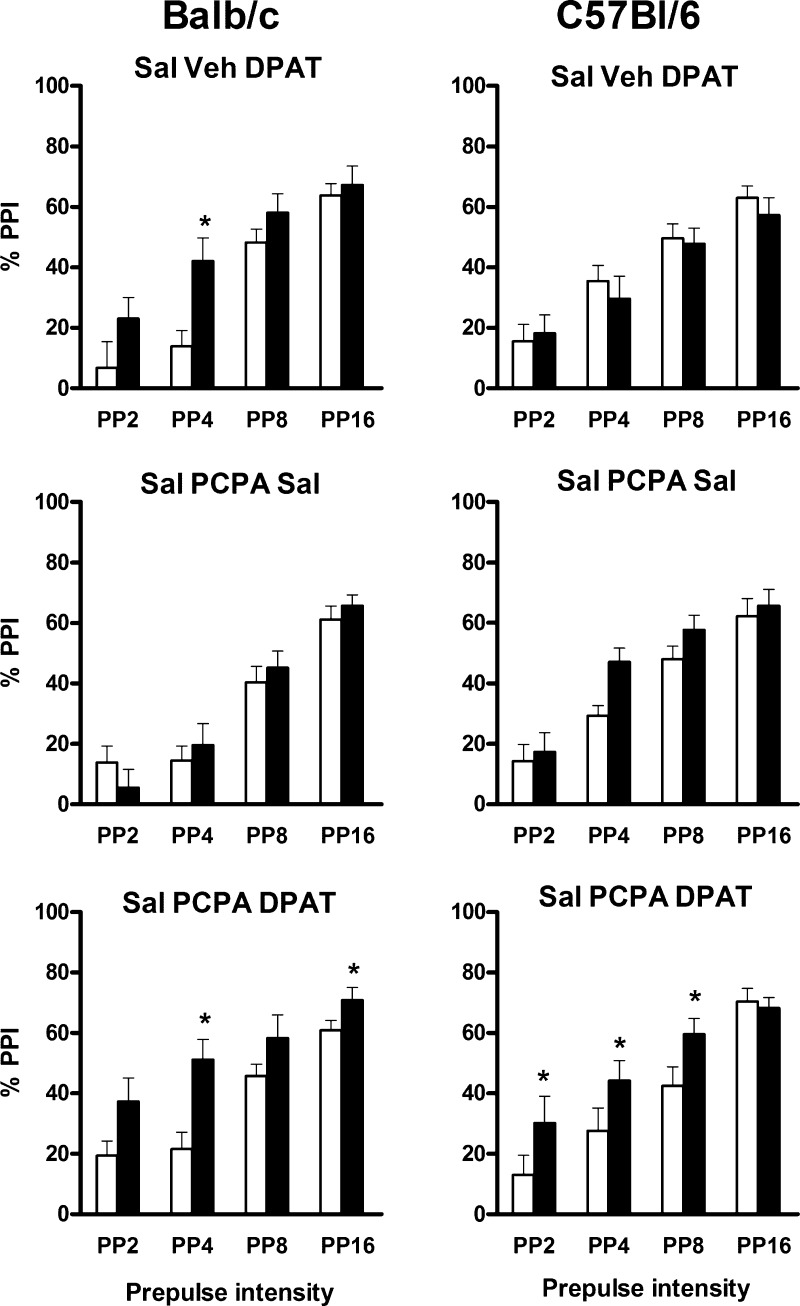
In mice chronically pretreated with vehicle, there was a strain-dependent effect of 8-OH-DPAT administration on PPI (F(1,19) = 7.1, P = 0.015) reflecting the expected increase in PPI in Balb/c mice (effect of dose: F(1,9) = 7.7, P = 0.021; 8-OH-DPAT × PP interaction: F(3,27) = 3.1, P = 0.043; P < 0.05 at PP4) with no effect in C57Bl/6 mice (Figure 4). The effect of 8-OH-DPAT in this cohort of Balb/c mice appeared to be smaller than that in Experiment 1 (Figure 1), which could have been caused by the repeated gavage treatment.
Figure 4.
Effects of pretreatment with the serotonin synthesis inhibitor, PCPA, on the action of 5 mg/kg 8-OH-DPAT on PPI in Balb/c mice (left panels) and C57Bl/6 mice (right panels). Data are presented for each level of prepulse (2, 4, 8, or 16 dB over baseline). Top panels show baseline PPI after injection of saline (white bars) and the effects of 8-OH-DPAT (DPAT, black bars) after treatment with SSV vehicle (Sal Veh DPAT). Middle panels show baseline PPI after injection of saline before (white bars) and after (black bars) treatment with PCPA (Sal PCPA Sal). Bottom panels show baseline PPI after injection of saline (white bars) and the effect of 8-OH-DPAT (DPAT, black bars) after treatment with PCPA (Sal PCPA DPAT). *P < 0.05 for differences with the first PPI session in the same animals. For numbers of animals per group, see Table 3. Injection of 8-OH-DPAT induced an increase of PPI in C57Bl/6 mice after treatment with PCPA but not saline. PCPA treatment did not alter the effects of 8-OH-DPAT in Balb/c mice.
In mice chronically treated with PCPA, there were no differences in PPI in saline-injected mice compared to the control session before PCPA treatment commenced, indicating that PCPA did not affect baseline PPI (Figure 4). After PCPA treatment, PPI was significantly increased by 8-OH-DPAT administration (F(1,19) = 15.7, P = 0.001). However, unlike the result in vehicle-pretreated mice (see above), there was no statistically differential effect between the strains (as indicated by a lack of 8-OH-DPAT × strain interaction) and further ANOVAs were done comparing the effects of 8-OH-DPAT in each strain after PCPA pretreatment versus vehicle pretreatment. In Balb/c mice, this analysis showed the expected increase of PPI after 8-OH-DPAT treatment (dose effect: F(1,18) = 15.5, P < 0.001; 8-OH-DPAT treatment × PP: F(3,54) = 4.8, P = 0.005), and a lack of a PCPA pretreatment × 8-OH-DPAT treatment interaction confirmed that there was no statistically significant change in the action of 8-OH-DPAT on PPI in Balb/c with or without PCPA pretreatment. In contrast, in C57Bl/6 mice, there was a significant interaction between PCPA pretreatment and 8-OH-DPAT treatment (F(1,20) = 6.6, P = 0.019). While there was no effect of 8-OH-DPAT in these mice after saline pretreatment (see above), after PCPA pretreatment, PPI was significantly increased in C57Bl/6 mice by 8-OH-DPAT injection (main effect: F(1,10) = 7.9, P = 0.019; 8-OH-DPAT × PP interaction: F(3,30) = 3.3, P = 0.033; P < 0.05 at PP2, PP4, and PP8).
In animals chronically pretreated with saline, startle amplitudes tended to be higher in C57Bl/6 mice than in Balb/c mice (F(1,19) = 5.5, P = 0.030). 8-OH-DPAT significantly reduced startle amplitudes in both strains in this condition (main effect: F(1,19) = 30.5, P < 0.001). In the animals chronically pretreated with PCPA, startle amplitudes were again higher in C57Bl/6 mice than in Balb/c mice (F(1,22) = 9.3, P = 0.006). In this group of mice, there were no differences in startle amplitudes after saline injection between PCPA treatment and baseline (Table 3). In the third group of mice, which was pretreated chronically with PCPA, 8-OH-DPAT significantly reduced startle (F(1,19) = 38.2, P < 0.001).
Table 3. Average Startle Amplitudes in Balb/c Mice and C57Bl/6 Mice after Treatment with Saline or 8-OH-DPAT with or without Treatment with Parachlorophenylalanine (PCPA)a.
| Balb/c | C57Bl/6 | |
|---|---|---|
| saline–vehicle–8-OH-DPAT | ||
| before | 155 ± 23 | 269 ± 4 |
| after | 80 ± 20 | 210 ± 44 |
| saline–PCPA–saline | ||
| before | 186 ± 26 | 289 ± 43 |
| after | 152 ± 23 | 302 ± 31 |
| saline–PCPA–8-OH-DPAT | ||
| before | 180 ± 22 | 234 ± 46 |
| after | 84 ± 12 | 174 ± 39 |
Data are mean arbitrary system units ± SEMs for n = 10–12 mice per group. For statistical comparisons, see text.
PCPA treatment caused a modest, but significant reduction of serotonin levels in the hippocampus (20–40% compared to control values). There were no differences in the effects of PCPA between Balb/c and C57Bl/6 mice or between mouse groups subsequently tested with 8-OH-DPAT or with saline (Figure 3).
Discussion
In this study, we used the previously described strain differences in the effects of 8-OH-DPAT on PPI between Balb/c mice and C57Bl/6 mice as a tool to explore the role of 5-HT1A receptors in PPI and to investigate if there were conditions under which we could alter strain-specific responses to resemble those of the opposite strain. Ultimately, the background to these studies was the validity of PPI as a cross-species phenomenon, which has been questioned by several disparate pharmacological study findings in humans, rats, and mice. Here, we show that either the properties of the drugs used to modulate PPI, or the background activity of the relevant neurotransmitter system, that is, 5-HT in this case, greatly affects the directions of the changes in PPI. These effects were independent of changes in startle elicited by some of the treatments.
The first series of experiments included a range of 5-HT1A receptor ligands which are commonly used as a full agonist (8-OH-DPAT), a silent antagonist (WAY100,635) or partial agonists. Grouping the results by their strain-specific actions (Figure 1) revealed a continuum from 8-OH-DPAT and BMY7378 inducing significant increases in PPI in Balb/c, but not C57Bl/6 mice, to NAN190 and WAY100,635 inducing increases in PPI in C57Bl/6 but not Balb/c mice. Buspirone, MDL73,005EF, and S15,535 had little effect on PPI in either strain.
The differential effects of 8-OH-DPAT in Balb/c and C57Bl/6 mice are similar to our previous findings.14,20 The similar action of BMY7378 on PPI has not been reported before. This drug has been introduced as a 5-HT1A receptor partial agonist, with effects similar to 8-OH-DPAT on 5-HT1A autoreceptors30,31 but antagonist properties on behavioral effects dependent on 5-HT1A heteroreceptors.32−34 By analogy, this would suggest that the actions of 8-OH-DPAT on PPI in Balb/c mice are mediated by 5-HT1A autoreceptors. However, it is then surprising that none of the other 5-HT1A receptor partial agonists increased PPI in this strain. It is possible that the high affinity of BMY7378 for α1D adrenoceptors35 plays a role in its effects on PPI but an involvement of this receptor subtype in PPI has not been demonstrated before and would need further experiments to confirm.
The effects of the various 5-HT1A receptor ligands on PPI in Balb/c mice contrasted sharply with their effects in C57Bl/6 mice. In the latter strain, both the 5-HT1A receptor antagonist, WAY100,635 and the partial agonist, NAN-190 increased PPI with the other drugs not having effects. One explanation for the effects of these drugs is that C57Bl/6 mice display high tonic serotonergic activity, which presumably via 5-HT1A receptor-mediated stimulation of dopamine release in these animals, leads to relatively low PPI. Indeed, C57Bl/6 mice have been shown to have low resting PPI compared to many other mouse strains,36 although this was not consistently seen in the present study when C57Bl/6 and Balb/c mice were compared (Figure 1). The presence of 5-HT1A receptors has been demonstrated in the ventral tegmental area (VTA)37 and administration of a selective 5-HT1A receptor antagonist decreased the number of spontaneously active A10 DA neurons and their degree of bursting in this region.38 By antagonizing 5-HT1A receptors, treatment with WAY100,635 might reduce mesolimbic dopamine release and, consequently, increase PPI similar to what has been shown with antipsychotic treatment in this strain.39 The similarity of the effects of NAN-190 with those of WAY100,635 suggest that NAN-190 acts as a 5-HT1A receptor antagonist in this environment. NAN-190 has been shown to display 5-HT1A receptor agonist properties in some in vivo paradigms but antagonist properties in others.40−43 However, an additional action of NAN-190 at dopamine autoreceptors44 and α1 adrenoceptors45,46 cannot be excluded. Both receptor systems are clearly involved in the regulation of PPI.6
While BMY7378 mimicked the action of 8-OH-DPAT on PPI in Balb/c mice and NAN-190 mimicked the action of WAY100,635 on PPI in C57Bl/6 mice, it is somewhat surprising that none of the other putative 5-HT1A receptor partial agonists (buspirone, MDL73,005EF, S15,535) had effects on PPI in either strain. Previous studies have shown either agonist or antagonist effects of buspirone, MDL73,005EF, and S15,535 depending on the experimental conditions.42−44,47−50 One explanation for their lack of effects on PPI could be that these compounds display a mix of agonist/antagonist properties in the experimental conditions used here that essentially cancel each other out. In other words, while a 5-HT1A receptor agonist action predominates for BMY7378 and a 5-HT1A receptor antagonist action predominates for NAN-190, the other compounds may have mixed actions that preclude a clear “net” effect on PPI under the present conditions. The lack of effect of buspirone on PPI in C57Bl/6 mice is consistent with an earlier report,39 although this compound was not tested in Balb/c mice. The effects of MDL73,005EF and S15,535 on PPI have not been described before.
To investigate further differences between Balb/c mice and C57Bl/6 mice in PPI regulation, we treated each strain of mice with the 5-HT precursor, 5-HTP to increase,27,28 or the tryptophan hydroxylase inhibitor, PCPA, to decrease serotonin synthesis and, presumably, serotonergic activity,29 and tested the effects of 8-OH-DPAT on PPI. 5-HTP pretreatment increased baseline PPI in C57Bl/6 mice, as measured after an acute saline injection. Compared to this 5-HTP-induced higher baseline, a parallel cohort of 5-HTP plus 8-OH-DPAT-treated mice showed a significantly lower level of PPI, suggesting that after 5-HTP pretreatment, it is possible to induce disruption of PPI in C57Bl/6 mice by acute treatment with 8-OH-DPAT. This is diametrically opposite to the commonly observed PPI-increasing effect of 8-OH-DPAT in mice, including in Balb/c mice in the present experiments (Figure 1), and is akin to the effect of 8-OH-DPAT in the majority of published studies in rats. 5-HTP treatment had no effect in Balb/c mice.
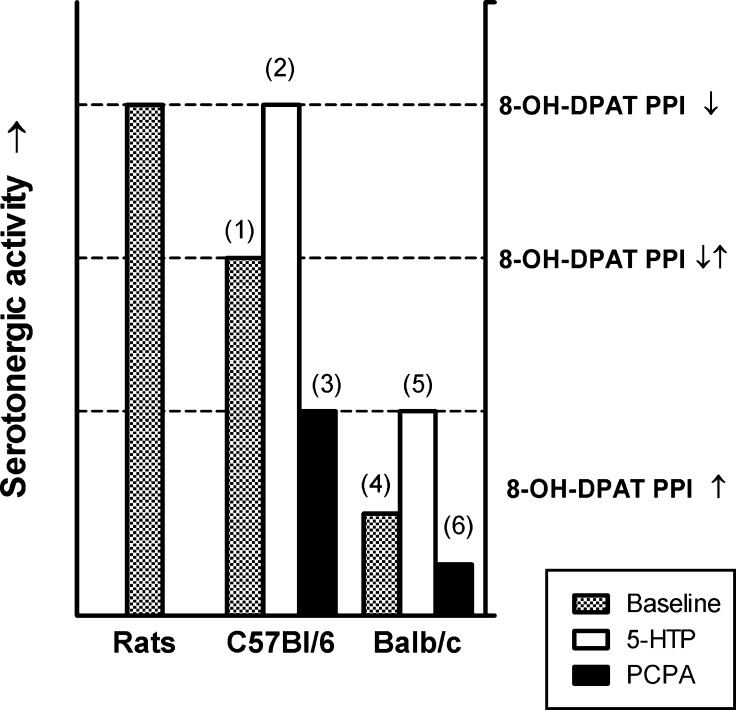
While 5-HTP pretreatment was used to enhance serotonin synthesis in the brain, the reverse experiment was to reduce serotonin synthesis by PCPA pretreatment. In C57Bl/6 mice pretreated with PCPA, 8-OH-DPAT induced an increase in PPI. As expected, 8-OH-DPAT already had this effect in control Balb/c mice and PCPA did not alter this. Together with the other results obtained in this study, these data indicate that in C57Bl/6 mice, the effects of 8-OH-DPAT can be made to increase PPI (in the case of PCPA pretreatment), decrease of PPI (in the case of 5-HTP pretreatment), or have no apparent effect (no pretreatment) (Figure 5). This shift in the action of 8-OH-DPAT resembles that of a shift of the effects of 5-HT1A receptor ligands from an increase in PPI in Balb/c mice (seen after 8-OH-DPAT and BMY7378) to an increase in PPI in C57Bl/6 mice (seen with NAN-190 and WAY100,635). Thus, the latter effect may be explained by high serotonergic activity that masks 8-OH-DPAT-induced increases in PPI in C57Bl/6 mice and that can be reinstated by reducing serotonergic activity with PCPA (Figure 5). As an extension of this idea, it can be speculated that the apparent lack of effect of 8-OH-DPAT on PPI in C57Bl/6 is in fact a mix of a “Balb/c-like” increase and a “ratlike” decrease of PPI. This would also explain how further increasing serotonergic activity in this strain with 5-HTP unmasks a “ratlike” effect, whereas reducing serotonergic activity with PCPA unmasks a “Balb/c-like” effect of 8-OH-DPAT (Figure 5). It would be of interest to test the effects of different 5-HT1A receptor ligands after 5-HTP or PCPA treatment in C57Bl/6 and Balb/c mice. The prediction would be, that the effects of these other drugs would be altered by increasing or decreasing endogenous 5-HT synthesis, similar to the shift in the effects of 8-OH-DPAT. In Balb/c mice, which are known to have a polymorphism in Tph2, resulting in lower serotonin synthesis in the brain,24−26 it was not surprising that PCPA treatment had no further effect. Surprisingly, however, unlike our prediction (see the introduction section), treatment with 5-HTP, in an attempt to “boost” serotonin synthesis, also did not alter the responses of these animals to 8-OH-DPAT. It is possible that higher doses or more prolonged 5-HTP treatment are needed in Balb/c mice to significantly enhance low serotonin synthesis and further experiments are needed to clarify this (Figure 5).
Figure 5.
Hypothesized interactions of the serotonin system, 5-HT1A receptor activation/inhibition, and prepulse inhibition. (1) In C57Bl/6 mice, serotonergic activity is higher but 8-OH-DPAT has no apparent effect on PPI because it induces both an increase and a decrease in PPI that cancel each other out. (2) Increases in serotonergic activity in response to treatment with 5-HTP in C57Bl/6 mice unmask a “ratlike” decrease/disruption of PPI. (3) A decrease in serotonergic activity in C57Bl/6 mice in response to treatment with PCPA unmasks a “Balb/c-like” increase in PPI. (4) Because of a polymorphism in the tryptophan hydroxylase 2 gene, Balb/c have lower serotonergic activity compared to C57Bl/6 mice and show an 8-OH-DPAT-induced increase in PPI. (5) 5-HTP treatment at the present dose is not able to increase serotonergic activity sufficiently in Balb/c mice to unmask a “C57Bl/6-like” effect of 8-OH-DPAT. (6) Reducing serotonergic activity in Balb/c mice further with PCPA does not alter PPI responses to 8-OH-DPAT. In this scheme, blocking high baseline serotonergic activity in C57Bl/6 with a 5-HT1A receptor antagonist elicits a “Balb/c-like” increase in PPI. This was observed with WAY100,635 and NAN-190 in the present experiments.
PPI is a stable phenomenon over repeated consecutive daily testing.51−53 It is therefore unlikely that the effects of 5-HTP or PCPA can be explained by changes in PPI over the repeated-dosing protocol or that differences in the lengths of the experiments played a major role in the results obtained with 5-HTP and PCPA. Nevertheless, further studies could include repeated saline injections to control for time-related changes in baseline PPI.
The neuroanatomical/functional explanations for these shifts in the directions of effects between one ligand under different conditions (see 8-OH-DPAT between control, 5-HTP pretreatment, and PCPA pretreatment conditions, Figure 5) or between different ligands with a range of partial agonist activities, remains to be elucidated. One possibility is that the effect of a given 5-HT1A receptor ligand on PPI is a mix of actions at 5-HT1A autoreceptors on raphe nucleus cell bodies and 5-HT1A heteroreceptors elsewhere in the brain. The latter could include 5-HT1A receptors in other systems involved in PPI regulation, such as the mesolimbic dopamine system in the VTA (e.g., ref (37); however, see ref (54)), or the prefrontal cortex (e.g., ref (55)). The present results suggest that the net result on PPI of activating this mix of 5-HT1A auto- and heteroreceptors at different sites may depend on endogenous serotonergic activity. It would be of great interest to distinguish the activation of these different populations of 5-HT1A receptors, for example, by microinjection studies,56 selective lesion studies,57 or using ligands with preferential pre- versus postsynaptic actions.58 Alternatively, recently introduced genetically modified mice with selective 5-HT1A auto- or heteroreceptor inactivation could be used to dissect the relative contribution of these receptor subpopulations in PPI regulation.59,60 Such experiments might also further clarify the marked differences between C57Bl/6 mice and Balb/c mice.
Increasingly, and also shown in the present study, it is becoming clear that PPI changes in one and the same species/strain can be in either direction, most likely mediated by differential involvement of regional populations of a given receptor, a number of regulatory inputs from brain regions other than the nucleus accumbens, or neurotransmitter systems other than dopamine. Thus, it is the relative contribution of these inputs that ultimately determines the direction of PPI changes and results in the predominant response seen in particular species or strains. This means that PPI can still be considered a “cross-species” phenomenon. However, the relative and species-specific contribution of one or more of a large number of modulatory systems needs to be taken into account when interpreting the data. This is important not only for the study of fundamental brain mechanisms involved in disruptions of PPI, for example, as seen in schizophrenia, but also for drug development. In the latter case, effects on PPI of new compounds that act on a number of neurotransmitter receptor systems are likely to be multidimensional and may make it more complicated to extrapolate the results from preclinical experiments to the clinic.
Methods
Animals
Male Balb/c mice and C57Bl/6 mice were obtained from Monash Animal Services, Monash University, Australia. The animals were 10–14 weeks of age at the time of PPI studies. They were housed in groups of 3–5 in standard mouse cages, with free access to standard pellet food and water. The mice were maintained on a 12-h light–dark cycle (lights on at 06:30), at a constant temperature of 22 ± 2 °C. All treatments and experimental protocols were in accordance with the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes (1990) set out by the National Health and Medical Research Council of Australia.
Prepulse Inhibition (PPI)
PPI of the acoustic startle response was measured with eight automated startle chambers (SR-Lab; San Diego Instruments, San Diego, CA) as previously described.61,62 Briefly, mice were placed individually into a transparent Plexiglas cylinder in a sound-attenuating cabinet. The cylinder had a piezoelectric movement sensor mounted underneath to record startle responses, expressed in arbitrary SR-Lab system units. Irrespective of their treatment, all mice underwent the same PPI session, which comprised 104 trials presented with variable intertrial intervals (8–27 s), including 32 pulse-alone trials (4 blocks of 8115 dB trials) and 64 prepulse–pulse trials. The 32 115 dB pulse-alone trials were delivered as one block of 8 at the start, one block of 8 at the end, and 16 trials pseudorandomly mixed into the main part of the PPI session. This sequence of pulse-alone trials was included to allow calculation of startle habituation although that parameter was not included in the present results. In addition to 16 115 dB pulse-alone stimuli, the middle 88 trials consisted of pseudorandom delivery of 8 trials during which no stimulus was delivered, and 64 prepulse–pulse trials. “No stimulus” trials were included to monitor if there were gross motor side effects of the drug treatments that could interfere with the startle responses. Prepulse–pulse trials consisted of a prepulse (PP) of an intensity of 2, 4, 8, or 16 dB above the 70 dB background (PP2, PP4, PP8, or PP16, respectively, 8 trials at each intensity), followed by a designated interstimulus interval (ISI) and then the 115 dB startle pulse. We used two ISIs, 30 and 100 ms, between the onset of the prepulse and the onset of the startle pulse.63 Because there were few effects of the various drugs on PPI at the 30 ms ISI, only data obtained with the 100 ms ISI are presented here.
In prepulse inhibition studies, startle must be presented. While PPI is calculated as percentage of startle responses, extremely high or low startle values may skew the PPI results. Average startle was calculated from all 4 blocks of pulse-alone trials. However, for the calculation of % PPI, only the 16 pulse-alone trials in the main component of the session were used. The % PPI was calculated as [(pulse-alone trials startle response amplitude – prepulse–pulse trials startle amplitude)/(pulse-alone trials startle amplitude)] × 100%.
Experiment 1
Drugs were dissolved or diluted in saline to doses that were selected on the basis of the literature (see below). All drugs were administered intraperitoneally in a volume of 10 mL/kg. In a randomized, crossover protocol, all mice in each cohort of C57Bl/6 and Balb/c mice received all doses of one particular drug, with 3–4 days allowed between each PPI session. Mice were not reused for other drug treatments or experiments after this sequence of PPI tests.
Drugs in Experiment 1 included the 5-HT1A receptor agonist, 8-OH-DPAT ((±)-8-hydroxy-2-(dipropylamino)tetralin, Tocris, Bristol, U.K.),14,20,22 the 5-HT1A receptor antagonist, WAY100,635 (N-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl]-N-2-pyridinylcyclohexane carboxamide maleate salt, Sigma-Aldrich),64 and the 5-HT1A receptor partial agonists buspirone (N-[4-[4-(2-pyrimidinyl)-1-piperazinyl]butyl]-8-azaspiro[4.5]decane-7,9-dione hydrochloride, Sigma),41,65 BMY7378 (8-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl]-8-azaspiro[4.5]decane-7,9-dione dihydrochloride, Sigma),30−34 MDL73,005EF (8-[2-(1,4-benzodioxan-2-ylmethylamino)ethyl]-8-azaspiro [4.5]decane-7,9-dione hydrochloride, Tocris),42,49 NAN-190 (1-(2-methoxyphenyl)-4-[4-(2-phthalimido)butyl]piperazine hydrobromide, Sigma),40−43 and S15,535 (1-(2,3-dihydro-1,4-benzodioxin-5-yl)-4-(2,3-dihydro-1h-inden-2-yl)-piperazine, Sigma).43,47,48,50
Based upon previous literature (see above) all drugs were administered at 0 (saline vehicle), 0.2, 1, and 5 mg/kg doses, except BMY7378. Initial experiments with the latter drug showed marked effects at the lowest dose, whereupon a second cohort of mice was treated with 0 (saline vehicle), 0.04, 0.2, and 1 mg/kg. Data from these two cohorts were then combined. The total number of BMY7378-treated mice per strain therefore depended on the dose: for all doses, n = 24, except 0.04 and 5 mg/kg for which n = 12. For all other drugs there were 8–12 animals per strain per cohort, except for the NAN-190 treatment, which was tested in the same dose range in two cohorts, the data from which were then combined to result in n = 24.
Experiment 2
Balb/c and C57Bl/6 mice were twice tested for PPI. The first session was a control session without 5-HTP treatment. The second session, the next day, was carried out 1 h after intraperitoneal injection of either 100 mg/kg of 5-HTP66,67 (Sigma) or saline. Immediately before each PPI session, mice were injected with either 5 mg/kg 8-OH-DPAT or saline. Thus, there were three cohorts of 10–12 mice of each strain: cohort 1 received 8-OH-DPAT on day 1 followed on day 2 by 8-OH-DPAT after saline pretreatment; cohort 2 received 8-OH-DPAT on day 1 followed on day 2 by 8-OH-DPAT after 5-HTP pretreatment; cohort 3 received saline on day 1 followed on day 2 by saline after 5-HTP pretreatment. Because of the short time-course of action of 5-HTP, in a separate session 1 week after the PPI tests, mice were once again injected with saline or 5-HTP and were killed 1 h later by cervical dislocation. This way, central 5-HT levels could be assessed with a similar time-course as the behavioral experiments. As a representative brain region with high levels of 5-HT, the hippocampus was rapidly dissected and frozen on dry ice for the analysis of 5-HT levels.
As outlined above, these studies with 5-HTP pretreatment were done in three parallel cohorts of mice. To ascertain that the apparent induction of an 8-OH-DPAT-induced PPI disruption in C57Bl/6 mice could also be seen in one and the same cohort, we did an additional pilot experiment (n = 4 mice). On the first day, C57Bl/6 mice received saline and were tested for PPI. The next day, mice were first treated with 5-HTP as described above and, 1 h later, received saline and again were tested for PPI. Immediately after this PPI session, animals were injected with 8-OH-DPAT and retested for PPI. The results from this pilot cohort showed that baseline (saline) PPI at the 100 ms ISI went from 36.3 ± 6.3% to 61.6 ± 8.8% after 5-HTP treatment, similar to the data in Figure 2. In these animals, 8-OH-DPAT treatment reduced PPI to 39.6 ± 8.6%, confirming that 8-OH-DPAT can induce a “ratlike” disruption of PPI in C57Bl/6 mice provided they are given 5-HTP pretreatment, which significantly boosts 5-HT levels in the brain (Figure 3).
Experiment 3
Balb/c and C57Bl/6 mice were twice tested for PPI. On the first day, the animals were injected intraperitoneally with saline (to control for potential injection effects) and tested for PPI. On day 2, 3, and 4, mice were treated by gavage with 100 mg/kg PCPA25,68 (Sigma) in standard suspension vehicle (SSV, 0.4% Tween 80, 0.5% benzylalcohol, 0.9% NaCl, 0.5% carboxymethyl-cellulose sodium, all compounds from Sigma) or SSV only. Twenty-four hours after the last PCPA treatment, mice were intraperitoneally injected with saline or 5 mg/kg 8-OH-DPAT and again tested for PPI. Thus, there were three cohorts of 10–12 mice of each strain for Experiment 3: cohort 1 received saline on day 1, SSV on days 2, 3, and 4, and 8-OH-DPAT on day 5; cohort 2 received saline on day 1, PCPA on days 2, 3, and 4, and saline on day 5; cohort 3 received saline on day 1, PCPA on days 2, 3, and 4, and 8-OH-DPAT on day 5. All cohorts were tested in parallel. Within 1 h after the PPI session on day 5, all mice were killed by cervical dislocation and the hippocampus was rapidly dissected and frozen on dry ice for the analysis of 5-HT levels.
5-HT ELISA
Brain samples from 5-HTP and PCPA-treated mice were stored at −80 °C until they were assayed for serotonin content using Serotonin ELISA kits (Labor Diagnostika Nord, Nordhorn, Germany). Samples were briefly thawed on ice and 500 μL of 0.1 N perchloric acid (BDH Laboratory Supplies, Poole, England) was added to each sample. The samples were then homogenized and centrifuged (5 min, 4 °C, 15 500g), and the supernatants were used in the assay. Standards of known serotonin concentrations were diluted in 0.1 N perchloric acid. The serotonin in the standards and samples was acetylated prior to the immunoassay itself to enable antibody detection. ELISA plates were read at 450 nm, and the serotonin content of the samples was extrapolated by comparing the optical density of the samples (mean of duplicates) to that of the standard curve (GraphPad Prism 4, GraphPad Software, San Diego, CA). Because the 5-HT assays were done over multiple plates and at different times, all data are expressed as percentages of the controls run on the same day/plate to compensate for variability across assay days.
Statistical Analysis
All data are expressed as mean ± standard error of the mean (SEM). Differences between groups were assessed by analysis of variance (ANOVA) with repeated measures where appropriate. Thus, between-group factors were strain (Balb/c vs C57Bl/6), while within-group repeated measures were dose or prepulse intensity. The effects of BMY7378 were analyzed for each dose separately, as not all animals received all doses. In Experiment 1, the effects of the various drug doses were compared to PPI and startle after saline injection in the same animals, thus using each mouse as its own control. In Experiments 2 and 3, changes in PPI after experimental treatments (vehicle, 5-HTP, or PCPA) were compared to those before treatment.
Acknowledgments
The author is a Senior Research Fellow of the National Health and Medical Research Council of Australia (NHMRC). The technical assistance of Emma Ruimschotel, Thijs Crommentuijn, and Sally Martin is gratefully acknowledged.
These studies were supported by the Percy Baxter Charitable Trust and Operational Infrastructure Funding from the Victorian State Government.
The authors declare no competing financial interest.
References
- Braff D. L.; Geyer M. A.; Swerdlow N. R. (2001) Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology 156, 234–258. [DOI] [PubMed] [Google Scholar]
- Kumari V.; Sharma T. (2002) Effects of typical and atypical antipsychotics on prepulse inhibition in schizophrenia: a critical evaluation of current evidence and directions for future research. Psychopharmacology 162, 97–101. [DOI] [PubMed] [Google Scholar]
- Koch M. (1999) The neurobiology of startle. Prog. Neurobiol. 59, 107–128. [DOI] [PubMed] [Google Scholar]
- Swerdlow N. R.; Geyer M. A. (1998) Using an animal model of deficient sensorimotor gating to study the pathophysiology and new treatments of schizophrenia. Schizophr. Bull. 24, 285–301. [DOI] [PubMed] [Google Scholar]
- Swerdlow N. R.; Caine S. B.; Braff D. L.; Geyer M. A. (1992) The neural substrates of sensorimotor gating of the startle reflex: a review of recent findings and their implications. J. Psychopharmacol. 6, 176–190. [DOI] [PubMed] [Google Scholar]
- Geyer M. A.; Krebs-Thomson K.; Braff D. L.; Swerdlow N. R. (2001) Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology 156, 117–154. [DOI] [PubMed] [Google Scholar]
- van den Buuse M. (2010) Modeling the positive symptoms of schizophrenia in genetically modified mice: Pharmacology and methodology aspects. Schizophr. Bull. 36, 246–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow N. R., Braff D. L., Bakshi V. P., and Geyer M. A. (1996) An animal model of sensorimotor gating deficits in schizophrenia predicts antipsychotic drug action. In Antipsychotics (Csernansky J. G., Ed.), Springer, Berlin. [Google Scholar]
- Swerdlow N. R.; Braff D.; Geyer M. A. (1999) Cross-species studies of sensorimotor gating of the startle reflex. Ann. N.Y. Acad. Sci. 877, 202–216. [DOI] [PubMed] [Google Scholar]
- Swerdlow N. R.; Martinez Z. A.; Hanlon F. M.; Platten A.; Farid M.; Auerbach P.; Braff D. L.; Geyer M. A. (2000) Towards understanding the biology of a complex phenotype: rat strain and substrain differences in the sensorimotor gating-disruptive effects of dopamine agonists. J. Neurosci. 20, 4325–4336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talledo J. A.; Sutherland Owens A. N.; Schortinghuis T.; Swerdlow N. R. (2009) Amphetamine effects on startle gating in normal women and female rats. Psychopharmacology 204, 165–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abel K. M.; Allin M. P. G.; Hemsley D. R.; Geyer M. A. (2003) Low dose ketamine increases prepulse inhibition in healthy men. Neuropharmacology 44, 729–737. [DOI] [PubMed] [Google Scholar]
- Brody S. A.; Geyer M. A.; Large C. H. (2003) Lamotrigine prevents ketamine but not amphetamine-induced deficits in prepulse inhibition in mice. Psychopharmacology 169, 240–246. [DOI] [PubMed] [Google Scholar]
- Gogos A.; Bogeski M.; Van den Buuse M. (2008) Role of serotonin-1A receptors in the action of antipsychotic drugs: comparison of prepulse inhibition studies in mice and rats and relevance for human pharmacology. Behav. Pharmacol. 19, 548–561. [DOI] [PubMed] [Google Scholar]
- Swerdlow N. R.; Braff D. L.; Geyer M. A. (2000) Animal models of deficient sensorimotor gating: what we know, what we think we know, and what we hope to know soon. Behav. Pharmacol. 11, 185–204. [DOI] [PubMed] [Google Scholar]
- Swerdlow N. R.; Weber M.; Qu Y.; Light G. A.; Braff D. L. (2008) Realistic expectations of prepulse inhibition in translational models for schizophrenia research. Psychopharmacology 199, 331–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bantick R. A.; Deakin J. F.; Grasby P. M. (2001) The 5-HT1A receptor in schizophrenia: a promising target for novel atypical neuroleptics?. J. Psychopharmacol. 15, 37–46. [DOI] [PubMed] [Google Scholar]
- Burnet P. W.; Eastwood S. L.; Harrison P. J. (1996) 5-HT1A and 5-HT2A receptor mRNAs and binding site densities are differentially altered in schizophrenia. Neuropsychopharmacology 15, 442–455. [DOI] [PubMed] [Google Scholar]
- Newman-Tancredi A. (2010) The importance of 5-HT1A receptor agonism in antipsychotic drug action: Rationale and perspectives. Curr. Opin. Invest. Drugs 11, 802–812. [PubMed] [Google Scholar]
- Van den Buuse M.; Martin S.; Holgate J.; Matthaei K.; Hendry I. (2007) Mice deficient in the alpha subunit of Gz show changes in pre-pulse inhibition, anxiety and responses to 5-HT1A receptor stimulation, which are strongly dependent on the genetic background. Psychopharmacology 195, 273–283. [DOI] [PubMed] [Google Scholar]
- Dulawa S. C.; Geyer M. A. (2000) Effects of strain and serotonergic agents on prepulse inhibition and habituation in mice. Neuropharmacology 39, 2170–2179. [DOI] [PubMed] [Google Scholar]
- Dulawa S. C.; Gross C.; Stark K. L.; Hen R.; Geyer M. A. (2000) Knockout mice reveal opposite roles for serotonin 1A and 1B receptors in prepulse inhibition. Neuropsychopharmacology 22, 650–659. [DOI] [PubMed] [Google Scholar]
- Dulawa S. C.; Hen R.; Scearce-Levie K.; Geyer M. A. (1997) Serotonin1B receptor modulation of startle reactivity, habituation, and prepulse inhibition in wildt-ype and serotonin1B knockout mice. Psychopharmacology 132, 125–134. [DOI] [PubMed] [Google Scholar]
- Calcagno E.; Canetta A.; Guzzetti S.; Cervo L.; Invernizzi R. W. (2007) Strain differences in basal and post-citalopram extracellular 5-HT in the mouse medial prefrontal cortex and dorsal hippocampus: relation with tryptophan hydroxylase-2 activity. J. Neurochem. 103, 1111–1120. [DOI] [PubMed] [Google Scholar]
- Cervo L.; Canetta A.; Calcagno E.; Burbassi S.; Sacchetti G.; Caccia S.; Fracasso C.; Albani D.; Forloni G.; Invernizzi R. W. (2005) Genotype-dependent activity of tryptophan hydroxylase-2 determines the response to citalopram in a mouse model of depression. J. Neurosci. 25, 8165–8172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siesser W. B.; Zhang X.; Jacobsen J. P. R.; Sotnikova T. D.; Gainetdinov R. R.; Caron M. G. (2010) Tryptophan hydroxylase 2 genotype determines brain serotonin synthesis but not tissue content in C57Bl/6 and BALB/c congenic mice. Neurosci. Lett. 481, 6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artaiz I.; Zazpe A.; Del Río J. (1998) Characterization of serotonergic mechanisms involved in the behavioural inhibition induced by 5-hydroxytryptophan in a modified light-dark test in mice. Behav. Pharmacol. 9, 103–112. [PubMed] [Google Scholar]
- Jacobsen J. P.; Nielsen E. Ø.; Hummel R.; Redrobe J. P.; Mirza N.; Weikop P. (2008) Insensitivity of NMRI mice to selective serotonin reuptake inhibitors in the tail suspension test can be reversed by co-treatment with 5-hydroxytryptophan. Psychopharmacology 199, 137–150. [DOI] [PubMed] [Google Scholar]
- Dailly E.; Chenu F.; Petit-Demoulière B.; Bourin M. (2005) Specificity and efficacy of noradrenaline, serotonin depletion in discrete brain areas of Swiss mice by neurotoxins. J. Neurosci. Methods 150, 111–115. [DOI] [PubMed] [Google Scholar]
- Hjorth S.; Bengtsson H. J.; Milano S.; Lundberg J. F.; Sharp T. (1995) Studies on the role of 5-HT1A autoreceptors and α1-adrenoceptors in the inhibition of 5-HT release--I. BMY7378 and prazosin. Neuropharmacology 34, 615–620. [DOI] [PubMed] [Google Scholar]
- Fornal C. A.; Marrosu F.; Metzler C. W.; Tada K.; Jacobs B. L. (1994) Effects of the putative 5-hydroxytryptamine1A antagonists BMY 7378, NAN 190 and (-)-propranolol on serotonergic dorsal raphe unit activity in behaving cats. J. Pharmacol. Exp. Ther. 270, 1369–1366. [PubMed] [Google Scholar]
- Mendelson S. D.; Quartermain D.; Francisco T.; Shemer A. (1993) 5-HT1A receptor agonists induce anterograde amnesia in mice through a postsynaptic mechanism. Eur. J. Pharmacol. 236, 177–182. [DOI] [PubMed] [Google Scholar]
- Millan M. J.; Rivet J. M.; Canton H.; Le Marouille-Girardon S.; Gobert A. (1993) Induction of hypothermia as a model of 5-hydroxytryptamine1A receptor-mediated activity in the rat: a pharmacological characterization of the actions of novel agonists and antagonists. J. Pharmacol. Exp. Ther. 264, 1364–1376. [PubMed] [Google Scholar]
- Sharp T.; Backus L. I.; Hjorth S.; Bramwell S. R.; Grahame-Smith D. G. (1990) Further investigation of the in vivo pharmacological properties of the putative 5-HT1A antagonist, BMY 7378. Eur. J. Pharmacol. 176, 331–340. [DOI] [PubMed] [Google Scholar]
- Goetz A. S.; King H. K.; Ward S. D.; True T. A.; Rimele T. J.; Saussy D. L. J. (1995) BMY 7378 is a selective antagonist of the D subtype of alpha 1-adrenoceptors. Eur. J. Pharmacol. 272, R5–6. [DOI] [PubMed] [Google Scholar]
- Paylor R.; Crawley J. N. (1997) Inbred strain differences in prepulse inhibition of the mouse startle response. Psychopharmacology 132, 169–180. [DOI] [PubMed] [Google Scholar]
- Doherty M. D.; Pickel V. M. (2001) Targeting of serotonin 1A receptors to dopaminergic neurons within the parabrachial subdivision of the ventral tegmental area in rat brain. J. Comp. Neurol. 433, 390–400. [DOI] [PubMed] [Google Scholar]
- Minabe Y.; Schechter L.; Hashimoto K.; Shirayama Y.; Ashby C. R. J. (2003) Acute and chronic administration of the selective 5-HT1A receptor antagonist WAY-405 significantly alters the activity of midbrain dopamine neurons in rats: an in vivo electrophysiological study. Synapse 50, 181–190. [DOI] [PubMed] [Google Scholar]
- Ouagazzal A.-M.; Jenck F.; Moreau J.-L. (2001) Drug-induced potentiation of prepulse inhibition of acoustic startle reflex in mice: a model for detecting antipsychotic activity?. Psychopharmacology 156, 273–283. [DOI] [PubMed] [Google Scholar]
- Przegaliński E.; Ismaiel A. M.; Chojnacka-Wójcik E.; Budziszewska B.; Tatarczyńska E.; Błaszczyńska E. (1990) The behavioural, but not the hypothermic or corticosterone, response to 8-hydroxy-2-(DI-n-propylamino)-tetralin, is antagonized by NAN-190 in the rat. Neuropharmacology 29, 521–526. [DOI] [PubMed] [Google Scholar]
- Moser P. C. (1991) The effect of putative 5-HT1A receptor antagonists on 8-OH-DPAT-induced hypothermia in rats and mice. Eur. J. Pharmacol. 193, 165–172. [DOI] [PubMed] [Google Scholar]
- Buisson-Defferier S.; Van den Buuse M. (1992) Cardiovascular effects of the 5-HT1A receptor ligand, MDL 73005EF, in conscious spontaneously hypertensive rats. Eur. J. Pharmacol. 223, 133–141. [DOI] [PubMed] [Google Scholar]
- Millan M. J.; Canton H.; Gobert A.; Lejeune F.; Rivet J. M.; Bervoets K.; Brocco M.; Widdowson P.; Mennini T.; Audinot V.; Honore P.; Renouard A.; Le Marouille-Girardon S.; Verriele L.; Gressier H.; Peglion J.-L. (1994) Novel benzodioxopiperazines acting as antagonists at postsynaptic 5-HT1A receptors and as agonists at 5-HT1A autoreceptors: a comparative pharmacological characterization with proposed 5-HT1A antagonists. J. Pharmacol. Exp. Ther. 268, 337–352. [PubMed] [Google Scholar]
- Gobert A.; Lejeune F.; Rivet J. M.; Audinot V.; Newman-Tancredi A.; Millan M. J. (1995) Modulation of the activity of central serotoninergic neurons by novel serotonin1A receptor agonists and antagonists: a comparison to adrenergic and dopaminergic neurons in rats. J. Pharmacol. Exp. Ther. 273, 1032–1046. [PubMed] [Google Scholar]
- Claustre Y.; Rouquier L.; Serrano A.; Bénavidès J.; Scatton B. (1991) Effect of the putative 5-HT1A receptor antagonist NAN-190 on rat brain serotonergic transmission. Eur. J. Pharmacol. 204, 71–77. [DOI] [PubMed] [Google Scholar]
- Sharp T.; Umbers V.; Hjorth S. (1996) The role of 5-HT1A autoreceptors and α1-adrenoceptors in the inhibition of 5-HT release - II NAN-190 and SDZ 216-525. Neuropharmacology 35, 735–741. [DOI] [PubMed] [Google Scholar]
- Dekeyne A.; Brocco M.; Adhumeau A.; Gobert A.; Millan M. J. (2000) The selective serotonin (5-HT)1A receptor ligand, S15535, displays anxiolytic-like effects in the social interaction and Vogel models and suppresses dialysate levels of 5-HT in the dorsal hippocampus of freely-moving rats. A comparison with other anxiolytic agents. Psychopharmacology (Berlin, Ger.) 152, 55–66. [DOI] [PubMed] [Google Scholar]
- Millan M. J.; Rivet J. M.; Canton H.; Lejeune F.; Gobert A.; Widdowson P.; Bervoets K.; Brocco M.; Peglion J. L. (1993) S 15535: a highly selective benzodioxopiperazine 5-HT1A receptor ligand which acts as an agonist and an antagonist at presynaptic and postsynaptic sites respectively. Eur. J. Pharmacol. 230, 99–102. [DOI] [PubMed] [Google Scholar]
- Moser P. C.; Tricklebank M. D.; Middlemiss D. N.; Mir A. K.; Hibert M. F.; Fozard J. R. (1990) Characterization of MDL 73005EF as a 5-HT1A selective ligand and its effects in animal models of anxiety: comparison with buspirone, 8-OH-DPAT and diazepam. Br. J. Pharmacol. 99, 343–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youn J.; Misane I.; Eriksson T. M.; Millan M. J.; Ogren S. O.; Verhage M.; Stiedl O. (2009) Bidirectional modulation of classical fear conditioning in mice by 5-HT1A receptor ligands with contrasting intrinsic activities. Neuropharmacology 57, 567–576. [DOI] [PubMed] [Google Scholar]
- Abel K.; Waikar M.; Pedro B.; Hemsley D.; Geyer M. A. (1998) Repeated testing of prepulse inhibition and habituation of the startle reflex: a study in healthy human controls. J. Psychopharmacol. 12, 330–337. [DOI] [PubMed] [Google Scholar]
- Schulz B.; Fendt M.; Pedersen V.; Koch M. (2001) Sensitization of prepulse inhibition deficits by repeated administration of dizocilpine. Psychopharmacology 156, 177–181. [DOI] [PubMed] [Google Scholar]
- Wolf R.; Dobrowolny H.; Matzke K.; Paelchen K.; Bogerts B.; Schwegler H. (2005) Prepulse inhibition is different in two inbred mouse strains (CPB-K and BALB/cJ) with different hippocampal NMDA receptor densities. Behav. Brain Res. 166, 78–84. [DOI] [PubMed] [Google Scholar]
- Prisco S.; Pagannone S.; Esposito E. (1994) Serotonin-dopamine interaction in the rat ventral tegmental area: an electrophysiological study in vivo. J. Pharmacol. Exp. Ther. 271, 83–90. [PubMed] [Google Scholar]
- Diaz-Mataix L.; Scorza M. C.; Bortolozzi A.; Toth M.; Celada P.; Artigas F. (2005) Involvement of 5-HT1A receptors in prefrontal cortex in the modulation of dopaminergic activity: role in atypical antipsychotic action. J. Neurosci. 25, 10831–10843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romaniuk A.; Koprowska M.; Krotewicz M.; Strzelczuk M.; Wieczorek M. (2001) Effects of 8-OHDPAT administration into the dorsal raphe nucleus and dorsal hippocampus on fear behavior and regional brain monoamines distribution in rats. Behav. Brain Res. 120, 47–57. [DOI] [PubMed] [Google Scholar]
- Gogos A.; Kusljic S.; Van den Buuse M. (2005) 8-OH-DPAT-induced effects on prepulse inhibition: Pre- vs. post-synaptic 5-HT1A receptor activation. Pharmacol., Biochem. Behav. 81, 664–672. [DOI] [PubMed] [Google Scholar]
- Depoortère R.; Bardin L.; Auclair A. L.; Kleven M. S.; Prinssen E.; Colpaert F.; Vacher B.; Newman-Tancredi A. (2007) F15063, a potential antipsychotic with D2/D3 antagonist, 5-HT1A agonist and D4 partial agonist properties: (II) Activity in models of positive symptoms of schizophrenia. Br. J. Pharmacol. 151, 253–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piszczek L. P., Schlax K., Wyrzykowska A., Piszczek A., Audero E., and Gross C. (2012) Dissecting the role of serotonin 1A hetero- and autoreceptors using a novel Cre-conditional allele. Neuroscience Meeting Planner. New Orleans, LA: Society for Neuroscience 2012, Poster 68.21/W64.
- Yadav P. N.; Abbas A. I.; Farrell M. S.; Setola V.; Sciaky N.; Huang X. P.; Kroeze W. K.; Crawford L. K.; Piel D. A.; Keiser M. J.; Irwin J. J.; Shoichet B. K.; Deneris E. S.; Gingrich J.; Beck S. G.; Roth B. L. (2011) The presynaptic component of the serotonergic system is required for clozapine’s efficacy. Neuropsychopharmacology 36, 638–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Buuse M.; Martin S.; Brosda J.; Leck K. J.; Matthaei K. I.; Hendry I. (2005) Enhanced effect of dopaminergic stimulation on prepulse inhibition in mice deficient in the alpha subunit of Gz. Psychopharmacology 183, 358–367. [DOI] [PubMed] [Google Scholar]
- van den Buuse M.; Wischhof L.; Lee R. X.; Martin S.; Karl T. (2009) Neuregulin 1 hypomorphic mutant mice: enhanced baseline locomotor activity but normal psychotropic drug-induced hyperlocomotion and prepulse inhibition regulation. Int. J. Neuropsychopharmacol. 12, 1383–1393. [DOI] [PubMed] [Google Scholar]
- Van den Buuse M.; Gogos A. (2007) Differential effects of antipsychotic drugs on serotonin-1A receptor mediated disruption of prepulse inhibition. J. Pharmacol. Exp. Ther. 320, 1224–1236. [DOI] [PubMed] [Google Scholar]
- Fletcher A.; Forster E. A.; Bill D. J.; Brown G.; Cliffe I. A.; Hartley J. E.; Jones D. E.; McLenachan A.; Stanhope K. J.; Critchley D. J.; Childs K. J.; Middlefell V. C.; Lanfumey L.; Corradetti R.; Laporte A. M.; Gozlan H.; Hamon M.; Dourish C. T. (1996) Electrophysiological, biochemical, neurohormonal and behavioural studies with WAY-100635, a potent, selective and silent 5-HT1A receptor antagonist. Behav. Brain Res. 73, 337–353. [DOI] [PubMed] [Google Scholar]
- Moser P. C. (1989) An evaluation of the elevated plus-maze test using the novel anxiolytic buspirone. Psychopharmacology 99, 48–53. [DOI] [PubMed] [Google Scholar]
- Cheng C. H.; Costall B.; Kelly M. E.; Naylor R. J. (1994) Actions of 5-hydroxytryptophan to inhibit and disinhibit mouse behaviour in the light/dark test. Eur. J. Pharmacol. 255, 39–49. [DOI] [PubMed] [Google Scholar]
- Goodwin G. M.; DeSouza R. J.; Wood A. J.; Green A. R. (1986) Lithium decreases 5-HT1A and 5-HT2 receptor and alpha 2-adrenoceptor mediated function in mice. Psychopharmacology (Berlin, Ger.) 90, 482–487. [DOI] [PubMed] [Google Scholar]
- Furukawa T.; Yamada K.; Kohno Y.; Nagasaki N. (1979) Brain serotonin metabolism with relation to the head twitches elicited by lithium in combination with reserpine in mice. Pharmacol., Biochem. Behav. 10, 547–549. [DOI] [PubMed] [Google Scholar]