Abstract

The illicit consumption of psychoactive compounds may cause short and long-term health problems and addiction. This is also true for amphetamines and cocaine, which target monoamine transporters. In the recent past, an increasing number of new compounds with amphetamine-like structure such as mephedrone or 3,4-methylenedioxypyrovalerone (MDPV) entered the market of illicit drugs. Subtle structural changes circumvent legal restrictions placed on the parent compound. These novel drugs are effectively marketed “designer drugs” (also called “research chemicals”) without any knowledge of the underlying pharmacology, the potential harm or a registration of the manufacturing process. Accordingly new entrants and their byproducts are identified postmarketing by chemical analysis and their pharmacological properties inferred by comparison to compounds of known structure. However, such a heuristic approach fails, if the structures diverge substantially from a known derivative. In addition, the understanding of structure–activity relations is too rudimentary to predict detailed pharmacological activity. Here, we tested a combined approach by examining the composition of street drugs using mass spectrometry and by assessing the functional activity of their constituents at the neuronal transporters for dopamine, serotonin, and norepinephrine. We show that this approach is superior to mere chemical analysis in recognizing novel and potentially harmful street drugs.
Keywords: Amphetamine, bath-salts, mass spectrometry, combo, psychostimulants, 2C-B, MDPV
The use of illicit psychoactive drugs such as amphetamine and cocaine is widespread, and it has been increasing on a worldwide basis.1 These drugs induce addiction and carry the risk of a number of serious side effects. Hence, they represent a serious health risk and impose a major burden on the healthcare systems.2
Amphetamines and cocaine-like drugs target the monoamine transporter family and bind with different affinities to the transporters for dopamine (DAT), norepinephrine (NET), and serotonin (SERT).3 Both drug types lead to a similar increase in the concentration of monoamine neurotransmitters in the synaptic cleft, but they differ markedly in their molecular mechanism of action.4 Amphetamine and its congeners are substrates of all three monoamine transporters, while cocaine and its derivatives are nontransported inhibitors.5 Amphetamines increase the concentrations of neurotransmitters by inducing a current through the transporter6 and thereby reversing the direction of transport.7 In addition, they competitively inhibit reuptake of the physiological substrate.8 Cocaine is a transport inhibitor: it docks to the outward facing conformation of the transporter at a site, which overlaps with the substrate binding site.9
Historically, both cocaine and amphetamines have been used in clinical medicine. However, their addictive properties were soon recognized, their medical use was tightly regulated and eventually abandoned, and finally they were found on the illicit drug market. Furthermore, alternatives have always been sought and marketed, in part by exploiting legal loopholes. MDMA (3,4-methylendioxymethamphetamine, “ecstasy”) is an early example; other so-called “designer drugs” can be found as part of a group of compounds called “bath salts” which include 3,4-methylendioxypyrovalerone (MDPV), 4-methylmethcathinone (mephedrone), and 3,4-methylendioxymethcathinone (methylone). Recently, these latter compounds have been classified as Schedule I controlled substances in the United States.10 Importantly, these three “bath salt” members are related to the family of plant-derived stimulant cathinones. Other analogues are still sold and widely distributed over the Internet as “legal highs” without apparently incurring a risk of prosecution. At present, these “bath salts” are not readily classified with respect to their pharmacological mechanism of action: mephedrone11 is believed to be a substrate/releaser while MDPV solely acts as an uptake inhibitor.12,13
Many modifications are apparently tolerated, if amphetamine is used as a starting point; the same is true for the tropane ring system and related moieties. There is a large incentive to explore the pertinent chemical space and to create structural modifications which exploit legal loopholes and are marketed by under the appealing brand “designer drugs”. In fact, the illicit drug market provides revenues that readily cover the costs of the underlying chemical innovation. Issues of quality control and contaminants are only considered minor sources of concern and hence do not raise production costs. In addition, more recently, combinations of these drugs have also been marketed deliberately, for instance a combination of MDPV and mephedrone.14
Novel psychostimulants or “combo applications” will only be recognized by the authorities, if drug dealers are arrested, illegal chemical laboratories are found, or the drugs are obtained from drug users. In this arms race, the illegal chemistry is always one step ahead. The crucial point is to reduce the time lag between market entry of a compound and its identification and classification. The prevention project “CheckIt!” in Austria provides an important window of opportunity to recognize novel drugs entering the market: drug users can anonymously test the content of the drugs, which they have bought, without risking criminal prosecution. This drug prevention initiative is based in Vienna, but it also reaches out to people at various venues where rave parties, other major events, and musical performances take place. Samples are analyzed on site (a few milligrams scratched into a test tube) by mass spectrometry (in a bus that has been changed into a mobile laboratory) and tested for any major psychostimulants known to be on the market. The drug user receives the information on the content of his sample immediately after the end of the analysis. If a sample does not contain the usual suspected drug, it will be classified as “unknown”. These samples are especially precious because they allow for documenting market entry of a novel drug. The state-of-the-art chemical analysis provides the structural information by examining the fragments generated during mass spectrometry. However, it suffers from a major limitation: it cannot gauge the biological activity of the novel drugs.15 The current project aims at overcoming this limitation: we established a bioassay that relies on different cell lines expressing the human isoforms of SERT, DAT, NET, and the rat transporter for GABA 1 (GAT1) as negative control to complement the highly sensitive analysis by mass spectrometry. We provide a proof-of-principle by examining four samples that had been sold as psychostimulants, mostly amphetamine-like drugs: for lack of pharmacological data, they were initially classified as unknown. Accordingly, we determined their ability to inhibit substrate uptake and to induce transporter-mediated release.
Results and Discussion
We tested four different anonymously supplied samples obtained from drug consumers. The consumers voluntarily contacted the Viennese project “CheckIt! Check your drugs” to have their purchased drugs analyzed. The drugs had been purchased by the drug consumers as either traditional amphetamines such as “ecstasy” (sample A) and “speed” (sample D) or novel amphetamine-like drugs of the type 'bath salt” such as “mephedrone” (sample C). Sample B was purchased as “2C-B”, which does not readily qualify as an amphetamine: “2C-B” (4-Bromo-2,5-dimethoxyphenethylamine) primarily targets serotonin receptors.16 Therefore, it serves as an ideal control, because “2C-B” is predicted to neither exert any effect on monoamine transporters nor on the GABA transporter-1. The initial analysis of the samples was done by mass spectrometry and did not reveal any known pharmacological active compound. Thus, the drugs were classified “of unknown content”.
Limits on the further analytical strategy were imposed by the following considerations: (i) the amount that was supplied by the drug consumers was obviously small (only a few milligrams were obtained from the purchased drug samples). (ii) The concentration of the unknown amphetamine-like compounds was unknown. (iii) One part of the sample had already been used for the initial mass spectrometric analysis and the sample was to be analyzed by high-resolution mass spectrometry. Hence, a substantial fraction of the residual material had to be set aside. We assessed the pharmacological nature of the compounds by studying their interaction profile at SERT, NET, DAT, and GAT1 employing HEK293 cells that expressed the human isoforms of the monoamine transporters and the rat isoform for GAT1. First, we analyzed if the compounds inhibited uptake of transporter substrates. In a second approach, we also determined if they acted as releasers, that is, they promoted substrate efflux from preloaded cells, which is the hallmark of an amphetamine-like action. We included the cell line expressing rat GAT1 for control purposes in the uptake inhibition experiments: although the transport direction can be reversed by GAT1 substrates,17 amphetamines do not exert any activity at this member of the NSS family.18 However, contaminants may increase cell permeability and hence inhibit substrate uptake by a nonspecific action. Because of the limited quantity of compound, each experiment was characterized in a single experiment done in triplicate; a second experiment was conducted to confirm the results. For the sake of completeness, it should be mentioned that Rothman et al.19 have developed high-throughput assays to assess transporter substrate activity in rat brain synaptosomes. While such assays might not be suitable for the rapid combined analyses described, the results from rat brain tissue can serve as a physiologically relevant comparator for data obtained from transfected cells expressing DAT, NET, or SERT.
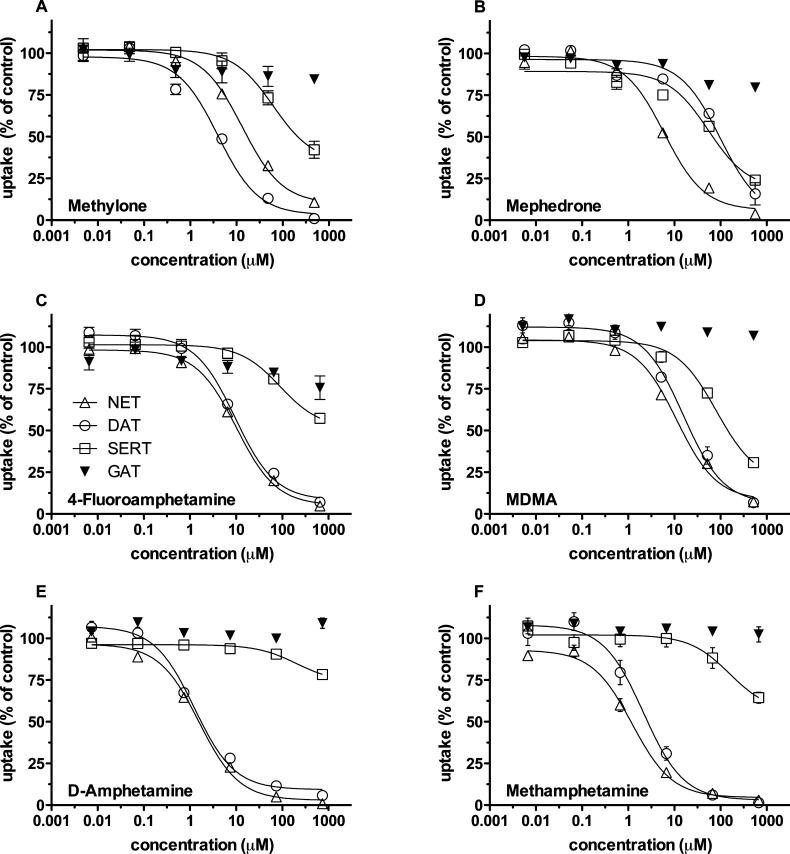
Concentration–response curves were generated for six reference compounds, that is, MDMA, 4-fluoramphetamine, d-amphetamine, methylone, mephedrone, and methamphetamine. This choice reflected the illicit market situation and included abundantly distributed amphetamines and more rarely found amphetamines. The selected compounds differ in their selectivity for individual transporters. This is evident from Figure 1, which allows for grasping the profile of each compound. The IC50 values are given in Table 1; both the IC50 values and the profile served as reference points for the analysis of the drugs of unknown content.
Figure 1.
Uptake inhibition by reference compounds. HEK293 cells stably expressing DAT, NET, SERT, or rGAT1 were used for uptake inhibition assays. Uptake was inhibited by increasing concentrations of reference compounds as indicated. Cells were incubated with the test compounds for 5 min before the tritiated substrates were added to the incubation buffer. The concentration of tritiated substrates was 0.03 μM in the case of [3H]5-HT while 0.05 μM was used for [3H]MPP+. The radioactive counts under basal conditions (i.e., no drugs added) were as follows (n = 26 randomly chosen values, the following values are given as mean ± SEM): HEK-NET: 52252 ± 1867 cpm, blank: 4921 ± 1933 cpm. HEK-DAT: 30168 ± 2209 cpm, blank: 2892 ± 141 cpm. HEK-SERT: 44339 ± 4245 cpm, blank: 3703 ± 185 cpm. HEK-GAT1: 14964 ± 628 cpm, blank: 3892 ± 272 cpm. These values were set 100% to normalize for interassay variation. Data are shown as means ± SEM of three independent experiments.
Table 1. Inhibition Profiles (IC50 values) of Different Amphetamines for Transport of [3H]5-HT by Human SERT and [3H]MPP+ by Human DAT and NETa.
| SERT | NET | DAT | GAT1 | |
|---|---|---|---|---|
| MDMA | 88.3 ± 12.1 | 12.4 ± 1.8 | 9.1 ± 3.8 | n.d. |
| 4-fluoramphetamine | 94.83 ± 9.2 | 10.3 ± 0.3 | 9.5 ± 0.1 | n.d. |
| d-amphetamine | 110.0 ± 14.7 | 1.5 ± 0.1 | 1.45 ± 0.2 | n.d. |
| methylone | 63.3 ± 6.4 | 13.9 ± 1.3 | 4.21 ± 0.3 | n.d. |
| mephedrone | 25.64 ± 4.0 | 6.8 ± 0.6 | 98.8 ± 9.1 | n.d. |
| methamphetamine | 182.1 ± 83.1 | 1.3 ± 0.1 | 1.17 ± 0.6 | n.d. |
Uptake of [3H]GABA by the rat GABA transporter-1 (GAT) was assessed as a control for possible non-specific toxic actions. All values are given as mean ± SEM in PM. n.d.: not detectable.
Next, we examined the unknown samples (termed samples A–D). The unknown samples were sequentially diluted by a factor of 10 to cover 6 orders of magnitude and tested for their ability to inhibit substrate uptake. Figure 2 shows the inhibitory profile of each sample. It is worth noting that none of the samples inhibited uptake of [3H]GABA. This ruled out a nonspecific action (e.g., due to cellular toxicity, pore formation, or other mechanisms that dissipate the ionic driving forces)
Figure 2.
Uptake inhibition by unknown samples. The four unknown samples (A–D) were serially diluted six times by a factor of 10. For uptake inhibition experiments, the cells were treated exactly as described under the figure legend for Figure 1. Data are shown as means ± SEM of two independent experiments performed in duplicate
Inspection of the graphs in Figure 2 reveals characteristic fingerprints of the compounds: The first of the four samples shows a significant effect on all three monoamine transporters at similar potency but exerted no effect at GAT1. However, even under the assumption that the consumer bought the sample as “ecstasy”, the inhibitory pattern resembled none of our reference compounds. At best, it came close to the observed pattern with MDMA with the difference that MDMA has a somewhat smaller effect at NET and DAT; this was not seen in sample A: in contrast, the effect on NET and DAT was slightly higher than that on SERT. Sample B did not exert any appreciable effect at any of the monoamine transporters, and GAT1 was also unaffected. This was not surprising since the customer bought the sample under the label “2C-B”. Therefore, this explains why no significant change from baseline was to be observed. When we examined sample C, we observed a diverse inhibition pattern with the strongest inhibition exerted at DAT, followed closely by NET. SERT was inhibited at lower potency, and, again, GAT1 was completely unaffected, a pattern seen with mephedrone (Figure 1A). Because of experimental uncertainty in dilution curves with limited amounts of data points, methylone must also be taken into account as an alternative that is still compatible with the data (cf. Figures 1B and 2C). Our assignment is consistent with the fact that sample C was sold under the name of “mephedrone”. The fingerprint of sample D resembled the inhibitory pattern of methamphetamine or d-amphetamine, that is, equipotent inhibition of DAT and NET and poor activity at SERT (cf. Figures 2D and 1E,F). This profile is also compatible with the fact that sample D was sold under the name “speed”. This finding was somewhat surprising because amphetamine or methamphetamine ought to have been detectable by the initial mass spectrometric analysis. Thus, after our initial pharmacological assessment of the unknown samples, we suspected amphetamine-like drugs in samples A, C, and D. As expected, sample B did not inhibit any of the transporters examined in this study.
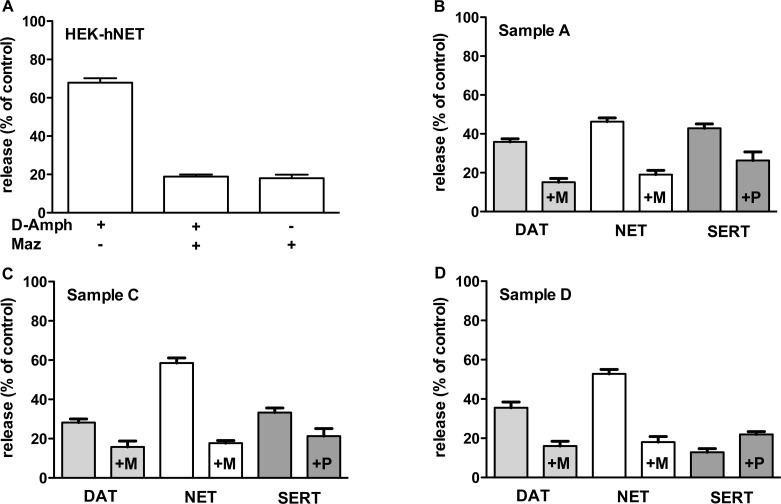
Inhibition of uptake indicates that a compound interacts with the transporter.3 However, it does not prove that a compound can induce transporter-mediated efflux.4 Therefore, we examined samples A, C, and D for their ability to induce efflux. A superfusion assay is the gold standard to test transporter-mediated efflux in synaptosomes or slices prepared from animal tissue2 or in heterologous expressing cell lines.20 Superfusion provides a robust assay format to assess transporter-mediated efflux, because confounding effects arising form back diffusion are eliminated.21 The disadvantage of superfusion, however, is the large volume of superfusate and hence the need of a larger amount of the compound under study. In this project, however, the amount of the samples was limiting. Accordingly, we resorted to the batch-release assay originally established by Rudnick and co-workers.22 In the batch-release assay, the amount of releasing compound needed is much smaller since the typical volume is 0.1 mL. We preloaded the cells with tritiated MPP+ for 20 min and initiated efflux over a time of 10 min by exchanging the buffer containing tritiated label by buffer that included the unknown samples. We tested the batch-release assay and confirmed that (i) our monoamine transporters expressing HEK293 cells responded to a concentration of amphetamines at IC50 value (for DAT, d-amphetamine 1 μM; for NET, d-amphetamine 1 μM; and for SERT, MDMA 10 μM) and (ii) this efflux was specific, that is, inhibited by a saturating concentration of selective inhibitors (for DAT and NET, mazindole 10 μM; and for SERT, paroxetine, 10 μM). A representative example of the assay is shown in Figure 3A. Next, we studied efflux induced by the unknown samples A, C, and D by using a dilution which inhibited the uptake of the pertinent substrate by 50%. We hypothesized that sample A would be equieffective in inducing efflux by all three transporters. In contrast, sample C was predicted to have a stronger effect on DAT and NET. Finally, sample D should be devoid of any effect on SERT-mediated efflux. Figure 3 shows the results from the batch-release assays. As expected, all efflux induced by samples A, C, and D was specific since application of the selective reuptake blockers mazindole (“+M”) and paroxetine (“+P”) inhibited efflux similar to the example shown in Figure 3A. As hypothesized, sample A promoted efflux through all three transporters with comparable efficacy. However, sample C only caused a pronounced substrate efflux through NET and elicited release by DAT, albeit to a lesser extent; furthermore, SERT-mediated efflux was low. The effects elicited by sample D were as predicted for amphetamine or methamphetamine; that is, the sample elicited efflux via DAT and NET, but it was ineffective in promoting release by SERT. In fact, sample D elicited even less efflux in the absence of paroxetine than in its presence. The intriguing result that efflux in the presence of paroxetine was even more pronounced could at best be explained as follows: in earlier publications, we described “efflux” caused by paroxetine and other reuptake inhibitors.20,21,23 However, what seemed to be “efflux” was finally confirmed to simply be pseudoefflux caused by (i) substrate diffusing out of the cells and (ii) inhibition of reuptake. In a subsequent study as well as this study, we intended to prevent this pseudoefflux by using the charged substrate MPP+. However, we cannot rule out that organic cation transporters expressed in the HEK293 cells used may play a role in this context.
Figure 3.
Release. HEK293 cells stably expressing DAT, NET, or SERT were used for batch-release assays. They were seeded into 96-well plates, and all were incubated for 20 min with [3H]MPP+ (0.05 μM) for the sake of simplicity. After a gentle wash, the cells were overlaid with buffer containing test compounds in the presence or absence of blocker (+M denotes mazindole, 10 μM; +P stands for paroxetine, 10 μM) for 10 min. The dilution of the test compounds was chosen to be at the IC50 value. At the end, the buffer was removed and the cells were lysed and counted for radioactivity. All data are expressed as percent of control, that is, HEK293 cells that had received buffer only. All experiments have been performed two to three times in duplicate.
The results of the batch-release assay supported the notion that the samples under investigation exert amphetamine-like actions, because their releasing effect was reduced by coapplication of specific uptake inhibitors.
Taken togther the data supported the classification of sample B as a compound unrelated to amphetamine, sample C as a putative member of the bath-salt family including methylone and mephedrone, and sample D as methamphetamine or d-amphetamine. However, the profile of sample A was most reminiscent of MDMA although its activity at DAT and NET was too strong. Therefore, we reanalyzed all samples by more extensive mass spectrometry than was possible under field (i.e., street) conditions. Table 2 summarizes the qualitative and quantitative results of this analysis. The identification of the compounds contained in the samples was ultimately based on a comparison of their UV spectra, MS data, and retention time to those of reference substances. The latter two are also listed in Table 2.
Table 2. Qualitative and Quantitative Results of the Mass Spectrometry Analysis.
| sample | purchased as | compounds identified | amount (mg/g) | molecular ion [M+H]+ | monoisotopic mass [M+H]+/ [M-H]- | retention time (min) |
|---|---|---|---|---|---|---|
| A | ecstasy | amphetamine | 48 | 136 | 136.10 | 4.01 |
| mCPP | 166 | 197 | 197.08 | 5.73 | ||
| metoclopramide | n.q.a | 300 | 300.14 | 5.51 | ||
| B | 2C-B | 2C-B | n.q.a | 260 | 260.02 | 4.85 |
| C | mephedrone | mephedrone | 610 | 178 | 178.12 | 5.02 |
| caffeine | 132 | 195 | 195.08 | 3.54 | ||
| D | speed | amphetamine | 60 | 136 | 136.10 | 4.03 |
| caffeine | 276 | 195 | 195.08 | 3.54 | ||
| paracetamol | 110 | 152 | 152.06 | 1.95 | ||
| acetylsalicylic acid | n.q.a | 179.04b | 1.81 |
Not quantified.
Acquired using negative ionization mode.
Figure 4 shows the mass spectra acquired during the standard HPLC-MS screening procedure. The prevalence of all identified substances listed in Table 2 was verified by measuring the exact monoisotopic molecular masses with a high resolution Q-TOF mass spectrometer and by calculating their chemical formula from the exact mass. Interestingly, sample A contained a mixture of two psychostimulants: amphetamine and m-chlorophenylpiperazine (mCPP): this fact explains the strong effect at SERT, which would not have been observed with amphetamine alone: mCPP has been recognized earlier as a potent 5-HT releaser.24−27 It is one of the most striking findings of the present study that sample A is a so-called “combo”, a mixture of two different psychostimulants. Such “combos” are often sold to customers without their knowledge. “Combos” have already been described in the literature;14 they can also be found on Internet portals in reports by drug consumers (www.erowid.org) and, most recently, even in the statistical report on drug use in Austria (www.oebig.at). Typically, combinations of drugs exert different side effects; synergism (i.e., overadditive effects) is not only seen for the intended actions but can result in more debilitating adverse effects.
Figure 4.
HPLC mass spectrometry. Mass spectra of drugs acquired during standard HPLC screening procedure under following conditions: ionization mode, ESI; polarity, +ev; probe temperature, 280 °C; cone, 60 V; scan time, 1 s; x-axis, mass to charge ratio (m/z); y-axis, relative abundance (%).
The exact nature of sample D was still not unraveled even after extensive reanalysis using a unit-resolution mass spectrometer (Figure 5). Therefore, sample D was reanalyzed using high resolution mass spectrometry to identify the two unknown substances found in the primary screening. Finally, amphetamine and acetylsalicylic acid were identified by direct injection into a high resolution Q-TOF-MS followed by MS/MS. The values shown in Table 2 are the results of this high-resolution mass spectrometry.
Figure 5.

HPLC mass spectrometry. HPLC chromatogram (UV detection trace at 215 nm) of the complex sample D containing acetylsalicylic acid, paracetamol, caffeine, amphetamine, and the internal standard using the separation conditions as described in the Methods section.
This result matches the predictions reasonably well. In addition, it also substantiates the claim of the drug dealer who sold the drug to the consumer under the brand name “speed”. The adulteration by acetylsalicylic acid is not uncommon;28 it is used to dilute psychostimulants, presumably because it is readily available and it elicits a strong taste sensation, which is suggestive of high drug content.
It is evident that medical professionals, street workers, and legal authorities face an uphill battle in their attempt to confront the recent shifts in the use of illicit drugs. One of the challenges is to remain up-to-date in recognizing novel psychostimulants or new combinations of compounds in various segments of the illicit marketplace. This is due to the limits imposed by their detection. As exemplified in the current study, we initially failed to identify rather common psychostimulant drugs by mass spectrometry, but the bioassay employed was indeed sensitive enough to detect the amphetamine-like actions of the drugs. In addition, this assay reliably discriminated the various amphetamine-like drugs from “2C-B” (or similar structural analogues). It also deciphered the “combo” of amphetamine and mCPP. Hence, it provides a relatively rapid screening tool that allows for sensitive pharmacological detection of novel amphetamine-like drugs and unknown “combo” applications. We are currently working on possibilities to also assess release and uptake in a mobile format on-site, in parallel to the mass spectrometry analysis. This may open an avenue to establish an early warning system given the surge of novel compounds that reach the markets, including formerly “legal highs”,11 clinical implications that these drugs possess,29 necessitates such a pharmacological assay as an important tool to quickly respond to the rapidly changing market conditions.
Methods
Materials
Dulbecco’s modified Eagle’s medium (DMEM) and trypsin were purchased from PAA Laboratories GmbH (Pasching, Austria). Fetal calf serum was purchased from Invitrogen. [3H]5-HT ([3H]5-hydroxytryptamine; serotonin; 28.3 Ci/mmol) and [3H]GABA (35 Ci/mmol) were purchased from PerkinElmer, Boston, MA. [3H]1-Methyl-4-phenylpyridinium (MPP+; 85 Ci/mmol) was supplied by American Radiolabeled Chemicals (St. Louis, MO). Serotonin (5-HT), s-(+)-3,4-methylenedioxymethamphetamine (MDMA), paroxetine, methamphetamine, and d-amphetamine were purchased from Sigma. Mephedrone and methylone were purchased from Serobac (Vienna, Austria). 4-Fluoramphetamine was obtained from Lipomed, Arlesheim, Switzerland. 1-Methyl-4-phenylpyridinium ion (MPP+) was purchased from Research Biochemicals International, Natick, MA.
Sample Collection and Preparation
The samples used in this study were obtained from drug users participating voluntarily and anonymously in the Checkit! program. Three to ten milligrams of substance were scraped into a test vial and weighed with an analytical balance. The substance was diluted in 1 mL of methanol and vortex mixed for 1 min. The solution was centrifuged for 3 min at 13 200 rpm/min. Ten microliters of the supernatant were diluted with 400 μL of internal standard solution (trazodone 50 μg/mL dissolved in 10 mM aqueous ammonium formate buffer).
LC-ESI-MS Conditions of the Standard Screening Procedure
The samples were analyzed by employing an LC-Packings Ultimate High Performance Liquid Chromatography (HPLC) system (Dionex, Netherlands) equipped with a Dionex PDA-100 photodiode array detector and coupled with a Finnigan Surveyor MSQ plus mass spectrometer (Thermo Electron Corporation, San Jose, CA) with an ESI-probe. Separation was performed on a 2.1 × 150 mm Luna PFP column (Phenomenex; Torrance, CA) using fast gradient elution with 10 mM aqueous ammonium formate buffer (pH 4.5) and acetonitrile (ACN), starting from 10% ACN and 90% buffer at 0.0 min to 90% ACN and 10% buffer at 6.0 min with a total run time of 7.5 min. The flow rate was set to 300 μL/min. Five microliters of sample solution was injected into the HPLC system. After separation, compounds were detected simultaneously by the PDA and the mass spectrometer. The operation of the LC-MS and chromatographic analysis was carried out by using Dionex Chromeleon 6.8 (SR 7) chromatography software. For the identification of the compounds, retention time, UV spectra, and mass spectra were obtained and compared to those of reference substances previously measured. The quantitation was achieved by UV-detection at a wavelength of 254 nm.
High Resolution ESI-Q-TOF Mass Spectrometric Analysis
Unidentified substance peaks acquired during standard screening were subjected to an in-depth analysis: the sample solution was again diluted with methanol to a concentration of approximately 10 μg/mL and injected directly into the Q-TOF-MS (maXis, Bruker Daltonik GmbH, Germany) instrument with a syringe pump at a flow rate of 5 μL/min. The instrument used under the described conditions provided a mass resolution of 50 000. Eligible molecular ions, known form the previous HPLC-MS analysis, were identified, and MS/MS was performed and recorded (ionization mode, positive and negative; capillary, 1.5 kV; temperature, 150 °C; collision energy, 10–30 eV). MS and MS/MS spectra were interpreted, and elementary formulars were calculated from the exact masses using Bruker Daltonics DataAnalysis 4.0 software.
Uptake and Release Assays
The generation of HEK293 cell lines expressing hSERT, hNET, hDAT, or rGAT1 (HEK-SERT, HEK-DAT, HEK-NET, or HEK-GAT1, respectively) is described earlier.7,17,30 hSERT was expressed under the control of a tetracycline inducible promoter.7
HEK293 cells stably expressing either neurotransmitter transporter were seeded onto poly-d-lysine-coated 48-well plates (0.5 × 105 cells/well), 24 h prior to the experiment. For inhibition experiments, the specific activity of the tritiated substrate was kept constant: [3H]GABA, 0.03 μM; [3H]MPP+, 0.03 μM; [3H]5-HT, 0.03 μM.
Assay conditions were as outlined.31 In brief, the cells were washed thrice with Krebs–Ringer–HEPES buffer (KHB; composition: 25 mM HEPES·NaOH, pH 7.4, 120 mM NaCl, 5 mM KCl, 1.2 mM CaCl2, and 1.2 mm MgSO4 supplemented with 5 mM d-glucose). Then, the diluted reference and sample compounds were added and incubated for 5 min to allow for equilibration with the transporters. Subsequently, the tritiated substrates were added and the reaction was stopped after 5 min. Cells were lysed with SDS 1% and counted in a beta-counter (Packard instruments). All determinations have been performed in duplicate or triplicate.
For release studies, HEK-SERT, HEK-NET, or HEK-DAT cells were grown in 96-well plates (4 × 104 cells per well). The cells were preloaded with 0.05 μM [3H]MPP+ for 20 min at 37 °C in a final volume of 0.1 mL/well. The cells were incubated with the test compounds after three gentle wash steps with Krebs–Ringer–HEPES buffer at room temperature. d-Amphetamine was used in release assays as reference compound for all three monoamine transporters. All compounds were used at the dilution where a 50% inhibition of substrate uptake was observed during the uptake assays. The specificity of drug-induced release was assessed by the addition of inhibitors mazindole (10 μM; for DAT and NET), paroxetine (10 μM; SERT), and tiagabine (10 μM; GAT1) to the test compound. After 10 min, the incubation buffer was removed and transferred into a counting vial; the cells remaining in the well were overlaid with a 1% SDS solution, thereby disintegrated and the resulting solution transferred into a counting vial. All samples were subjected to standard liquid scintillation counting (Packard Instruments). All determinations have been performed in triplicate. The sum of the counts in the incubation buffer and the cell lysate reveal 100% of [3H]MPP+ included in the assay. Hence, this sum is the control value to which the released [3H]MPP+ is calculated as percentage: the data shown in Figure 3 are expressed as released [3H]MPP+ in percent of control, that is, the sum of [3H]MPP+ released to the incubation buffer and the [3H]MPP+ in the cell lysate.
Data Analysis
Data from uptake inhibition experiments were fitted by nonlinear, least-squares curvilinear regression to an equation for a rectangular hyperbola. The fit was not improved by employing a logistic equation (Hill equation). The program used to perform the fit was GraphPad Prism version 5.0d for MacOsX, GraphPad Software, San Diego, CA, www.graphpad.com.
Glossary
Abbreviations
- 5-HT
5-hydroxytryptamine (serotonin)
- MDMA
s-(+)-3,4-methylenedioxy-methamphetamine
- MPP+
1-methyl-4-phenylpyridinium ion
- NSS
neurotransmitter:sodium symporters
- HEK
human embryonic kidney
- DAT
dopamine transporter
- SERT
serotonin transporter; HEPES
- SDS
sodium dodecylsulfonic acid
- Q-TOF-MS
quadrupole time-of-flight mass spectrometer
Author Contributions
Participated in research design: M.H., A.L., R.R., R.S., and H.H.S. Conducted experiments: M.H., A.L., and R.R. Performed data analysis and interpretation: M.F., M.H., A.L., R.R., R.S., and H.H.S. Wrote or contributed to the writing of the manuscript: M.F., A.L., R.R., R.S., H.H.S.
The support of the Austrian Science Fund/FWF is gratefully acknowledged: F3506 to HHS and F3510 to MF.
The authors declare no competing financial interest.
References
- Carroll F. I.; Lewin A. H.; Mascarella S. W.; Seltzman H. H.; Reddy P. A. (2012) Designer drugs: a medicinal chemistry perspective. Ann. N.Y. Acad. Sci. 1248, 18–38. [DOI] [PubMed] [Google Scholar]
- Steinkellner T.; Yang J.-W.; Montgomery T. R.; Chen W.-Q.; Winkler M.-T.; Sucic S.; Lubec G.; Freissmuth M.; Elgersma Y.; Sitte H. H.; Kudlacek O. (2012) Ca2+/Calmodulin-dependent Protein Kinase IIα (αCaMKII) Controls the Activity of the Dopamine Transporter: Implications for Angelman Syndrome. J. Biol. Chem. 287, 29627–29635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen A. S.; Andersen J.; Jørgensen T. N.; Sørensen L.; Eriksen J.; Loland C. J.; Strømgaard K.; Gether U. (2011) SLC6 neurotransmitter transporters: structure, function, and regulation. Pharmacol. Rev. 63, 585–640. [DOI] [PubMed] [Google Scholar]
- Sitte H. H.; Freissmuth M. (2010) The reverse operation of Na(+)/Cl(−)-coupled neurotransmitter transporters--why amphetamines take two to tango. J. Neurochem. 112, 340–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman R. B.; Baumann M. H. (2002) Serotonin releasing agents. Neurochemical, therapeutic and adverse effects. Pharmacol., Biochem. Behav. 71, 825–836. [DOI] [PubMed] [Google Scholar]
- Schicker K.; Uzelac Z.; Gesmonde J.; Bulling S.; Stockner T.; Freissmuth M.; Boehm S.; Rudnick G.; Sitte H. H.; Sandtner W. (2012) Unifying concept of serotonin transporter-associated currents. J. Biol. Chem. 287, 438–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilber B.; Scholze P.; Dorostkar M. M.; Sandtner W.; Holy M.; Boehm S.; Singer E. A.; Sitte H. H. (2005) Serotonin-transporter mediated efflux: A pharmacological analysis of amphetamines and non-amphetamines. Neuropharmacology 49, 811–819. [DOI] [PubMed] [Google Scholar]
- Scholze P.; Zwach J.; Kattinger A.; Pifl C.; Singer E. A.; Sitte H. H. (2000) Transporter-mediated release: a superfusion study on human embryonic kidney cells stably expressing the human serotonin transporter. J. Pharmacol. Exp. Ther. 293, 870–878. [PubMed] [Google Scholar]
- Beuming T.; Kniazeff J.; Bergmann M. L.; Shi L.; Gracia L.; Raniszewska K.; Newman A. H.; Javitch J. A.; Weinstein H.; Gether U.; Loland C. J. (2008) The binding sites for cocaine and dopamine in the dopamine transporter overlap. Nat. Neurosci. 11, 780–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Administration D. E. (2011) Schedules of Controlled Substances: Temporary Placement of Three Synthetic Cathinones Into Schedule I. Fed. Regist. 76, 65471–65475. [PubMed] [Google Scholar]
- Baumann M. H.; Ayestas M. A.; Partilla J. S.; Sink J. R.; Shulgin A. T.; Daley P. F.; Brandt S. D.; Rothman R. B.; Ruoho A. E.; Cozzi N. V. (2012) The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue. Neuropsychopharmacology 37, 1192–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann M. H.; Partilla J. S.; Lehner K. R.; Thorndike E. B.; Hoffman A. F.; Holy M.; Rothman R. B.; Goldberg S. R.; Lupica C. R.; Sitte H. H.; Brandt S. D.; Tella S. R.; Cozzi N. V; Schindler C. W. (2012) Powerful Cocaine-Like Actions of 3,4-Methylenedioxypyrovalerone (MDPV), a Principal Constituent of Psychoactive “Bath Salts” Products. Neuropsychopharmacology 10.1038/npp.2012.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmler L. D.; Buser T. A.; Donzelli M.; Schramm Y.; Dieu L.-H.; Huwyler J.; Chaboz S.; Hoener M. C.; Liechti M. E. (2012) Pharmacological characterization of designer cathinones in vitro. Br. J. Pharmacol. 10.1111/j.1476-5381.2012.02145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schifano F.; Albanese A.; Fergus S.; Stair J. L.; Deluca P.; Corazza O.; Davey Z.; Corkery J.; Siemann H.; Scherbaum N.; Farre’ M.; Torrens M.; Demetrovics Z.; Ghodse A. H. (2011) Mephedrone (4-methylmethcathinone; “meow meow”): chemical, pharmacological and clinical issues. Psychopharmacology (Berlin, Ger.) 214, 593–602. [DOI] [PubMed] [Google Scholar]
- EMCDDA. (2011) 2011 Annual report on the state of the drugs problem in Europe. http://www.emcdda.europa.eu/publications/annual-report/2011.
- Villalobos C. A.; Bull P.; Sáez P.; Cassels B. K.; Huidobro-Toro J. P. (2004) 4-Bromo-2,5-dimethoxyphenethylamine (2C-B) and structurally related phenylethylamines are potent 5-HT2A receptor antagonists in Xenopus laevis oocytes. Br. J. Pharmacol. 141, 1167–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitte H. H.; Singer E. A.; Scholze P. (2002) Bi-directional transport of GABA in human embryonic kidney (HEK-293) cells stably expressing the rat GABA transporter GAT-1. Br. J. Pharmacol. 1, 93–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidel S.; Singer E. A.; Just H.; Farhan H.; Scholze P.; Kudlacek O.; Holy M.; Koppatz K.; Krivanek P.; Freissmuth M.; Sitte H. H. (2005) Amphetamines Take Two to Tango: an Oligomer-Based Counter-Transport Model of Neurotransmitter Transport Explores the Amphetamine Action. Mol. Pharmacol. 67, 140–151. [DOI] [PubMed] [Google Scholar]
- Rothman R. B.; Baumann M. H.; Dersch C. M.; Romero D. V; Rice K. C.; Carroll F. I.; Partilla J. S. (2001) Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse 39, 32–41. [DOI] [PubMed] [Google Scholar]
- Scholze P.; Zwach J.; Kattinger A.; Pifl C.; Singer E. A.; Sitte H. H. (2000) Transporter-Mediated Release: A Superfusion Study on Human Embryonic Kidney Cells Stably Expressing the Human Serotonin Transporter 1. J. Pharmacol. Exp. Ther. 293, 870–878. [PubMed] [Google Scholar]
- Scholze P.; Sitte H. H.; Singer E. A. (2001) Substantial loss of substrate by diffusion during uptake in HEK-293 cells expressing neurotransmitter transporters. Neurosci. Lett. 309, 173–176. [DOI] [PubMed] [Google Scholar]
- Wall S. C.; Gu H.; Rudnick G. (1995) Biogenic amine flux mediated by cloned transporters stably expressed in cultured cell lines: amphetamine specificity for inhibition and efflux. Mol. Pharmacol. 47, 544–550. [PubMed] [Google Scholar]
- Sitte H. H.; Scholze P.; Schloss P.; Pifl C.; Singer E. A. (2000) Characterization of carrier-mediated efflux in human embryonic kidney 293 cells stably expressing the rat serotonin transporter: a superfusion study. J. Neurochem. 74, 1317–1324. [DOI] [PubMed] [Google Scholar]
- Baumann M. H.; Rutter J. J.; Auerbach S. B. (1993) Intravenous administration of the serotonin agonist m-chlorophenylpiperazine (mCPP) increases extracellular serotonin in the diencephalon of awake rats. Neuropharmacology 32, 1381–1386. [DOI] [PubMed] [Google Scholar]
- Baumann M. H.; Ayestas M. A.; Dersch C. M.; Rothman R. B. (2001) 1-(m-chlorophenyl)piperazine (mCPP) dissociates in vivo serotonin release from long-term serotonin depletion in rat brain. Neuropsychopharmacology 24, 492–501. [DOI] [PubMed] [Google Scholar]
- Pettibone D. J.; Williams M. (1984) Serotonin-releasing effects of substituted piperazines in vitro. Biochem. Pharmacol. 33, 1531–1535. [DOI] [PubMed] [Google Scholar]
- Eriksson E.; Engberg G.; Bing O.; Nissbrandt H. (1999) Effects of mCPP on the extracellular concentrations of serotonin and dopamine in rat brain. Neuropsychopharmacology 20, 287–296. [DOI] [PubMed] [Google Scholar]
- Pavlic M.; Schubert B.; Libiseller K.; Oberacher H. (2010) Comprehensive identification of active compounds in tablets by flow-injection data-dependent tandem mass spectrometry combined with library search. Forensic Sci. Int. 197, 40–47. [DOI] [PubMed] [Google Scholar]
- Sammler E. M.; Foley P. L.; Lauder G. D.; Wilson S. J.; Goudie A. R.; O’Riordan J. I. (2010) A harmless high?. Lancet 376, 742. [DOI] [PubMed] [Google Scholar]
- Bulling S.; Schicker K.; Zhang Y.-W.; Steinkellner T.; Stockner T.; Gruber C. W.; Boehm S.; Freissmuth M.; Rudnick G.; Sitte H. H.; Sandtner W. (2012) The mechanistic basis for noncompetitive ibogaine inhibition of serotonin and dopamine transporters. J. Biol. Chem. 287, 18524–18534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sucic S.; Dallinger S.; Zdrazil B.; Jørgensen T. N.; Holy M.; Kudlacek O.; Seidel S.; Cha J. H.; Gether U.; Newman A. H.; Ecker G. F.; Freissmuth M.; Sitte H. H. (2010) The N Terminus of Monoamine Transporters Is a Lever Required for the Action of Amphetamines. J. Biol. Chem. 285, 10924–10938. [DOI] [PMC free article] [PubMed] [Google Scholar]