Abstract
A 7-day infusion of serotonin (5-hydroxytryptamine, 5-HT) causes a sustained fall in elevated blood pressure in the male deoxycorticosterone acetate (DOCA)-salt rat. As hypertension is a long-term disease, we presently test the hypothesis that a longer (30 day) 5-HT infusion could cause a sustained fall in blood pressure in the established hypertensive DOCA-salt rat. This time period (∼4 weeks) was also sufficient to test whether 5-HT could attenuate the development of DOCA-salt hypertension. 5-HT (25 μg/kg/min; sc) or vehicle (Veh) was delivered via osmotic pump to (1) established DOCA-salt rats for one month, (2) Sprague–Dawley rats prior to DOCA-salt administration for one month, and blood pressure and heart rate measured telemetrically. On the final day of 5-HT infusion, free platelet poor plasma 5-HT concentrations were significantly higher in 5-HT versus Veh-infused rats, and mean arterial pressure was significantly lower in 5-HT-infused (135 ± 4 mmHg vs Veh-infused 151 ± 7 mmHg) established DOCA-salt rats. By contrast, 5-HT-infusion did not prevent the development of DOCA-salt hypertension (144 ± 7 mmHg vs Veh = 156 ± 6 mmHg). Isometric contraction of aortic strips was measured, and neither the potency nor maximum contraction to the alpha adrenergic receptor agonist phenylephrine (PE) or 5-HT were modified by infusion of 5-HT (established or preventative infusion), and maximum aortic relaxation to acetylcholine (ACh) was modestly but not significantly enhanced (∼15% improvement). This study demonstrates 5-HT is capable of lowering blood pressure in established DOCA-salt hypertensive rats over the course of one month in a mechanism that does not significantly modify or is dependent on modified vascular responsiveness. This finding opens the possibility that elevation of 5-HT levels could be useful in the treatment of hypertension.
Keywords: 5-HT, blood pressure, vascular reactivity, hypertension
Hypertension is characterized by an elevation in arterial pressure, increased vascular resistance, cardiac hypertrophy, increased sympathetic nervous system tone, and altered renal function.1 Despite the myriad of deleterious changes, the etiology of essential hypertension remains largely unknown. Interestingly, several lines of evidence suggest an important association between 5-HT and hypertension.
5-HT is a vasoactive amine synthesized primarily in the enterochromaffin cells of the gastrointestinal tract, and the raphe nucleus in the central nervous system.2−4 Free plasma 5-HT in both human and animal models of hypertension are elevated compared to normotensive human and animal controls.5,6 5-HT receptor antagonists (5-HT2A receptor antagonist ketanserin, 5-HT2B receptor antagonist LY272015) lower blood pressure in select animal models of hypertension but are ineffective in others.7−9 The arteries of hypertensive animals are hyperresponsive to the constrictor effects of 5-HT, and selective 5-HT reuptake inhibitors elevate blood pressure.8−10 Collectively, this evidence suggests that elevated levels of free plasma 5-HT might be acting to increase vascular resistance and blood pressure, thereby contributing to essential hypertension.
Surprisingly, experimental efforts to elevate free, platelet poor plasma 5-HT chronically (7 days) actually lowered blood pressure in the deoxycorticosterone acetate (DOCA)-salt and sham rat,11 and a similar chronic reduction in blood pressure stimulated by 5-HT has been observed in the spontaneously hypertensive rat.12 Thus, instead of contributing to essential hypertension, the elevated levels of 5-HT observed in hypertensive patients may actually represent an adaptation to elevated blood pressure, an idea yet to be examined. The exact mechanism(s) of a 5-HT-induced fall in blood pressure are not yet fully understood, but important mechanistic observations have been made, primarily in the rat model and using a one week infusion of 5-HT. In both the normotensive and DOCA-salt hypertensive rat, the fall in blood pressure was eliminated by administration of the nitric oxide synthase inhibitor NG-nitro-l-arginine.11 The fall in blood pressure is enabled by a fall in total peripheral resistance (TPR), not cardiac output, as flow probe measurements support,13 and the functioning of the serotonin transporter (SERT) accounts for ∼50% of the fall in blood pressure stimulated by 5-HT in the male rat.14 Separate efforts are being made to establish (1) the vascular bed(s) most greatly affected by 5-HT; and (2) the 5-HT receptors or mechanisms by which the fall in blood pressure is established. This present study takes a different approach, one designed to identify those mechanisms that might be affected by 5-HT in developing versus established hypertension, providing additional directions for future research.
Using gold-standard radiotelemetry for blood pressure measurement, we investigated whether a longer term (30 day) infusion of 5-HT could reduced blood pressure of the DOCA-salt model in which hypertension is established. We also investigated whether 5-HT could prevent the development of DOCA-salt hypertension, in the hopes that any differences between 5-HT administration pre- and posthypertension might provide mechanistic insight. We chose a 30-day infusion because this is the longest time an Alzet pump (nonrefillable) can be used and also covers the time of development of DOCA-salt (typically a 4 week window) that would allow us to test whether 5-HT could prevent DOCA-salt hypertension. In both studies, we used a dose of 5-HT (25 μg/kg/min) that was chosen based on a dose response curve done and published in 2011.15 The dose used in the present study is a maximal concentration of 5-HT, and this was also the dose used in the original 7 day studies,11,12 making for a fair comparison and building of the outcomes of these studies. The two in vivo experiments carried out in the present paper are not identical, as one determines the functions of 5-HT in established hypertension, while the other investigates whether 5-HT can inhibit the development of hypertension. Both are useful in determining the ultimate therapeutic usefulness of 5-HT. We anticipated that 5-HT could reduce the development of hypertension because chronic treatment with the committed 5-HT precursor L-5-hydroxytryptophan (5-HTP) prevented the development of DOCA-salt hypertension in the rat.16 To connect these important in vivo physiological experiments with published work, we continue to use the DOCA salt model of hypertension.
The DOCA model is the experimental model of choice because with it, one can (1) define when hypertension begins; (2) define how fast blood pressure rises (mix of different DOCA doses and salts); (3) demonstrate vascular changes observed in hypertensive humans (remodeling, vascular hyperreactivity and loss of endothelial cell function); (4) model the human condition of elevated mineralocorticoids, a population that is increaesingly appreciated as clinically relevant.17,18 Finally, the DOCA-salt rat is the original model in which we demonstrated the ability of chronic (one week) administration of 5-HT to reduce blood pressure.11 In the present study, in addition to telemetric measures of blood pressure, we measured isometric contraction of the isolated artery from all rats. Loss of vascular contraction to agonists of the adrenergic receptor would support a fall in TPR, as would potentiation of endothelial cell dependent relaxation. Collectively, our goal is to gain insight as to the mechanisms of exogenous 5-HT to induce a chronic fall in blood pressure.
Results and Discussion
The ability of 5-HT, known primarily as a vasoconstrictor, to reduce blood pressure raises an interesting conundrum. How does a vasoconstrictor in vitro translate to a vasodepressor in vivo? Moreover, can this knowledge be used to develop new therapeutic treatment, given that adherence to pharmacological therapy and control of elevated blood pressure is so poor? We addressed the potential clinical relevance of 5-HT use by taking two independent but related approaches toward examining the ability of chronically elevated free 5-HT to decrease blood pressure. We asked these questions: (1) Is 5-HT capable of decreasing blood pressure in the established DOCA-salt hypertensive rat over the course of one-month; and (2) is 5-HT capable of inhibiting the development of DOCA-salt hypertension over one month? The extension of a reduction in blood pressure to a month is relevant given that hypertension is a chronic disease.
Our model, the DOCA-salt rat, is characterized by hypervolemia, high levels of mineralocorticoid, and high salt intake19,20 and typically is monitored over a 4 week time course. Initiation of DOCA-salt hypertension is characterized by (1) retention of salt and water, contributing to a hypervolemic state and (2) increased vasopressin release. Several factors contribute to the maintenance of established DOCA-salt hypertension and include (1) enhanced sympathetic nerve activity, (2) vasopressin release, (3) increased endothelin expression, (4) attenuation of the baroreceptor reflex, (5) vascular remodeling, and (6) nitric oxide depletion due to endothelial cell damage/dysfunction.19−22 Our experimental protocol infuses 5-HT during both the established and developmental phases of DOCA-salt hypertension, thus allowing for mechanistic insight.
5-HT Infusion in Established DOCA-Salt Hypertensive Rats
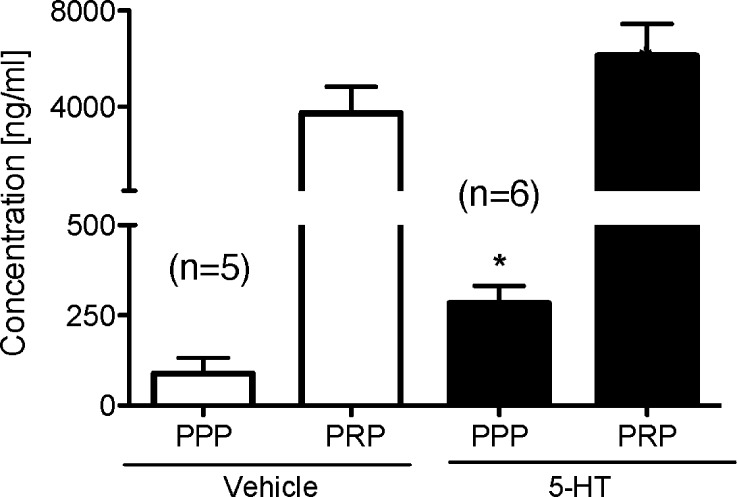
Sprague–Dawley rats underwent DOCA-salt surgery (uninephrectomy, DOCA pellet 200 mg/kg, and water supplemented with 1% NaCl and 0.2% KCl) and were given 4 weeks to become established DOCA-salt hypertensive rats. These rats then received either 5-HT (25 μg/kg/min) or vehicle (via osmotic pump) for one month. Figure 1 shows the final, terminal concentrations of 5-HT, as measured by high performance liquid chromatography (HPLC), separated as platelet poor plasma (PPP; free 5-HT) and platelet rich plasma (PRP; platelet bound 5-HT). PPP was significantly higher in rats that received 5-HT (282.9 ± 48.7 ng/mL) compared to those rats that received vehicle (87.9 ± 43.9 ng/mL) (p < 0.05), validating our infusion protocol as delivering exogenous 5-HT. For comparison, in the one week model of infusion using the same 5-HT dose, free plasma 5-HT levels (PPP) in the normotensive sham rats were 2.7 ± 0.29 ng/mL in Veh-infused rats (7 days) and 47.1 ± 23 ng/mL in 5-HT-infused rats (7 days).11 Further, HPLC analysis revealed no differences between the 5-HT content contained within the osmotic pump and a freshly made 5-HT standard, suggesting a one month 5-HT infusion did not degrade the 5-HT infused into established DOCA-salt rats.
Figure 1.
Plasma (platelet poor plasma; PPP) and platelet (platelet rich plasma; PRP) 5-HT content in established DOCA-salt hypertensive rats following 28-day infusion of vehicle or 5-HT. Bars represent means ± SEM for the number of animals in parentheses. *Statistically significant difference (p < 0.05) vs vehicle.
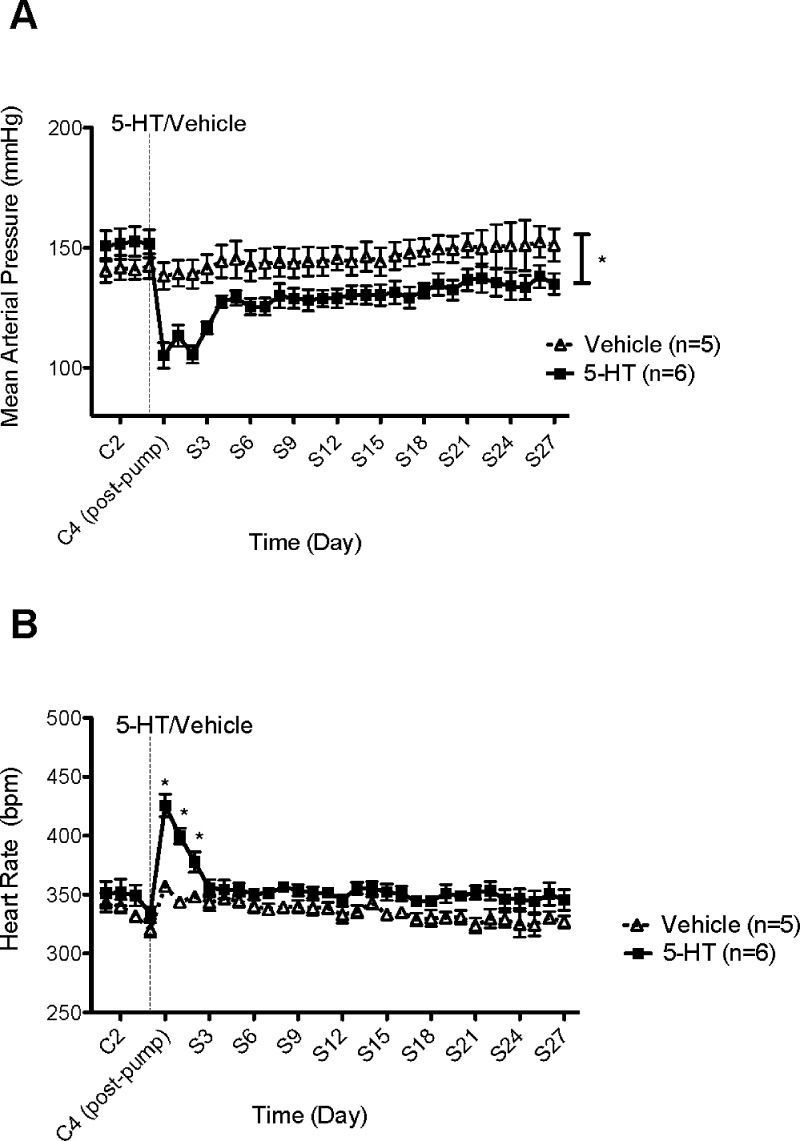
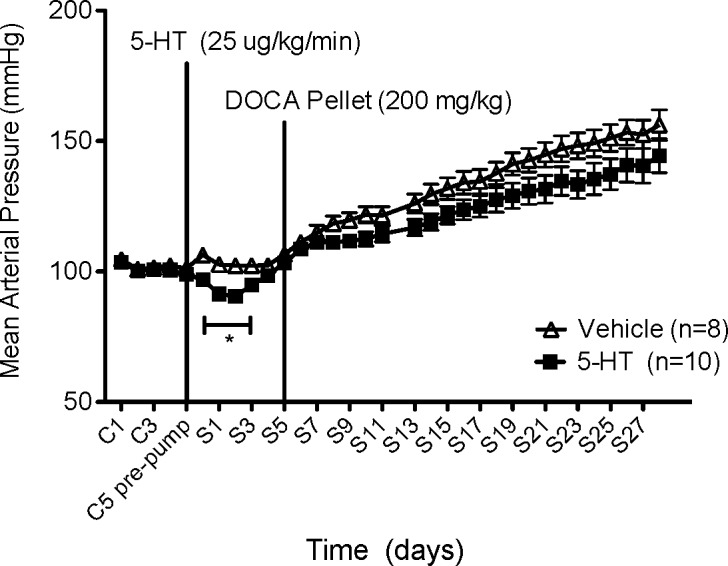
The effect of this infusion protocol on blood pressure is shown in Figure 2A. Mean arterial pressure (MAP) was significantly reduced (105 ± 5 mmHg) compared to rats receiving vehicle (138 ± 6 mmHg) at S2. Despite modest recovery in 5-HT-infused rats over the one-month study, MAP never returned to control levels (152 ± 6 mmHg at C2 vs 135 ± 4 mmHg at S27). By contrast, blood pressure did not change in rats receiving vehicle (142 ± 5 mmHg at C2 vs 151 ± 7 mmHg at S27) (p > 0.05). Further, heart rate was significantly increased (426 ± 10 bpm) compared to rats receiving vehicle (357 ± 3 bpm) immediately following 5-HT infusion (C4 postpump) (Figure 2B). However, HR returned to baseline by day 3 of 5-HT infusion. Changes in heart rate suggest that 5-HT, during its chronic infusion, did not abolish the baroreceptor reflex, as the fall in blood pressure was accompanied by a reflex increase in heart rate. We cannot exclude the possibility that 5-HT increases HR directly. This study demonstrates that 5-HT is capable of maintaining lower blood pressure(s) following 2 months of DOCA-salt treatment, a period when hypertension is considered irreversible.21
Figure 2.
(A) Effect of chronic 5-HT or vehicle infusion on mean arterial pressure (MAP) in established DOCA-salt hypertensive rats. Data points indicate 24 h averaged MAP ± SEM for the number of animals in parentheses. The vertical line denotes 5-HT or vehicle osmotic pump implant. Time is represented by days on the x-axis. C represents control recordings. S represents 5-HT/vehicle infusions. (B) Effect of chronic 5-HT or vehicle infusion on heart rate (HR) in established DOCA-salt hypertensive rats. Data points indicate 24 h averaged HR (bpm) ± SEM for the number of animals in parentheses. The vertical line denotes 5-HT or vehicle osmotic pump implant. Time is represented by days on the x-axis. C represents control recordings. S represents 5-HT/vehicle infusions. *Statistically significant difference (p < 0.05) vs vehicle.
Isometric Contractility in Established DOCA-Salt Hypertensive Rats Infused with 5-HT or Vehicle
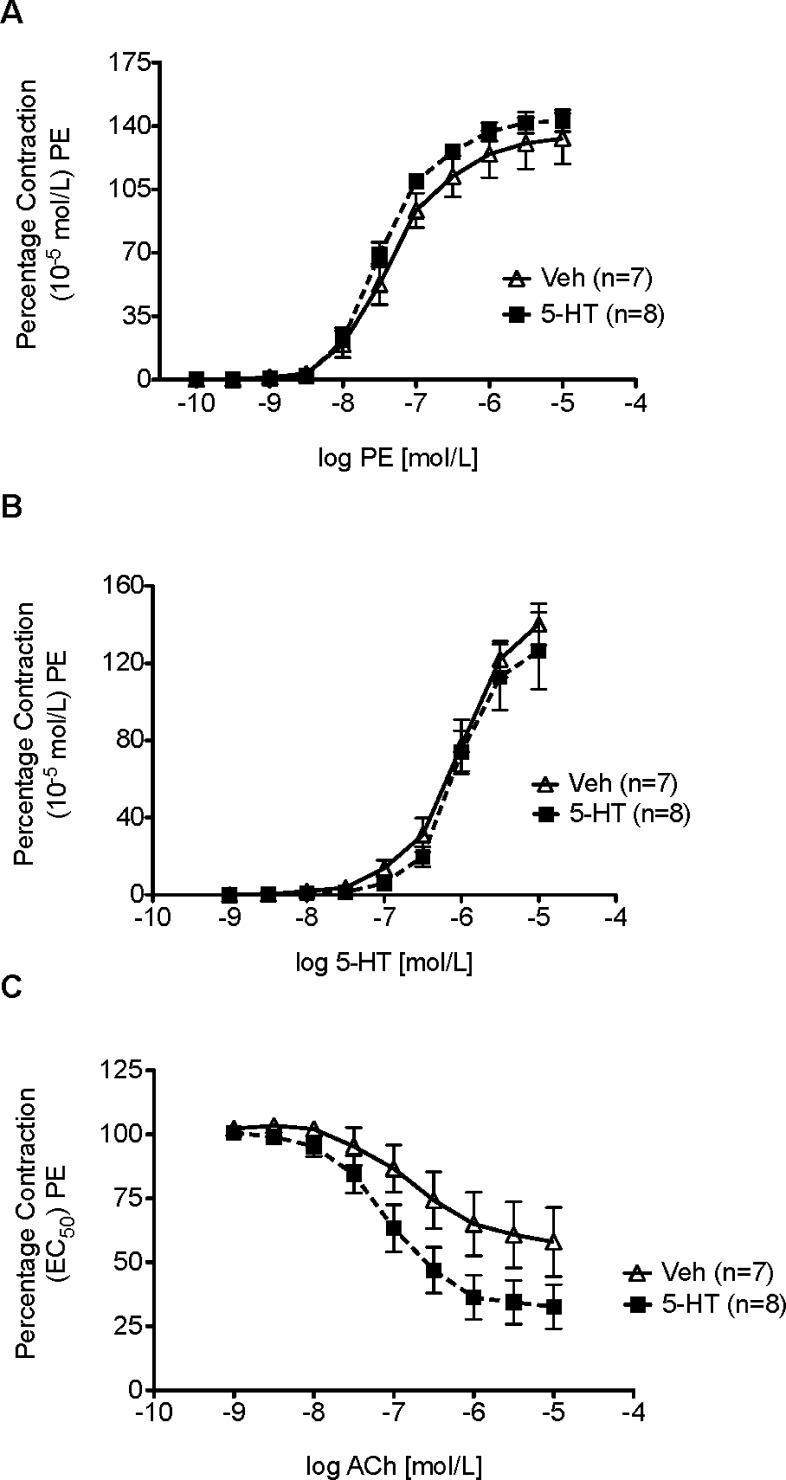
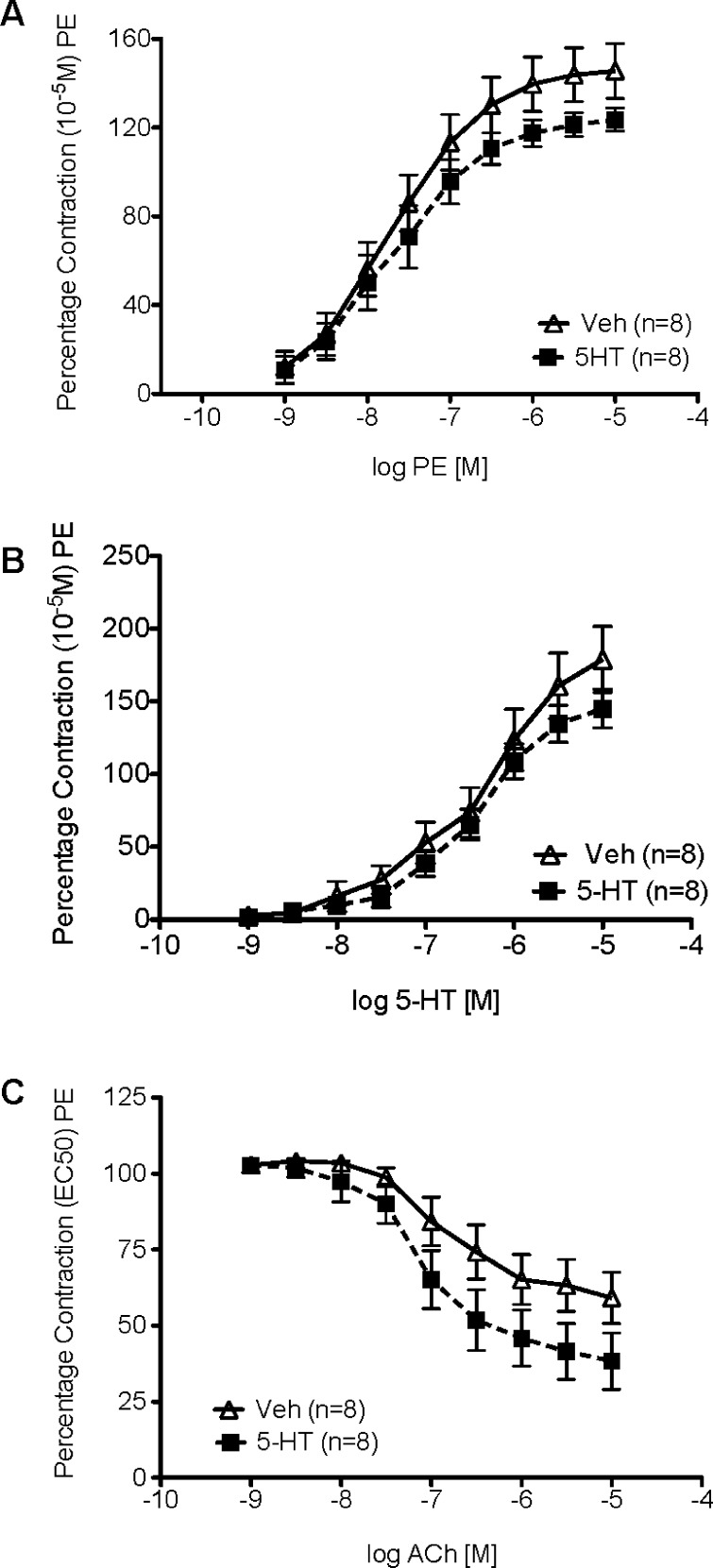
Aortic strips were mounted in warmed and aerated tissue baths for measurement of isometric contractile force to determine whether a one month infusion of 5-HT or vehicle affected the response to the α adrenergic receptor agonist, phenylephrine (PE), 5-HT, or Ach (Figure 3). The magnitude of initial contraction to PE (10–5 M) was not statistically different (p > 0.05) in established DOCA-salt 5-HT-infused (1165 ± 107.8 mg) compared to Veh-infused hypertensive rats (1415 ± 126.7 mg). These values were used to normalize subsequent contractile responses.
Figure 3.
Cumulative concentration response curve to (A) phenylephrine (PE), (B) 5-HT, or (C) acetylcholine (ACh) in the thoracic aorta of established DOCA-salt hypertensive rats. Data points represent means ± SEM for the number of animals in parentheses.
The potency of PE in contracting aortic strips from established DOCA-salt rats treated with 5-HT (−log EC50 [M] = 7.45 ± 0.05) was not different when compared to rats treated with vehicle (7.34 ± 0.1) (Figure 3A). Similarly, the maximum contraction to PE was not different (5-HT-infused 143 ± 6% vs Veh-infused 133 ± 14% PE-induced contraction, p > 0.05). The potency of [5-HT-infused −log EC50 [M] = 5.96 ± 0.14 vs Veh-infused 5.97 ± 0.09, p > 0.05] and maximum contraction to 5-HT [5-HT-infused 126 ± 20% vs Veh-infused 140 ± 11%; p > 0.05] was not changed by the one month infusion of 5-HT (Figure 3B). Finally, 5-HT infusion did not improve aortic sensitivity to ACh [5-HT-infused −log EC50 [M] = 7.06 ± 0.17 vs Veh-infused 6.76 ± 0.34, p > 0.05) (Figure 3C). However, as was observed in the one-week model, 5-HT infusion modestly, but not significantly, improved ACh-induced relaxation [5-HT-infused 33 ± 8.7% PE contraction remaining vs. Veh-infused 58 ± 13.5%, p < 0.05). 5-HT is known to release endothelial derived relaxing factors,23 but the ability of 5-HT to potentiate the relaxant effect of other substances (such as ACh) is less well understood.
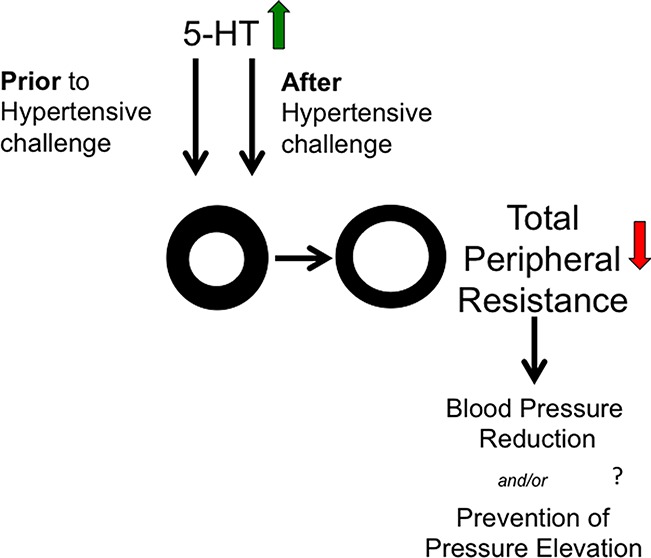
Importantly, the lack of a change in potency to the effects of PE and 5-HT demonstrated the potential of blood vessels to contract to normal stimuli (PE), and that those blood vessels did not lose there contractile response to 5-HT (desensitization) with either infusion protocol. This is a significant observation given that 5-HT clearly causes a fall in TPR.13 As such, the mechanisms by which TPR falls are relevant, and one example of this is the inability to respond to contractile stimuli. The unchanged response to PE is of particular interest given that sympathetic tone and activation of alpha adrenergic receptors is predominantly responsible for the establishment of normal TPR. 5-HT, in vitro, can cause arterial desensitization that is both homologous and heterologous, and thus, a loss of arterial contractility is a reasonable mechanism to examine.24 Our data suggest that this does not occur and that the endothelial cell may be protected by infusion of 5-HT. 5-HT can act as an antioxidant25 and potentially protects the endothelial cell from the oxidative stress so well recognized in this model.26,27 Collectively, our findings support that loss of vascular response (contraction to adrenergic or serotonergic stimulus) is not responsible for the fall in blood pressure during 5-HT infusion in the established hypertensive rat. This would suggest that 5-HT causes inhibition of the sympathetic nervous system or production of endothelin, two mechanisms known to support the established hypertension of this model.21,28
5-HT Infusion prior to the Development of DOCA-Salt Hypertension
In this second series of experiments, Sprague–Dawley rats received either 5-HT (25 μg/kg/min) or vehicle (via osmotic pump) for 5 days and then underwent DOCA-salt surgery (uninephrectomy, DOCA pellet 200 mg/kg, and water supplemented with 1% NaCl and 0.2% KCl) to determine whether 5-HT was capable of inhibiting the development of DOCA-salt hypertension. 5-HT infusion was continued for one month, covering the time period during which DOCA was given.
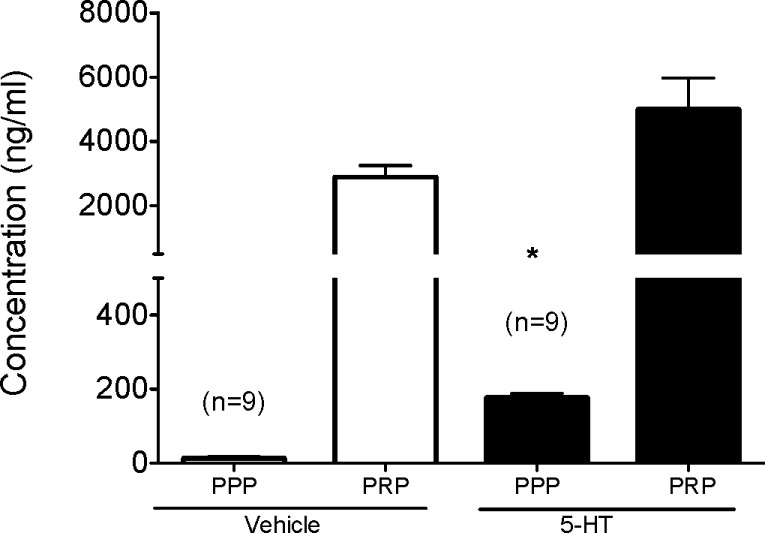
Free plasma 5-HT (PPP) was significantly (p < 0.05) higher in DOCA-salt rats that received 5-HT (177.1 ± 10.9 ng/mL) compared to those rats that received vehicle (13.3 ± 3.9 ng/mL) (Figure 4). This validated our infusion protocol and suggested that any change or lack of change in the development of DOCA-salt hypertension was not due to insufficient delivery and/or degradation of exogenous 5-HT during the study, an important observation given that 5-HT can be readily oxidized as an indole. The overall levels of 5-HT in this infusion were somewhat lower than that of the established hypertensive rat, suggesting that development of blood pressure itself may modify peripheral 5-HT metabolism. However, the fold increase in free plasma 5-HT in the 5-HT infused rats was nearly 10× that in vehicle infused rats, suggesting that exogenous 5-HT was delivered. Platelet rich plasma 5-HT concentration was modestly but not significantly elevated, but this was not suprising. Chronic infusion of 5-HT delivers 5-HT to markedly diverse tissues, such as the trachea and prostate.29 Thus, platelets are but one of the many sites that can take up 5-HT.
Figure 4.
Plasma (platelet poor plasma; PPP) and platelet (platelet rich plasma; PRP) 5-HT content in Sprague–Dawley rats following a one month infusion with either 5-HT or vehicle, followed by administration of a DOCA-salt pellet. Bars represent means ± SEM for the number of animals in parentheses. *Statistically significant difference (p < 0.05) vs vehicle.
Despite elevated levels of free 5-HT, there was no significant difference in the development of DOCA-salt hypertension or absolute magnitude of blood pressure reached in 5-HT vs vehicle-infused rats (Figure 5). Initially, 5-HT reduced blood pressure, but the absolute magnitude of MAP reached in 5-HT-infused rats (144 ± 6.5 mmHg) was not significantly different compared with Veh-infused rats (156 ± 5.9 mmHg). Data collection was briefly stopped at S12, but MAP was then continuously monitored for the remainder of the one month study (S13–S28). The inability of 5-HT to inhibit the development of DOCA-salt hypertension was an unexpected finding given that previous studies demonstrated that peripheral administration of 5-HTP, the committed precursor to 5-HT, prevented the development of DOCA-salt hypertension.16
Figure 5.
Effect of chronic 5-HT or vehicle infusion on mean arterial pressure (MAP) in Sprague–Dawley rats following a one month infusion with either 5-HT or vehicle, followed by administration of a DOCA-salt pellet. Data points indicate 24 h averaged MAP ± SEM for the number of animals in parentheses. The vertical line denotes 5-HT or vehicle osmotic pump implant and DOCA pellet implant. Time is represented by days on the x-axis. C represents control recordings. S represents 5-HT/vehicle infusions. *Statistically significant difference (p < 0.05) vs vehicle.
Isometric Contractility in DOCA-Salt Hypertensive Rats Infused with 5-HT or Vehicle Prior to DOCA-Salt Administration
As was observed in the first study, the initial contraction of aortic strips to a maximal concentration of PE (10–5 M) was not statistically different (p > 0.05) in DOCA-salt 5-HT-infused hypertensive rats (1094 ± 106.8 mg) compared to DOCA-salt Veh-infused hypertensive rats (939 ± 121.6 mg). These values were used to normalize subsequent contractile responses.
Neither the potency of PE [5-HT-infused −log EC50 [M] = 7.64 ± 0.10 vs Veh-infused = 7.65 ± 0.20, p > 0.05] nor maximal contraction to PE were modified by a one month infusion of 5-HT (5-HT-infused: 123 ± 5.2% of initial PE-induced contraction vs Veh-infused 146 ± 12.1%, p > 0.05, Figure 6A). Similarly, pharmacological parameters for contraction to 5-HT were not changed by 5-HT infusion [potency: 5-HT-infused −log EC50 [M] = 6.39 ± 0.11 vs Veh-infused 6.32 ± 0.16; p > 0.05; maximum 5-HT-infused 145 ± 13% PE contraction remaining vs Veh-infused 178 ± 23%, p > 0.05]. While 5-HT infusion did not improve aortic sensitivity to ACh in 5-HT-infused (−log EC50 [M] = 7.09 ± 0.19) compared to Veh-infused established DOCA-salt rats (EC50 = 6.85 ± 0.24, p > 0.05, Figure 6C), ACh-induced maximum relaxation was slightly, although not significantly greater in 5-HT-infused (38 ± 9.2% remaining) vs Veh-infused rats (59 ± 8.4% remaining, p > 0.05). These findings further emphasize the idea that loss of constriction is not the mechanism by which 5-HT reduces blood pressure, and both models support this idea. The ability of 5-HT to modestly improve ACh-induced relaxation is fascinating given the absolute dependence of 5-HT-induced fall in blood pressure on NOS.11 We must consider, given the variable degree of reduction of the magnitude of ACh-induced relaxation, that these experiments may have been underpowered to detect an improvement with 5-HT infusion. That this observation was made in two independent studies underscores the potential importance of this observation, and the specific interaction of 5-HT with NOS is a subject for future investigation.
Figure 6.
Cumulative concentration response curve to (A) phenylephrine (PE), (B) 5-HT, or (C) acetylcholine (ACh) in the thoracic aorta of DOCA-salt rats treated with either 5-HT or vehicle during the development of DOCA-salt hypertension. Data points represent means ± SEM for the number of animals in parentheses.
Clinical Significance
Several clinical scenarios correlate well with our investigation of elevated levels of free 5-HT and changes to blood pressure. Cardiopulmonary bypass, hemodialysis,30 and anaphylactic shock (31) are all associated with release of 5-HT from either the platelet or enterochromaffin cell and are associated with significant falls in blood pressure. Pharmacological inhibition of SERT via treatment with an SSRI (selective serotonin reuptake inhibitor) increases extracellular concentrations of 5-HT and changes in blood pressure are noted as side effects of treatment with these drugs.32 Further, 5-HTP, the committed substrate to 5-HT synthesis, is taken as a sleep aid, potentially exposing patients to elevated levels of free 5-HT.33 Taken together, these data suggest that there are several clinically relevant situations in which patients would have significantly elevated free plasma 5-HT levels, which could potentially modify blood pressure.
Perspectives and Limitations
One potential limitation of this study is the dose of DOCA-salt (200 mg/kg) used in our protocol to test the hypothesis of whether 5-HT was capable of preventing the development of DOCA-salt hypertension. Previously, a dose of 100 mg/kg of DOCA-salt was used to demonstrate 5-HTP (a committed precursor to 5-HT) was capable of preventing the development of DOCA-salt hypertension.16 The higher dose of 200 mg/kg of DOCA-salt (used throughout this study) may have masked the ability of 5-HT to inhibit the development of DOCA-salt hypertension and created results inconsistent with those previously found with 5-HTP. Another potential limitation of this study is the length of our infusion protocol. Clinically, patients often require antihypertensive medications for a lifetime, and thus, a one month infusion remains a relatively short exposure. Newly available infusion pumps may be able to extend this exposure even further.34 Another limitation stems from an assumption we make. Namely, we assume that it is free, nonplatelet bound 5-HT that is the biologically active 5-HT that produces the fall in blood pressure. Platelets are reservoirs of 5-HT, possessing SERT proteins that concentrate 5-HT to millimolar concentrations. It is possibility that the stability of the platelet in a rat in which hypertension is developing may be different than that with established hypertension. While the fragility of platelets has been published to be increased in DOCA-salt established hypertension and 5-HT content reduced,35 the status of the platetet is not known during the development of hypertension. Human platelet content of 5-HT is reduced in established hypertensive patients.5 A formal measure of platelet number during these phases of hypertension has not been performed. Thus, we cannot exclude the possibility that the function of the platelet itself may be changed and participate in the disease.
We also cannot exclude the idea that 5-HT affects the sympathetic nervous system tone to reduce blood pressure, through inhibition of the neuroeffector transmission or ganglionic function.12 One interesting speculation is that 5-HT is able to penetrate the blood-brain barrier to modify central function, and this is supported by work published over 40 years ago.36−38 5-HT itself may alter the permeability of the blood brain barrier and increase its own passage into the CNS. While this remains a speculation, it is an interesting line of evidence to pursue in ascertaining the mechanism(s) by which 5-HT decreases blood pressure.
In summary, this study reinforces the finding that chronic 5-HT-infusion lowers blood pressure in the established DOCA-salt hypertensive rat, and demonstrates that 5-HT is capable of lowering blood pressure over the course of one month. Although 5-HT was unable to prevent the development of DOCA-salt hypertension, it suggests a potentially beneficial role for 5-HT in the cardiovascular system.
Methods
Animal
The Michigan State University Institutional Animal Care and Use Committee (IACUC) approved all protocols. Male Sprague–Dawley rats (250–300 g; Charles River Breeding Laboratories, Portage, MI) underwent one of two separate experimental protocols: (1) Male Sprague–Dawley rats (250–300 g) underwent left uninephrectomy, and a DOCA pellet was implanted s.c. (200 mg/kg). Water was supplemented with 1% NaCl and 0.2% KCl for the duration of the study. After 4 weeks, these rats were considered established DOCA-salt hypertensive rats and were administered either 5-HT or vehicle via osmotic pump (s.c.) for one month. (2) Male Sprague–Dawley rats (250–300 g) underwent left uninephrectomy and were then administered either vehicle or 5-HT vehicle via osmotic pump (s.c.) for one month. After 5 days of treatment, Sprague–Dawley rats received a DOCA pellet (200 mg/kg), and their water was supplemented with 1% NaCl and 0.2% KCl for the remaining duration of the one month infusion.
Anesthesia and Analgesia
All rats were anesthetized with isoflurane (2% in 100% O2) and ventilated mechanically. At the time of surgery, the incision site was treated with topical antibiotic containing analgesic to prevent irritation and infection. Incisions were closed with silk suture. Rats were treated with amoxicillin (150 mg/kg/i.m.) following surgery and 3 days thereafter. All rats were treated with rimadyl (5 mg/kg, s.c. for 2 days) for general analgesia.
Surgical Methods: Telemeter Placement
Radiotelemeters (DSI PhysioTel PA series transmitter model PA-C40) were implanted subcutaneously in the lower abdomen and catheters introduced into the left femoral artery. Pressure sensing tips were advanced into the thoracic aorta. All rats were given 7 days to recover prior to any measure. Mean arterial pressure (MAP) and heart rate (HR) were recorded at 10-min intervals (10 s recording) for the duration of the study.
Surgical Methods: Alzet Osmotic Pump
A small incision was made at the base of the neck. Blunt dissection was used to create a small subcutaneous pocket between the scapulae. The pump (Alzet Osmotic Pump, model 2ML4 (28 days) or model 2ML1 (7 day) Duret Corporation, Cupertino, CA, 2.5 μL/h 28 days, 10 μL/h 7 days) was inserted and the skin sutured closed. To each pump, a 5-HT creatinine complex (25 μg/kg/min) and 1% ascorbic acid, antioxidant (0.02 g/pump), or vehicle (1% ascorbic acid, antioxidant) was loaded. The solution was dissolved in 1 M HCl, and a pH-balance (∼7) was achieved with 4 M NaOH.
Plasma 5-HT Measurements
Blood collection and platelet poor plasma (PPP)/platelet rich plasma (PRP) preparation were conducted according to our previously published methods.11 Briefly, 5 mL of blood was collected from left cardiac ventricle using a heparin-coated syringe. The blood was transferred into an EDTA anticoagulant vacutainer tube. Ten μM pargyline and 10 μM ascorbic acid were added. The EDTA tubes were spun at 160g for 30 min at 4 °C for PRP. Two mL of supernatant containing plasma and buffy coat were pipetted into EDTA-coated plastic tubes and mixed with a 1:1 dilution of 0.5 M EDTA. Ten μM pargyline and 10 μM ascorbic acid were added. These tubes were centrifuged for 20 min at 1350g at 4 °C for PPP. To the remaining pellet (platelet layer), 1 mL of platelet buffer (145 mM NaCl, 5 mM KCl, 1 mM CaCl2, 1 mM MgSO4 10 mM d-glucose) and 1 μM ADP were added. Ten μM pargyline and 10 μM ascorbic acid were added. These tubes were then vortexed and allowed to sit on ice for 15 min for platelets to become activated and degranulate. The tubes were then centrifuged at 730g for 10 min at 4 °C. Ten percent trichloroacetic acid (TCA) was added to deproteinize both sets of samples and allowed to sit on ice for 10 min. These tubes were centrifuged at 4500g for 20 min at 4 °C. Finally, the samples were ultracentrifuged at 280 000g for 2 h. 5-HT concentrations were measured using high-performance liquid chromatography (HPLC) at 0.2 V and 0.6 mL/min flow rate.
Isolated Tissue Bath
Rats were anesthetized with pentobarbital (60 mg/kg i.p.), and the thoracic aorta was removed and placed in physiological salt solution (PSS) containing (mM) NaCl 130; KCl 4.7; KH2PO4 1.8; MgSO4·7H2O 1.7; NaHCO3 14.8; dextrose 5.5; CaNa2EDTA 0.03 and CaCl2 1.6 (pH 7.2). The endothelium-intact thoracic aorta was cleaned and cut into helical strips. It was mounted in a warmed (37 °C) and aerated (95% O2, 5% CO2) tissue bath connected to Grass isometric transducer (FT03; Grass instruments, Quincy, MA), which was connected to an ADInstruments PowerLab (ADInstruments, Colorado Springs, CO). Tissues were placed under optimal resting tension (1500 mg; previously determined) and allowed to equilibrate for 1 h. The initial contraction was stimulated by a maximal concentration (10–5 M) of phenylephrine (PE). Cumulative concentration response curves to PE (10–10–10–5 M) and 5-HT (10–9–10–5 M) were generated. The aorta was also half-maximally contracted to PE, followed by generation of an acetylcholine (ACh) concentration response curve (10–9–10–5 M) to determine endothelial cell function. Concentration response curves were performed in the following order: (1) PE, (2) 5-HT, (3) ACh. Tissues were thoroughly washed in between each curve, and tissues rested between each curve. Potency of an agonist was expressed as −log EC50 [M] calculated by nonlinear regression in GraphPad Prism (GraphPad Software Inc., San Diego, CA), where EC50 is the effective concentration of the agonist (M) that induces 50% of the maximal response.
Statistical Analysis
For in vivo data analysis, within-group differences were assessed by a one-way ANOVA with post hoc multiple comparisons using Student–Newman–Keuls test (GraphPad Prism 5). An unpaired Student's t test was performed to measure point-to-point differences. In all cases, a p value of <0.05 was considered significant. All results are presented as means ± SEM.
Glossary
Abbreviations
- 5-HT
5-hydroxytryptamine
- PPP
platelet poor plasma
- PRP
platelet rich plasma
- DOCA
deoxycorticosterone acetate
- HPLC
high performance liquid chromatography
Author Contributions
R.P.D. drafted this manuscript and performed experiments; T.S. helped perform telemetry and blood pressure measures, as did H.G.; R.B. performed HPLC measures of 5-HT; N.R.T. helped with contractile experiments; S.W.W. helped with contractile experiments and manuscript writing.
This work was supported by funding from NIH to R.P.D. (HL099024) and S.W.W. (HL107495)
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
References
- Campese V. M.; Ku E.; Park J. (2011) Sympathetic renal innervation and resistant hypertension. Int. J. Hypertens. 2011, 814354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erspamer V.; Asero B. (1952) Identification of enteramine, the specific hormone of the enterochromaffin cell system, as 5-hydroxytryptamine. Nature 169, 800–801. [DOI] [PubMed] [Google Scholar]
- Rapport M. M.; Green A. A.; Page I. H. (1948) Serum vasoconstrictor (serotonin): IV Isolation and characterization. J. Biol. Chem. 176, 1243–1251. [PubMed] [Google Scholar]
- Dahlstroem A.; Fuxe K. (1964) Evidence for the existence of monoamine-containing neurons in the central nervous system. 1. Demonstration of monoamines in the cell bodies of brain stem neurons. Acta Physiol. Scand. 232(Suppl), 1–55. [PubMed] [Google Scholar]
- Brenner B.; Harney J. T.; Ahmed B. A.; Jeffus B. C.; Unal R.; Mehta J. L.; Kilic F. (2007) Plasma serotonin levels and the platelet serotonin transporter. J. Neurochem. 102, 206–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetkovska N.; Pletscher A.; Ferracin F.; Amstein R.; Buhler F. R. (1990) Impaired uptake of 5-hydroxytryptamine in platelet in essential hypertension: clinical relevance. Cardiovasc. Drugs Ther. 4, 105–109. [DOI] [PubMed] [Google Scholar]
- Watts S. W.; Fink G. D. (1999) 5-HT2B-receptor antagonist LY-272015 is antihypertensive in DOCA-salt-hypertensive rats. Am. J. Physiol.: Heart Circ. Physiol. 276, 944–952. [DOI] [PubMed] [Google Scholar]
- Watts S. W. (2005) 5-HT in systemic hypertension: foe, friend or fantasy. Clin. Sci. 08, 399–412. [DOI] [PubMed] [Google Scholar]
- Vanhoutte P.; Amery A.; Birkenhager W.; Breckenridge A.; Buhler F.; Distler A.; Dormandy J.; Doyle A.; Frohlich E.; Hansson L.; Hedner T.; Hollenberg N.; Jensen H.-E.; Lund-Johansen P; Meyer P.; Opie L.; Robertson I.; Safar M.; Schalekamp M.; Symoens J.; Trap-Jensen J.; Zanchetti A. (1988) Serotonergic mechanisms in hypertension: focus on the effects of ketanserin. Hypertension 11, 111–133. [DOI] [PubMed] [Google Scholar]
- Thompson L. P.; Webb R. C. (1987) Vascular responsiveness to serotonin metabolites in mineralocorticoid hypertension. Hypertension. 9, 277–281. [DOI] [PubMed] [Google Scholar]
- Diaz J.; Ni W.; Thompson J.; King A.; Fink G. D.; Watts S. W. (2008) 5-hydroxytryptamine lowers blood pressure in normotensive and hypertensive rats. J. Pharmacol. Exp. Ther. 325, 1031–1038. [DOI] [PubMed] [Google Scholar]
- Watts S. W.; Morrison S. F.; Davis R. P.; Barman S. M. (2012) Serotonin and blood pressure regulation. Pharmacol. Rev. 64(2), 359–388(also ref (28)). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. P.; Pattison J.; Thompson J. M.; Tiniakov R.; Scrogin K.; Watts S. W. (2012) 5-hydroxytryptamine (5-HT) reduces total peripheral resistance during chronic infusion: direct arterial mesenteric relaxation is not involved. BMC Pharmacol. 12, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. P.; Linder A. E.; Watts S. W. (2011) Lack of the serotonin transporter (SERT) reduces the ability of 5-hydroxytrytamine to lower blood pressure. Naunyn Schmuedebergs Arch. Pharmacol. 388(5), 543–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan T.; Watts S. W.; Davis R. P. (2011) Drug delivery: enabling technology for drug discovery and development. iPRECIO microinfusion pump: programmable, refillable and implantable. Front Pharmacol. 2, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fregly M. J.; Lockley O. E.; Summers C. (1987) Chronic treatment with L-5-hydroxytryptophan prevents the development of DOCA-salt-induced hypertension in rats. J. Hypertens. 5(5), 621–628. [DOI] [PubMed] [Google Scholar]
- Clark D.; Ahmed M. I; Calhoun D. A. (2012) Resistant hypertension and aldosterone: an update. Can. J. Cardiol. 28, 318–325. [DOI] [PubMed] [Google Scholar]
- Acelaiado M. C.; Pison R.; Dudenbostel T.; Dell’Italia L. J.; Cartmill F.; Zhang B.; Cofield S. S.; Oparil S.; Calhoun D. A. (2012) Refractory hypertension: definition, prevalence, and patient characteristics. J. Clin. Hypertens. 14, 7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk J.; McNeill J. H. (1992) The Pathogenesis of DOCA-Salt Hypertension. J. Pharmacol. Toxicol. Methods 3, 161–170. [DOI] [PubMed] [Google Scholar]
- Crofton J. T.; Share L.; Shade R. E.; Lee-Kwon W. J.; Manning M.; Sawyer W. H. (1979) The importance of vasopressin in the development and maintenance of DOCA-salt hypertension in the rat. Hypertension 1, 31–38. [DOI] [PubMed] [Google Scholar]
- Yemane H.; Busauskas M.; Burris S. K.; Knuepfer M. M. (2009) Neurohumoral mechanisms in deoxycortictosterone acetate (DOCA)-salt hypertension in rats. Exp. Physiol. 95.1, 51–55. [DOI] [PubMed] [Google Scholar]
- Van de Voorde J.; Leusen I. (1986) Endothelium-dependent and independent relaxation of aortic rings from hypertensive rats. Am. J. Physiol. 250, H711–H717. [DOI] [PubMed] [Google Scholar]
- Cohen R. A.; Shepherd J. T.; Vanhoutte P. M. (1983) 5-Hydroxytryptamine can mediate endothelium-dependent relaxation of coronary arteries. Am. J. Physiol. 245, H1077–H1080. [DOI] [PubMed] [Google Scholar]
- Watts S. W.; Schenck K. W.; Cohen M. L. (1994) Selective desensitization of 5-hydroxytryptamine 2A receptor mediated contraction in guinea pig trachea. Can. J. Physiol. Pharmacol. 72(5), 463–470. [DOI] [PubMed] [Google Scholar]
- Munoz-Castaneda J. R.; Montilla P.; Padillo F. J.; Bujalance I.; Munoz M. C.; Muntane J.; Tunez I. (2006) Role of serotonin in cerebral oxidative stress in rats. Acta Neurobiol. Exp. 66, 1–6. [DOI] [PubMed] [Google Scholar]
- Iyer A.; Chan V.; Brown L. (2010) The DOCA-Salt Hypertensive Rat as a Model of Cardiovascular Oxidative and Inflammatory Stress. Curr. Cardiol. Rev. 6(4), 291–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordellini S.; Nigro D.; Carvalho M. H.; Fortes Z. B.; Scivoletto R. (1988) Reactivity of macro- and microvessels of DOCA-salt hypertensive rats: role of the endothelial cell. Braz. J. Med. Biol. Res. 21(4), 845–9. [PubMed] [Google Scholar]
- Lariviere R.; Day R.; Schriffrin E. L. (1993) Increased expression of endothelin-1 gene in blood vessel of deoxycorticosterone acetate-salt hypertensive rats. Hypertension 21, 916–920. [DOI] [PubMed] [Google Scholar]
- Linder A. E.; Beggs K. M.; Burnett R. J.; Watts S. W. (2009) Body distribution of infused serotonin in rats. Clin. Exp. Pharmacol. Physiol. 36, 599–601. [DOI] [PubMed] [Google Scholar]
- Borgdoff P.; Fekkes D.; Tangelder G. J. (2002) Hypotension caused by extracorporal circulation. Circulation 106, 2588–2593. [DOI] [PubMed] [Google Scholar]
- Meuer S.; Ecker U.; Hadding U.; Bitter-Suermann D. (1981) Platelet serotonin release by C3a and C5a: two independent pathways of activation. J. Immunol. 126, 1506–1509. [PubMed] [Google Scholar]
- Rodriguez de la Torre B.; Dreher J.; Malevany I.; Bagli M.; Kolbinger M.; Omran H.; Lüderitz B.; Rao M. L. (2001) Serum levels and cardiovascular effects of tricyclic antidepressants and selective serotonin reuptake inhibitors in depressed patients. Ther. Drug Monit. 4, 435–440. [DOI] [PubMed] [Google Scholar]
- Das Y. T.; Bagchi M.; Bagchi D.; Preuss H,G. (2004) Safety of 5-hydroxy-l-tryptophan. Toxicol. Lett. 150, 111–122. [DOI] [PubMed] [Google Scholar]
- Abe C.; Tashiro T.; Tanaka K.; Ogihara R.; Morita H. (2009) A novel type of implantable and programmable infusion pump for small laboratory animals. J. Pharmacol. Toxicol. Methods 59(1), 7–12. [DOI] [PubMed] [Google Scholar]
- Umegaki K.; Nakamura K.; Ikeda M.; Inoue Y.; Tomita T. (1989) Changes in platelet function due to hypertension: comparison of experimental hypertension with spontaneous hypertension in rats. J. Hypertens. 7, 13–19. [DOI] [PubMed] [Google Scholar]
- Bulat M.; Supek K. (1968) Passage of 5-hydroxytryptamine through the blood brain barrier, its metabolism in the brain and elimination of 5-hydroxytryptamine from brain tissue. J. Neurochem. 15, 383–389. [DOI] [PubMed] [Google Scholar]
- Westergaard W. (1975) The effect of serotonin on the blood brain barrier to proteins. Acta Neuropathol. 32, 27–42.167542 [Google Scholar]
- Westergaard E. (1978) The effect of serotonin on the blood brain barrier to proteins. J. Neural Transm., Suppl. 14, 9–15. [PubMed] [Google Scholar]