Abstract
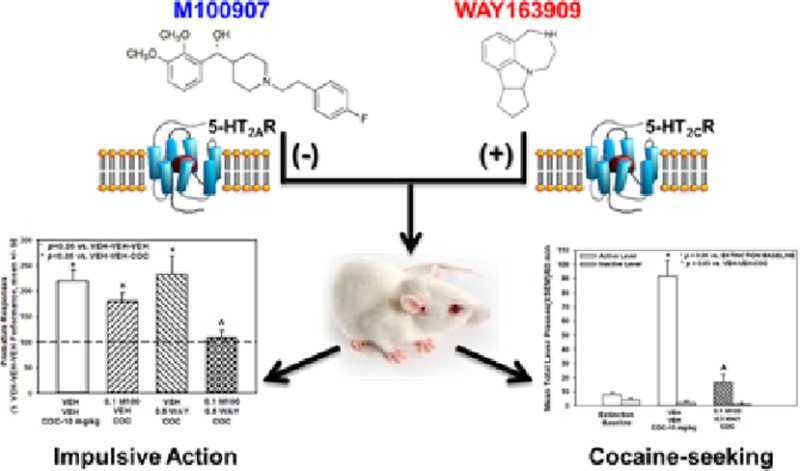
Relapse to cocaine dependence, even after extended abstinence, involves a number of liability factors including impulsivity (predisposition toward rapid, unplanned reactions to stimuli without regard to negative consequences) and cue reactivity (sensitivity to cues associated with cocaine-taking which can promote cocaine-seeking). These factors have been mechanistically linked to serotonin (5-hydroxytryptamine, 5-HT) signaling through the 5-HT2A receptor (5-HT2AR) and 5-HT2CR; either a selective 5-HT2AR antagonist or a 5-HT2CR agonist suppresses impulsivity and cocaine-seeking in preclinical models. We conducted proof-of-concept analyses to evaluate whether a combination of 5-HT2AR antagonist plus 5-HT2CR agonist would have synergistic effects over these liability factors for relapse as measured in a 1-choice serial reaction time task and cocaine self-administration/reinstatement assay. Combined administration of a dose of the selective 5-HT2AR antagonist M100907 plus the 5-HT2CR agonist WAY163909, each ineffective alone, synergistically suppressed cocaine-induced hyperactivity, inherent and cocaine-evoked impulsive action, as well as cue- and cocaine-primed reinstatement of cocaine-seeking behavior. The identification of synergism between a 5-HT2AR antagonist plus a 5-HT2CR agonist to attenuate these factors important in relapse indicates the promise of a bifunctional ligand as an anti-addiction pharmacotherapeutic, setting the stage to develop new ligands with improved efficacy, potency, selectivity, and in vivo profiles over the individual molecules.
Keywords: 1-Choice serial reaction time task, 5-HT2A receptor, 5-HT2C receptor, cocaine, cue reactivity, impulsivity, self-administration, serotonin
Dependence on the psychostimulant cocaine is marked by problematic vulnerability to relapse even years into abstinence.1,2 Two important liability factors that contribute to relapse are impulsivity (predisposition toward rapid, unplanned reactions to stimuli without regard to negative consequences)3 and cue reactivity (sensitivity to cues associated with cocaine-taking that can promote cocaine-seeking).4,5 Impulsivity and cue reactivity appear to be interrelated in human cocaine users,6 and new pharmacotherapeutic strategies that effectively diminish both are likely to enhance abstinence in the highly vulnerable population of cocaine-dependent individuals.
The underlying neurobiology of these liability factors includes a regulatory role for serotonin (5-HT; 5-hydroxytryptamine) neurotransmission. The actions of 5-HT in neurons are transduced by at least 14 subtypes of 5-HT receptors (5-HTXRs) which are presently grouped into seven families (5-HT1R–5-HT7R) according to their structural and functional characteristics.7−9 Selective blockade of the 5-HT2AR10−12 or activation of the 5-HT2CR10,13,14 consistently reduces impulsivity as measured by premature responses assessed in the 1- or 5-choice serial reaction time (1- or 5-CSRT) task, a preclinical assay that assesses the inability to withhold a prepotent response and aligns with human measures of impulsive action (e.g., continuous performance task).15,16 These same ligands also reliably suppress both cue- and cocaine-evoked reinstatement (cocaine-seeking) after a period of cocaine self-administration and extinction.17−20
We conducted proof-of-concept analyses to evaluate whether a combination of 5-HT2AR antagonist plus 5-HT2CR agonist would have synergistic effects over these liability factors for relapse. The 1-CSRT task was employed to measure impulsivity, while cue-evoked and cocaine-primed reinstatement of cocaine-seeking behavior was assessed after extinction from cocaine self-administration. The piperidine molecule M10090721 (Figure 1A) has been shown to bind to 5-HT2AR with high affinity (IC50 = 3.3 nM) and is greater than 100 times more selective for the 5-HT2AR over either the 5-HT2CR or 5-HT2BR.23,24 WAY163909 (Figure 1B) exhibits high affinity (Ki = 10.5 nM) and full efficacy (90% relative to 5-HT) at the 5-HT2CR and a low affinity (Ki = 212 nM) at the 5-HT2AR; WAY163909 is a weak partial agonist at the 5-HT2BR at concentrations 23-fold greater.22 M10090719,24−26 and WAY16390914,17,22,27 have been identified as a selective 5-HT2AR antagonist and 5-HT2CR agonist, respectively, in a wide array of cellular and behavioral models. The doses of M100907 and WAY163909, pretreatment time intervals, and order of injections to be employed in the analyses were chosen based upon our extensive experience and previous studies.12,14,17,19 Our results indicate that a combination of subthreshold doses of the two compounds effectively and synergistically suppress impulsivity and cocaine-seeking evoked by cues and cocaine exposure, suggesting a new strategy to achieve therapeutic advantage in cocaine addiction.
Figure 1.
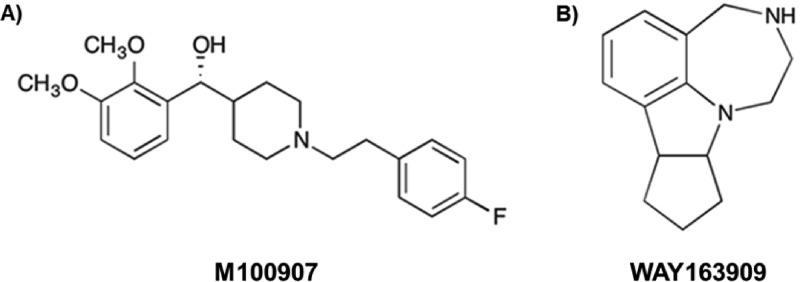
Structures of [A] the 5-HT2AR antagonist M100907 and [B] the 5-HT2CR agonist WAY163909 are shown.
Results and Discussion
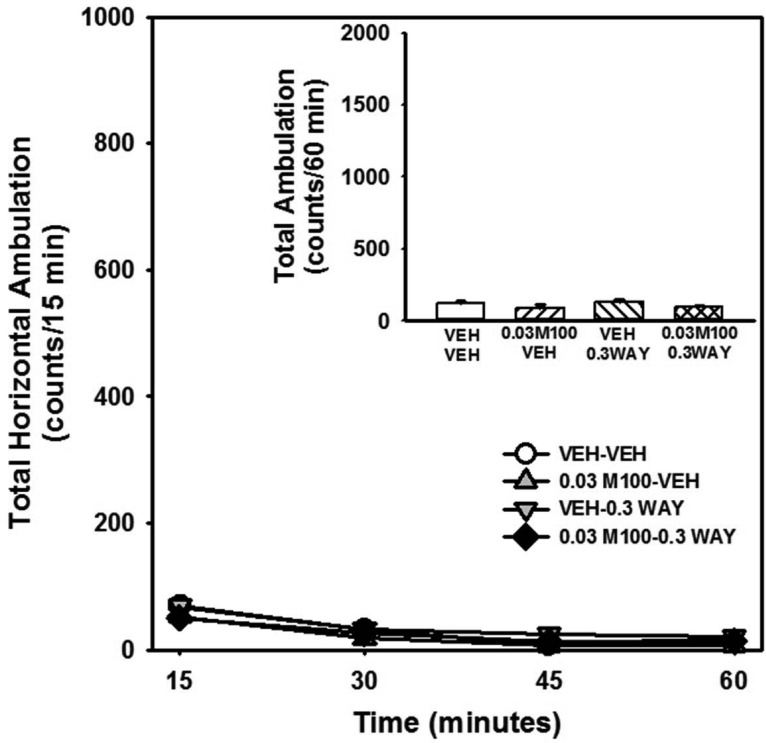
M100907 + WAY163909 Do Not Synergize to Alter Spontaneous Motor Activity
The evaluation of combined treatment with the selective 5-HT2AR antagonist M100907 plus the selective, full 5-HT2CR agonist WAY163909 required that we identify doses of M100907 and WAY163909 that were subthreshold in each assay (summarized in Table 1). We have shown that M100907 did not alter spontaneous motor activity in doses from 0.02 to 2 mg/kg under experimental conditions employed here.28 Further, we have shown that, while doses of 3 and 10 mg/kg of WAY163909 suppressed spontaneous motor activity in rats, doses of 0.1–1 mg/kg of WAY163909 were inactive.17 In the present study, we conducted an initial evaluation of the combined effects of these compounds in simple motor activity assays. Figure 2 displays the horizontal ambulations after injection with vehicle, M100907 (0.03 mg/kg), WAY163909 (0.3 mg/kg), or the combination of the two ligands; data are presented as total horizontal ambulations in 15 min bins (Figure 2) or for the entire 60 min session (Figure 2, inset). No main effect of treatment [F(3,110) = 2.01, n.s.], a main effect of time [F(3,110) = 22, p < 0.05] but no treatment × time interaction [F(9,110) = 0.33, n.s.] were observed for horizontal ambulations divided into four 15 min bins. A priori comparisons indicated that neither M100907 nor WAY163909 at the chosen dose altered horizontal ambulations versus vehicle+vehicle (VEH-VEH) at any 15 min interval (n.s.; Figure 2) as predicted by our previous observations.17,28 Furthermore, the combination of M100907 plus WAY163909 did not significantly alter horizontal ambulations versus VEH-VEH at any 15 min interval (n.s.; Figure 2). No main effect of treatment was observed for horizontal ambulations totaled for the entire 60 min test session [F(3,28) = 2.11, n.s.; Figure 2, inset]. The effects of M100907 plus WAY163909 on vertical activity were similar to horizontal ambulations (data not shown). Thus, subthreshold doses of M100907 (0.03 mg/kg)28 and WAY163909 (0.3 mg/kg)17 were identified, and the combined administration of these doses of M100907 plus WAY163909 did not evoke additive or synergistic effects on spontaneous motor activity (Figure 2; Table 1).
Table 1. Threshold Doses of the 5-HT2AR Antagonist M100907 and the 5-HT2CR Agonist WAY163909 in Motor Activity, Impulsivity and Cue Reactivity Assays.
| subthreshold
dosea |
|||
|---|---|---|---|
| M100907 | WAY163909 | synergism | |
| Motor Activityb | |||
| spontaneous motor activity | 0.03 mg/kg | 0.3 mg/kg | no |
| cocaine-induced motor activity | 0.03 mg/kg | 0.3 mg/kg | yes |
| Impulsivityc | |||
| inherent impulsive action | 0.01 mg/kg | 0.1 mg/kg | yes |
| cocaine-induced impulsive action | 0.1 mg/kg | 0.5 mg/kg | yes |
| Cue Reactivityd | |||
| cue-induced reinstatement | 0.001 mg/kg | 0.1 mg/kg | yes |
| cocaine-primed reinstatement | 0.1 mg/kg | 0.3 mg/kg | yes |
The “subthreshold dose” is the employed dose of ligand (i.p.) at which the noted behavior was unaltered (vs control).
Doses up to 2 mg/kg of M10090728 and 1 mg/kg of WAY16390917 were ineffective to alter spontaneous or cocaine-evoked motor activity (Figure 3).
Figure 2.
M100907 plus WAY163909 do not synergize to alter spontaneous motor activity. Rats were injected (i.p.) with M100907 (M100; 0.03 mg/kg) or vehicle (VEH; 1% Tween) 30 min prior, and WAY163909 (WAY; 0.3 mg/kg) or vehicle (VEH; saline, 1 mL/kg) 15 min prior to the start of the test session. The time course of horizontal ambulations is divided into 15 min time bins across the 60 min session; [inset] the mean total horizontal ambulations (counts/60 min) (±SEM).
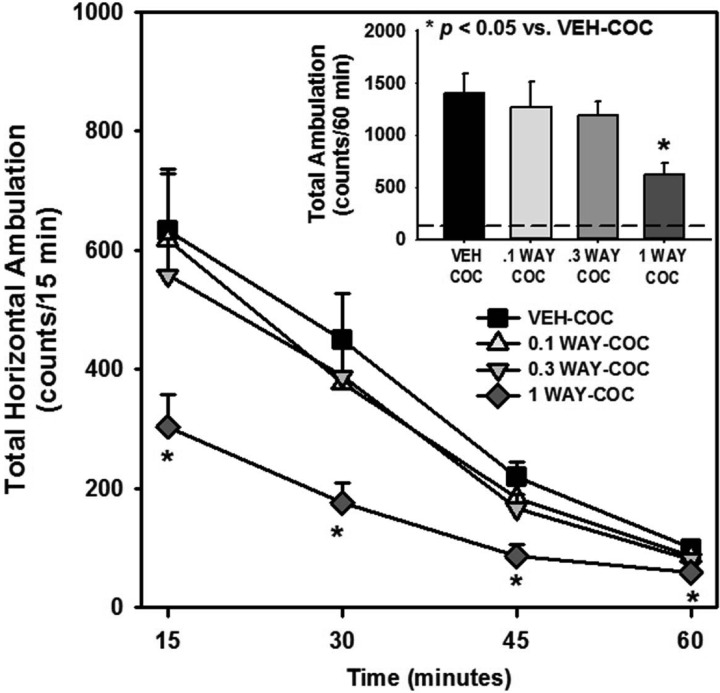
M100907 + WAY163909 Synergistically Suppress Cocaine-Induced Hyperactivity
Our studies have shown that M100907 (0.02–2 mg/kg) dose-dependently suppressed hyperactivity induced by acute cocaine administration.28 While the efficacy of WAY163909 to suppress cocaine-evoked hyperactivity is predicted based upon prior studies with other 5-HT2CR agonists,29,30 we first evaluated the ability of WAY163909 (0.1, 0.3, and 1 mg/kg) to alter horizontal ambulations evoked by cocaine (15 mg/kg); these WAY163909 doses were chosen as below those (3 and 10 mg/kg) noted to significantly suppress spontaneous motor activity.17 Figure 3 displays the horizontal ambulations after treatment with vehicle or WAY163909 prior to administration of cocaine; data are presented as total horizontal ambulations in 15 min bins (Figure 3) or for the entire 60 min session (Figure 3, inset). A main effect of treatment [F(3,44) = 3.83, p < 0.05], time [F(3,44) = 93.80, p < 0.05], and a treatment × time interaction [F(12,132) = 2.33, p < 0.05] were observed for horizontal ambulations divided into four 15 min bins. A priori comparisons revealed that a dose of 1 mg/kg, but not 0.1 or 0.3 mg/kg, of WAY163909 reduced cocaine-evoked horizontal ambulations at all four 15 min intervals (1 WAY-COC vs VEH-COC; p < 0.05; Figure 3). A main effect of treatment was observed for horizontal ambulations totaled for the entire 60 min test session [F(3,44) = 3.83, p < 0.05; Figure 3, inset]; a priori comparisons showed that 1 mg/kg, but not 0.1 or 0.3 mg/kg, of WAY163909 significantly suppressed cocaine-evoked horizontal ambulations (1 WAY-COC vs VEH-COC; p < 0.05; Figure 3, inset; dashed line indicates VEH-VEH levels). Thus, we identified subthreshold doses of M100907 (0.03 mg/kg)28 and WAY163909 (0.3 mg/kg; Figure 3) which do not alter cocaine-induced motor activity when tested alone (Table 1).
Figure 3.
WAY163909 dose-dependently suppresses cocaine-evoked hyperactivity. Rats received an i.p. injection of WAY163909 (WAY; 0.1, 0.3, or 1 mg/kg) or vehicle (VEH; saline, 1 mL/kg) 15 min prior to i.p. injection of cocaine (COC; 15 mg/kg) or vehicle (VEH; saline, 1 mL/kg). The time course of horizontal ambulations is divided into 15 min time bins across the 60 min session; [inset] the mean total horizontal ambulations (counts/60 min) (±SEM). The dashed line represents the mean VEH-VEH response. *p < 0.05 vs VEH-COC.
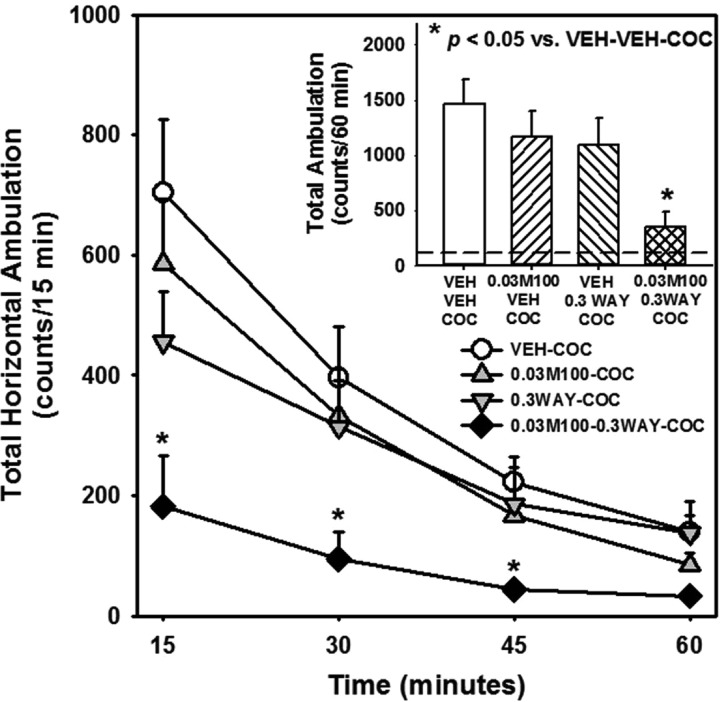
We next tested the hypothesis that combined treatment with subthreshold doses of M100907 (0.03 mg/kg) plus WAY163909 (0.3 mg/kg) suppresses cocaine-evoked hyperactivity. Figure 4 displays horizontal ambulations after treatment with vehicle, M100907 (0.03 mg/kg), WAY163909 (0.3 mg/kg), or the combination prior to cocaine (15 mg/kg); data are presented as total horizontal ambulations in 15 min bins (Figure 4) or for the entire 60 min session (Figure 4, inset). A main effect of treatment [F(3,92) = 11, p < 0.05], time [F(3,92) = 25.38, p < 0.05], but no treatment × time interaction [F(9,92) = 1.38, n.s.] were observed for horizontal ambulations divided into four 15 min bins. A priori comparisons indicated neither M100907 nor WAY163909 alone altered horizontal ambulations relative to VEH-VEH-COC at any 15 min interval (Figure 4). However, M100907 plus WAY163909 significantly reduced cocaine-evoked horizontal ambulations during each of the 15 min intervals (M100-WAY-COC vs VEH-VEH-COC; p < 0.05; Figure 4). A main effect of treatment was observed for horizontal ambulations totaled for the entire 60 min test session [F(3,23) = 4.43; p < 0.05; Figure 4, inset]; a priori comparisons showed that while neither ligands tested alone at chosen doses altered horizontal ambulations, the combination of M100907 plus WAY163909 significantly decreased cocaine-evoked horizontal ambulations (M100-WAY-COC vs VEH-VEH-COC; p < 0.05; Figure 4, inset; dashed line indicates VEH-VEH-VEH levels). The effects of M100907 plus WAY163909 on cocaine-evoked vertical activity were similar to cocaine-evoked horizontal ambulations (data not shown). Hence, the combination of subthreshold doses of M100907 (0.03 mg/kg) plus WAY163909 (0.3 mg/kg) synergistically attenuated cocaine-evoked hyperactivity (Figure 4; Table 1).
Figure 4.
M100907 plus WAY163909 synergistically suppress cocaine-evoked hyperactivity. Rats received treatment injections (i.p.) of M100907 (M100; 0.03 mg/kg) or vehicle (VEH; 1% Tween) at 30 min prior, and WAY163909 (WAY; 0.3 mg/kg) or vehicle (VEH; saline, 1 mL/kg) 15 min prior to receiving an i.p. injection of cocaine (COC; 15 mg/kg) or vehicle (VEH; saline, 1 mL/kg). The time course of horizontal ambulations is divided into 15 min time bins across the 60 min session; [inset] the mean total horizontal ambulations (counts/60 min) (±SEM). The dashed line represents the mean VEH-VEH response. *p < 0.05 vs VEH-VEH-COC.
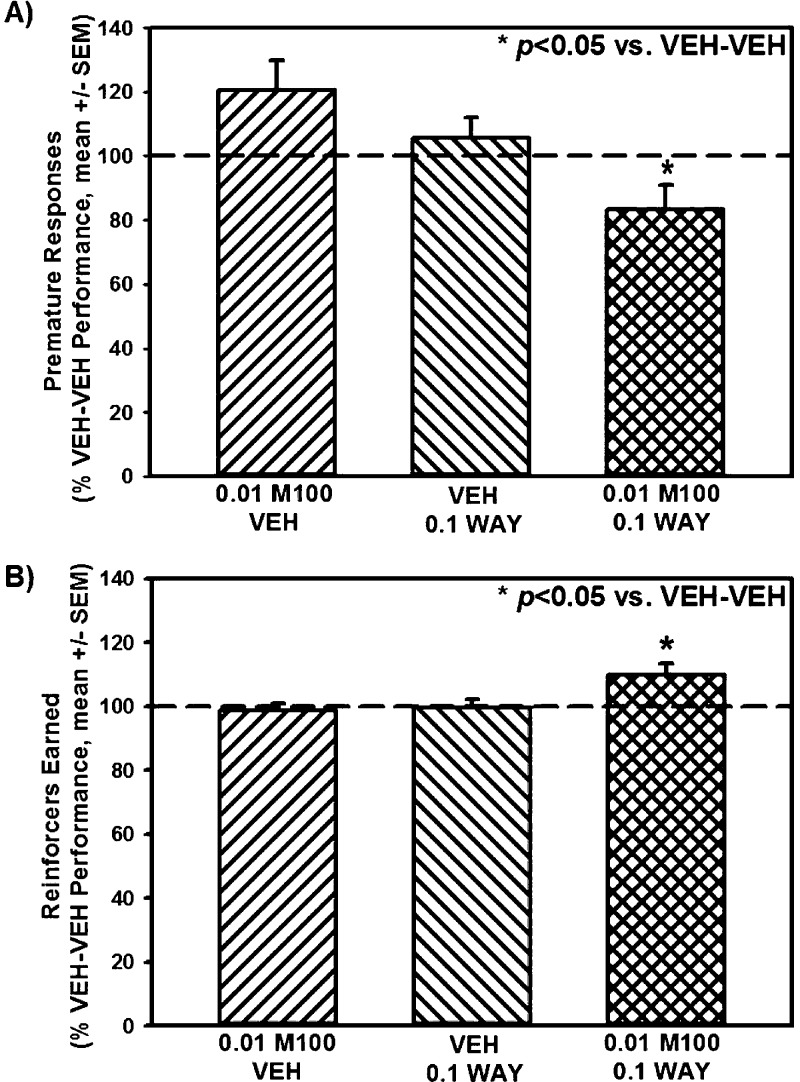
M100907 + WAY163909 Synergistically Suppress Inherent Impulsive Action
Selective blockade of the 5-HT2AR (e.g., M100907)10−12 or activation of the 5-HT2CR (e.g., WAY163909)10,13,14 consistently reduces inherent impulsive action measured in 1- or 5-CSRT tasks. We have published the observation that M100907 (0.01–0.5 mg/kg) dose-dependently suppresses inherent impulsive action as measured by premature responses in the 1-CSRT task with a subthreshold dose identified as 0.01 mg/kg (Table 1).12 WAY163909 also dose-dependently suppressed premature responses in the 1-CSRT task with a subthreshold dose identified as 0.1 mg/kg (Table 1).14 Our goal in the present series of studies was to test the hypothesis that combined treatment with the subthreshold doses of M100907 (0.01 mg/kg) plus WAY163909 (0.1 mg/kg) would synergize to suppress inherent impulsive action in the 1-CSRT task. Figure 5 displays the number of premature responses (Figure 5A) and reinforcers earned (Figure 5B) on the 1-CSRT task after treatment with vehicle, M100907 (0.01 mg/kg), WAY163909 (0.1 mg/kg), or the combination; data are presented as a percentage of the number of premature responses or reinforcers earned on the VEH-VEH session that preceded each dose/combination evaluated. A main effect of treatment on premature responses was observed [F(3,51) = 5.18; p < 0.05; Figure 5A]; a priori comparisons showed that M100907 plus WAY163909 significantly decreased premature responses versus VEH-VEH (p < 0.05; Figure 5A; dashed line indicates VEH-VEH levels). Neither M100907 nor WAY163909 alone altered the number of premature responses (n.s.; Figure 5A) as predicted by our previous observations.12,14 A main effect of treatment on the number of reinforcers earned was also observed [F(3,51) = 4.64; p < 0.05; Figure 5B]; a priori comparisons showed that M100907 plus WAY163909 significantly increased the number of reinforcers earned versus VEH-VEH (p < 0.05; Figure 5B; dashed line indicates VEH-VEH levels). Neither M100907 nor WAY163909 alone altered the number of reinforcers earned versus VEH-VEH (n.s.; Figure 5B).12,14 No main effect of treatment on percent accuracy [F(3,51) = 0.74; n.s.], percent omissions [F(3,51) = 0.61; n.s.], latency to start [F(3,51) = 0.10; n.s.], time to finish [F(3,51) = 0.33; n.s.], or time to retrieve the reinforcer [F(3,51) = 0.01; n.s.] was observed (data not shown). Taken together, combined treatment with subthreshold doses of M100907 (0.01 mg/kg) plus WAY163909 (0.1 mg/kg) synergize to reduce inherent impulsive action as defined by decreased premature responses and increased reinforcers earned in the 1-CSRT task (Figure 5; Table 1).
Figure 5.
WAY163909 plus M100907 synergistically suppress inherent impulsive action. Rats received i.p. injections of M100907 (M100; 0.01 mg/kg) or vehicle (VEH; 1% Tween) at 30 min prior, and WAY163909 (WAY; 0.1 mg/kg) or vehicle (VEH; saline, 1 mL/kg) 15 min prior to the start of the 1-CSRT task session. Data represent the number of [A] premature responses and [B] reinforcers earned on the 1-CSRT task test day expressed as percentage of control treatment (VEH-VEH; dashed line) (mean ± SEM). *p < 0.05 vs VEH-VEH.
M100907 + WAY163909 Synergistically Suppress Cocaine-Evoked Impulsive Action
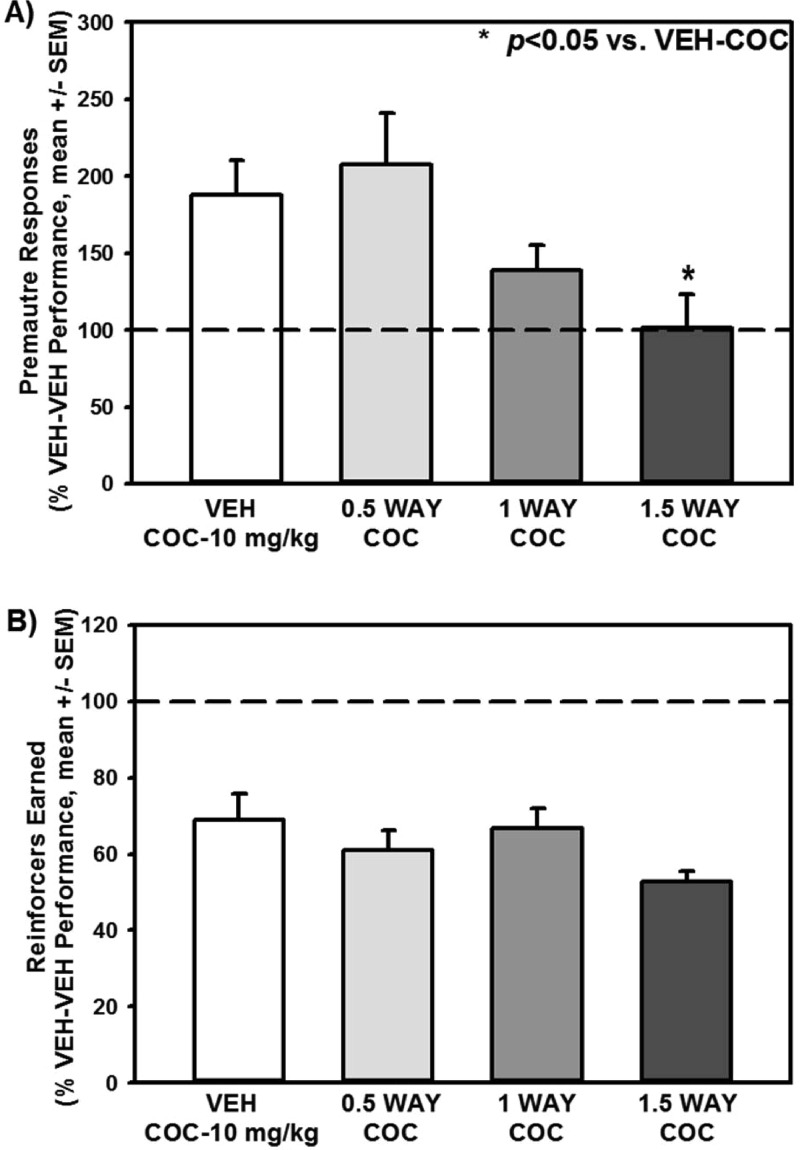
The 5-HT2AR and 5-HT2CR are potential pharmacotherapeutic targets for cocaine dependence; therefore, it is important to understand the influence of M100907 and WAY163909 over impulsive action emergent upon systemic administration of cocaine.12 In preparation for the combination studies, we were required to first establish the effects of WAY163909 on impulsive action evoked by cocaine in the 1-CSRT task. Figure 6 displays the number of premature responses (Figure 6A) and reinforcers earned (Figure 6B) on the 1-CSRT task after treatment with vehicle or WAY163909 (0.5, 1, and 1.5 mg/kg) prior to administration of cocaine (10 mg/kg); data are presented as a percentage of the number of premature responses or reinforcers earned on the VEH-VEH session that preceded each dose/combination evaluated. A main effect of treatment on premature responses was observed [F(3,47) = 4.14; p < 0.05; Figure 6A]. WAY163909 at 1.5 mg/kg, but not 0.5 or 1 mg/kg, significantly suppressed cocaine-induced increases in premature responses (1.5 WAY-COC vs VEH-COC; p < 0.05; Figure 6A) to levels seen for VEH-VEH (dashed line). No main effect of treatment on the number of reinforcers earned was observed [F(3,47) = 1.16; n.s; Figure 6B]; WAY163909 did not alter cocaine-evoked decreases in reinforcers earned (Figure 6B; dashed line indicates VEH-VEH levels). A main effect of treatment on percent omissions was observed; a priori comparisons revealed WAY163909 at the 1.5 mg/kg dose significantly increased percent omissions versus VEH-COC [F(3,47) = 5.96; p < 0.05; data not shown]. This was most likely not due to motility or motivational deficits31 as no main effect of treatment on percent accuracy [F(3,47) = 0.75; n.s.], latency to start [F(3,47) = 0.93; n.s.], time to finish [F(3,47) = 1.58, n.s.], or time to retrieve the reinforcer [F(3,47) = 0.53; n.s.] was observed (data not shown). Thus, a dose of 0.5 mg/kg of WAY163909 was identified as subthreshold to alter cocaine-evoked impulsive action in the 1-CSRT task (Figure 6; Table 1) and was used for the following synergism study.
Figure 6.
WAY163909 dose-dependently suppresses cocaine-evoked impulsive action. Rats received treatment injections (i.p.) of WAY163909 (WAY; 0.5, 1, 1.5 mg/kg) or vehicle (VEH; saline, 1 mL/kg) 15 min prior to an injection with cocaine (COC; 10 mg/kg, i.p) or vehicle (VEH; saline, 1 mL/kg), which occurred immediately before the start of the 1-CSRT task session. Data represent the number of [A] premature responses and [B] reinforcers earned on the 1-CSRT task test day expressed as percentage of control treatment (VEH-VEH; dashed line) (mean ± SEM). *p < 0.05 vs VEH-COC.
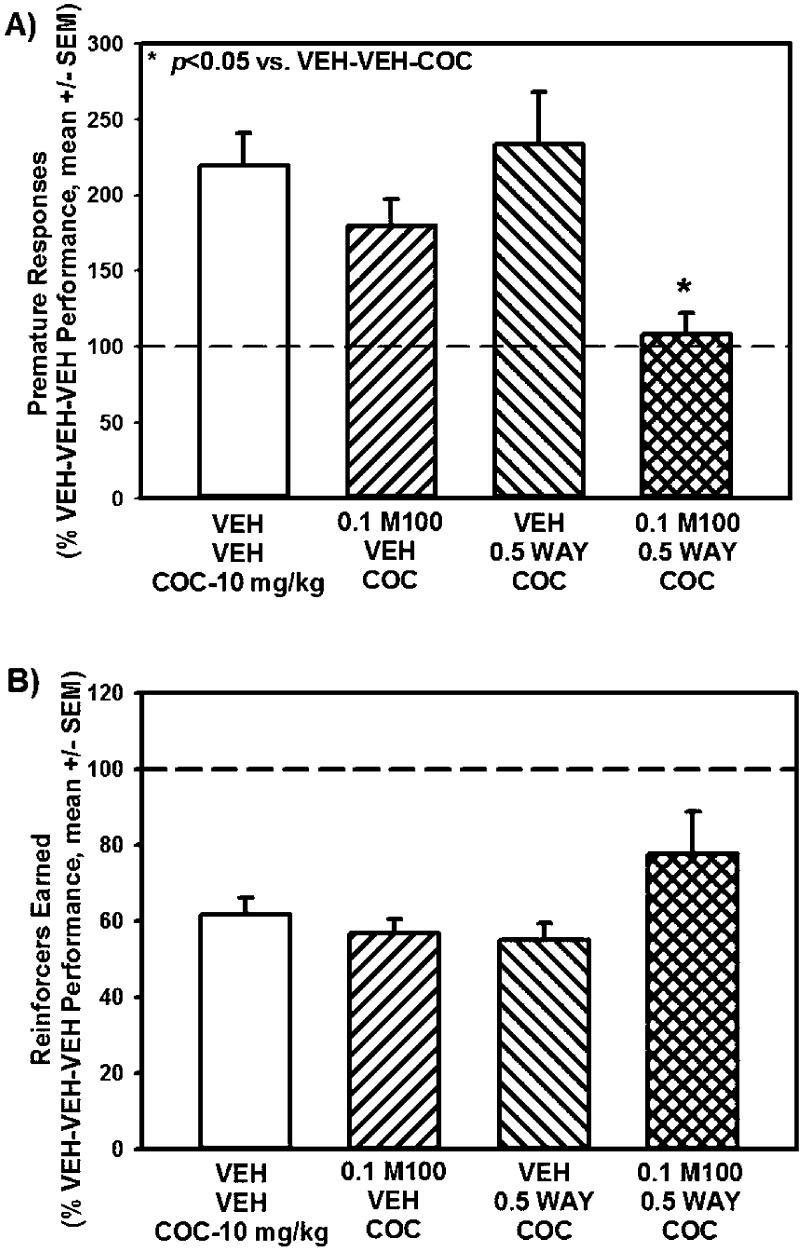
The apparent potency of M100907 to suppress inherent impulsive action in the 1-CSRT task was greater than that which suppressed cocaine-evoked impulsive action.12 For this reason, we chose 0.1 mg/kg of M100907 as the threshold dose to be employed here as this dose did not alter cocaine-evoked impulsive action in a previous study.12 In the present study, the subthreshold doses of 0.1 mg/kg of M100907 plus 0.5 mg/kg of WAY163909 were employed to test the hypothesis that combined treatment would synergize to suppress cocaine-evoked impulsive action in the 1-CSRT task. Figure 7 displays the number of premature responses (Figure 7A) and reinforcers earned (Figure 7B) on the 1-CSRT task following treatment with vehicle, M100907 (0.1 mg/kg), WAY163909 (0.5 mg/kg), or the combination administered prior to cocaine (10 mg/kg); data are presented as a percentage of the number of responses made on the VEH-VEH-VEH session that preceded each dose/combination evaluated. A main effect of treatment on premature responses was observed [F3,35 = 3.57; p < 0.05; Figure 7A; dashed lines indicates VEH-VEH-VEH levels]. M100907 plus WAY163909, but not M100907 or WAY163909 alone at chosen doses, significantly decreased cocaine-evoked premature responses (M100-WAY-COC vs VEH-VEH-COC; p < 0.05; Figure 7A) to levels seen for VEH-VEH-VEH (dashed line). No main effect of treatment on the number of reinforcers earned was observed [F3,35 = 1.39; n.s.; Figure 7B]; treatment with M100907, WAY163909 or the combination did not alter cocaine-evoked decreases in reinforcers earned (p > 0.05; Figure 7B; dashed line indicates VEH-VEH-VEH levels). In this experiment, no main effect of treatment on percent accuracy (F(3,35) = 0.50; n.s.), percent omissions (F(3,35) = 0.59; n.s.), latency to start (F(3,35) = 1.10; n.s.), time to finish (F(3,35) = 0.01; n.s.) or time to retrieve the reinforcer (F(3,35) = 1.28; n.s.) was observed (data not shown). Thus, subthreshold doses of M100907 plus WAY163909 synergistically normalized cocaine-evoked premature responses to basal levels in the 1-CSRT task (Figure 7; Table 1).
Figure 7.
WAY163909 plus M100907 synergistically suppress cocaine-evoked impulsive action. Rats received treatment injections (i.p.) of M100907 (M100; 0.1 mg/kg) or vehicle (VEH; 1% Tween) at 30 min prior, and WAY163909 (WAY; 0.5 mg/kg) or vehicle (VEH; saline, 1 mL/kg) 15 min prior to receiving an injection of cocaine (COC; 10 mg/kg) or vehicle (VEH; saline, 1 mL/kg) which occurred immediately before the start of the 1-CSRT task session. Data represent the number of [A] premature responses and [B] reinforcers earned on the 1-CSRT task test day expressed as percentage of control treatment (VEH-VEH-VEH; dashed line) (mean ± SEM). *p < 0.05 vs VEH-VEH-COC.
M100907 + WAY163909 Synergistically Suppress Cue-Evoked Reinstatement
We have published the observation that M100907 (0.01–0.8 mg/kg) dose-dependently suppressed cue-evoked reinstatement after extinction from cocaine self-administration with a subthreshold dose identified as 0.001 mg/kg (Table 1).19 All tested doses (0.5, 1, and 1.5 mg/kg) of WAY163909 suppressed cue-evoked reinstatement in a previous study;17 thus, we employed a dose of WAY163909 (0.1 mg/kg) which was 5-fold lower than the lowest previously tested effective dose for combination studies with the subthreshold dose of M100907 (0.001 mg/kg). In the present study, rats readily acquired cocaine self-administration (0.75 mg/kg/0.1 mL infusion) in daily 180 min sessions to stability (i.e., seven infusions/h on an FR 5 schedule for at least three sessions) and displayed <10% variation in the number of infusions received (i.e., cocaine intake) (data not shown). Across the last three sessions of stable self-administration on an FR 5 schedule (data not shown), there was no main effect of session on the number of active [F(2,21) = 0.1; n.s.], inactive lever responses [F(2,21) = 1.58; n.s.] or the number of infusions received [F(2,21) = 0.18; n.s.]. The average daily cocaine intake over the last three sessions of training was 33.03 ± 2.1 mg/kg. A main effect of extinction session on active lever presses [F(12,74) = 17.22; p < 0.05], but not on inactive lever presses [F(12,74) = 1.43; n.s.], was observed. Presses on the previously active lever decreased across extinction sessions with all rats achieving the extinction criterion (<15 active lever responses/session for three consecutive sessions) by session 13 of extinction training (data not shown). The “extinction baseline” was calculated as the mean total lever presses of all rats on the active (8.0 ± 1.4) or inactive lever (4.5 ± 0.9) during the final 60 min extinction session.
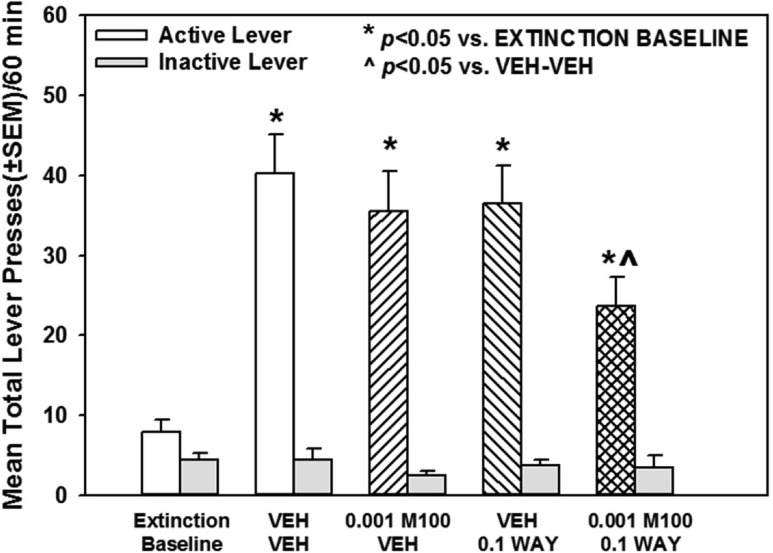
Figure 8 illustrates the number of lever presses during the 60 min cue-evoked reinstatement session on the lever previously associated with cocaine delivery after treatment with vehicle, M100907 (0.001 mg/kg), WAY163909 (0.1 mg/kg), or the combination. A main effect of treatment on active lever responses during the 60 min cue-evoked reinstatement session was observed [F(4,35) = 9.94; p < 0.05; Figure 8]. A priori comparisons indicated that lever presses on the previously active lever increased significantly during the 60 min cue-evoked reinstatement session (VEH-VEH) versus the extinction baseline (p < 0.05; Figure 8). Treatment with M100907 plus WAY163909 significantly decreased lever presses during the 60 min cue-evoked reinstatement session versus VEH-VEH (p < 0.05; Figure 8); neither M100907 nor WAY163909 alone altered active lever presses during the 60 min cue-evoked reinstatement session versus VEH-VEH (n.s.; Figure 8). There was no main effect of treatment on inactive lever responses [F(4,35) = 0.35; n.s.; Figure 8] or the latency to the first lever press during the 60 min cue-evoked reinstatement session versus VEH-VEH [F(3,28) = 0.93; n.s.; data not shown]. Thus, M100907 plus WAY163909 administered at subthreshold doses synergistically attenuated cue-evoked reinstatement (Figure 8; Table 1).
Figure 8.
M100907 plus WAY163909 synergistically suppress cue-evoked reinstatement of cocaine-seeking behavior. Rats received i.p. injections of M100907 (M100; 0.001 mg/kg) or vehicle (VEH; 1% Tween) at 30 min prior, and WAY163909 (WAY; 0.1 mg/kg) or vehicle (VEH; saline, 1 mL/kg) 15 min prior to the start of the reinstatement session. Data represent the mean (±SEM) total number of responses on the active (white bars) or inactive lever (gray bars) following extinction (“Extinction Baseline”) and upon ligand treatment on the test day for cue-evoked reinstatement of extinguished cocaine-seeking behavior. Reinstatement was initiated by a single nonresponse contingent presentation of cocaine-paired cues (pump and stimulus light). Each active lever press resulted in the presentation of the conditioned stimuli in the absence of cocaine delivery. *p < 0.05 vs Extinction Baseline; ^p < 0.05 vs VEH-VEH.
M100907 + WAY163909 Synergistically Suppress Cocaine-Primed Reinstatement
Cocaine-primed reinstatement following extinction is suppressed by 5-HT2CR agonists (e.g., RO 60–0175, MK212).20,30,32,33 Here, we assessed the ability of WAY163909 to alter cocaine-primed reinstatement after extinction from cocaine self-administration. In the present study, a separate cohort of rats readily acquired cocaine self-administration (0.75 mg/kg/0.1 mL infusion) in daily 180 min sessions to stability (i.e., seven infusions/h on an FR 5 schedule for at least three sessions) and displayed <10% variation in the number of infusions received (i.e., cocaine intake) (data not shown). Across the last three sessions of stable self-administration on an FR 5 schedule (data not shown), there was no main effect of session on the number of active [F(2,63) = 0.14; n.s.], inactive lever responses [F(2,63) = 0.04; n.s.] or the number of infusions received [F(2,63) = 0.13; n.s.]. The average daily cocaine intake over the last three sessions of training was 29.3 ± 0.9 mg/kg. A main effect of extinction session on active lever presses [F(14,160) = 23.92; p < 0.05], but not on inactive lever presses [F(14,160) = 0.60; n.s.], was observed. Presses on the previously active lever decreased across extinction sessions with all rats achieving the extinction criterion by session 15 of extinction training (data not shown). The “extinction baseline” was calculated as the mean total lever presses of all rats on the active (9.3 ± 0.6) or inactive lever (5.3 ± 1.2) during the final 60 min extinction session.
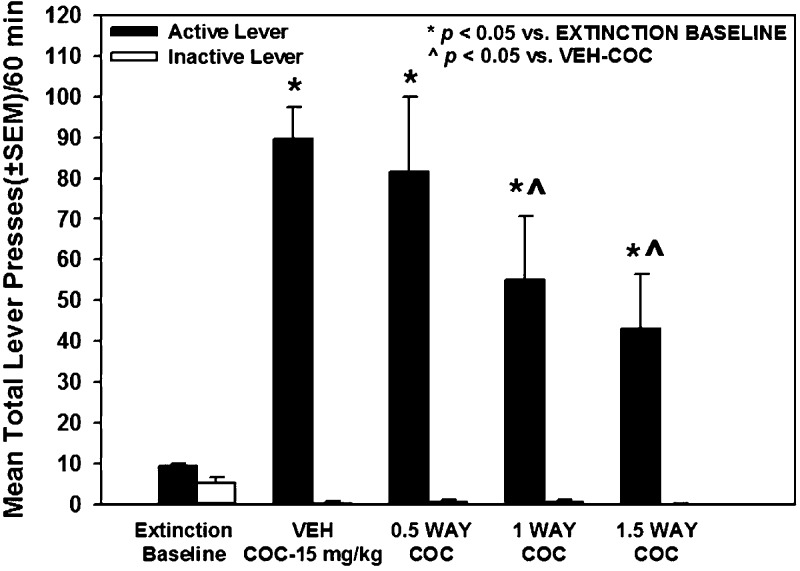
Figure 9 illustrates the number of lever presses during the 60 min cocaine-primed (15 mg/kg) reinstatement session after treatment with vehicle or WAY163909 (0.5, 1, or 1.5 mg/kg). A main effect of treatment on active lever responses was observed [F(4,39) = 18.91; p < 0.05; Figure 9]. A priori comparisons indicated that lever presses on the previously active lever increased significantly following the cocaine injection (VEH-COC) versus the extinction baseline (p < 0.05; Figure 9). Treatment with WAY163909 at 1 and 1.5 mg/kg significantly decreased lever presses during the cocaine-primed reinstatement session versus VEH-COC (p < 0.05; Figure 9). There was no main effect of treatment on inactive lever responses during cocaine-primed reinstatement session versus VEH-COC [F(4,39) = 2.21; n.s.; Figure 9]. There was a main effect of treatment on latency to the first lever press during the reinstatement session versus VEH-COC [F(3,18) = 4.27; p < 0.05; data not shown]; a priori comparisons revealed that 1.5 mg/kg WAY163909 significantly increased latency to the first lever press versus VEH-COC (p < 0.05; data not shown). Neither 0.5 nor 1 mg/kg of WAY163909 altered the latency to the first lever press versus VEH-COC (p < 0.05; data not shown). Thus, we have identified a subthreshold dose WAY163909 (0.5 mg/kg) which did not alter cocaine-primed reinstatement when tested alone (Figure 9; Table 1).
Figure 9.
WAY163909 dose-dependently suppresses cocaine-primed reinstatement of cocaine-seeking behavior. Rats received treatment injections (i.p.) of WAY163909 (WAY; 0.5, 1, 1.5 mg/kg) or vehicle (VEH; saline, 1 mL/kg) 15 min prior to injection with cocaine (COC; 15 mg/kg). Data represent the mean (±SEM) total number of responses on the active (black bars) or inactive lever (gray bars) following extinction (“Extinction Baseline”) and upon ligand treatment on the test day for cocaine-primed reinstatement of extinguished cocaine-seeking behavior. Cocaine-paired cues were not delivered during the 60 min cocaine-primed reinstatement test session and responses had no consequences. *p < 0.05 vs Extinction Baseline; ^p < 0.05 vs VEH-VEH.
The same cohort of rats employed in the M100907 plus WAY163909 cue-evoked reinstatement study (Figure 8) were utilized to test the hypothesis that combined treatment would synergize to suppress cocaine-primed reinstatement. The dose of 0.5 mg/kg of M100907 suppressed reinstatement evoked by nonresponse contingent administration of 10 or 20 mg/kg of cocaine.32 Here, we employed a dose of M100907 (0.1 mg/kg) which was 5-fold lower for combination studies with a subthreshold dose of WAY163909 (0.3 mg/kg).
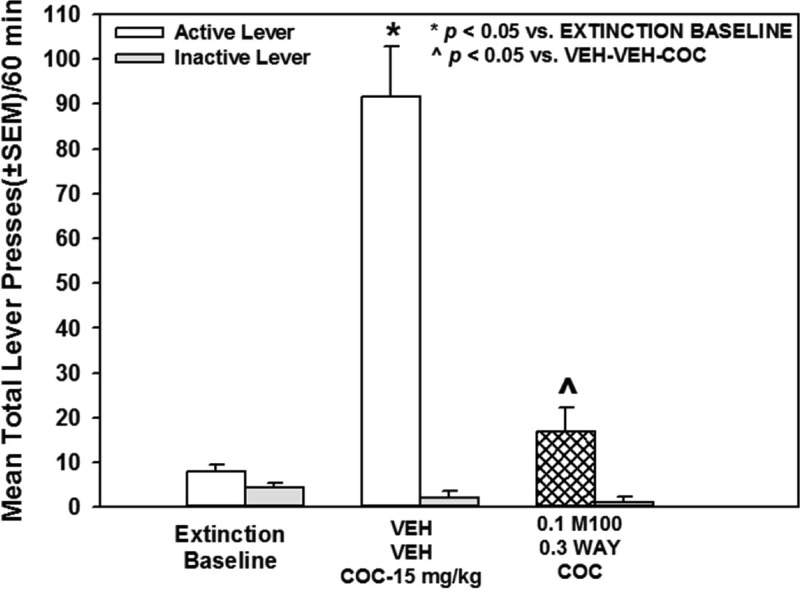
Figure 10 illustrates the mean total lever presses (±SEM) during the 60 min cocaine-primed (15 mg/kg) reinstatement session after treatment with vehicle or the combination of M100907 (0.1 mg/kg) plus WAY163909 (0.3 mg/kg). A main effect of treatment on active lever responses was observed [F(2,12) = 40.25; p < 0.05; Figure 10]. A priori comparisons indicated that lever presses on the previously active lever increased significantly following the cocaine injection (VEH-VEH-COC) versus the extinction baseline (p < 0.05; Figure 10). Treatment with M100907 plus WAY163909 significantly decreased lever presses during the cocaine-primed reinstatement session versus VEH-VEH-COC (p < 0.05; Figure 10). There was no main effect of treatment on inactive lever responses [F(2,12) = 1.52; n.s.; Figure 10] or the latency to the first lever press during the reinstatement session versus VEH-VEH-COC (data not shown). Thus, the combination of M100907 plus WAY163909 administered at subthreshold doses synergistically diminished cocaine-primed reinstatement (Figure 10; Table 1).
Figure 10.
M100907 plus WAY163909 synergistically suppress cocaine-primed reinstatement of cocaine-seeking behavior. Rats received i.p. injections of M100907 (M100; 0.1 mg/kg) or vehicle (VEH; 1% Tween) at 30 min prior, and WAY163909 (WAY; 0.3 mg/kg) or vehicle (VEH; saline, 1 mL/kg) 15 min prior to injection with cocaine (COC; 15 mg/kg). Data represent the mean (±SEM) total number of responses on the active (white bars) or inactive lever (gray bars) following extinction (“Extinction Baseline”) and upon ligand treatment on the test day for cocaine-primed reinstatement of extinguished cocaine-seeking behavior. Cocaine-paired cues were not delivered during the 60 min cocaine-primed reinstatement test session and responses had no consequences. *p < 0.05 vs Extinction Baseline; ^p < 0.05 vs VEH-VEH.
In summary, we have identified combinations of subthreshold doses of M100907 plus WAY163909 that did not alter spontaneous motor activity, but did synergistically suppress cocaine-induced hyperactivity, inherent and cocaine-evoked impulsive action, as well as cue-evoked and cocaine-primed reinstatement of cocaine-seeking behavior. The subthreshold doses of each ligand were chosen based upon empirical criteria established in each assay while the pretreatment intervals and order of administration were selected based on our extensive experience and previous publications.12,14,17,28 The synergism between the 5-HT2AR antagonist M100907 plus 5-HT2CR agonist WAY163909 was achieved at doses that are much lower than the doses of each ligand necessary to affect the given behavior when administered alone (Table 1). Employing 0.03 mg/kg of M100907 and 0.3 mg/kg of WAY163909, we demonstrated that spontaneous motor activity (Figure 2) and cocaine-evoked hyperactivity (Figure 3) were unaffected by the chosen dose of M100907 or WAY163909 administered alone; when combined, this ligand/dose combination suppressed cocaine-evoked motor activity by ∼75% (Figure 4), but did not alter spontaneous motor activity (Figure 2). Combination of even lower, inactive doses of M100907 (0.01 mg/kg) plus WAY163909 (0.1 mg/kg) synergized to suppress inherent impulsive action (Figure 5) while the combination of M100907 (0.1 mg/kg) plus WAY163909 (0.5 mg/kg) synergistically normalized cocaine-evoked impulsivity to basal levels. The doses of M100907 and WAY163909 employed to assess synergism on inherent impulsivity (Figure 5) are 3-fold lower than those employed in the spontaneous locomotor studies (Figure 2). The doses of M100907 (0.1 mg/kg) and WAY163909 (0.5 mg/kg) employed to assess synergism on cocaine-evoked impulsivity (Figure 7) are 2-fold lower than those which suppress cocaine-evoked locomotor activity (see McMahon and Cunningham, and Figure 3).28 Further, neither M100907 nor WAY163909 alone or in combination altered measures of motility (i.e., latency to start, time to finish, time to retrieve the reinforcer) in the 1-CSRT task. Lastly, cue-evoked reinstatement was synergistically suppressed by very low doses of M100907 (0.001 mg/kg) plus WAY163909 (0.1 mg/kg) (Figure 8). Cocaine-primed reinstatement was synergistically attenuated by the combination of doses of M100907 (0.1 mg/kg) plus WAY163909 (0.3 mg/kg) that were comparable to those required to synergistically suppress cocaine-evoked impulsivity. That this synergism to suppress cocaine-mediated behaviors was observed at higher doses of both ligands than those effective to suppress spontaneous motor activity, inherent impulsivity, and cue-evoked reinstatement may not be surprising given the complex impact of acute cocaine administration on the neurochemical pathways involved in generating impulsive action and reinstatement of cocaine-seeking behavior.12,14
Collectively, these data suggest that the synergistic efficacy of dual 5-HT2AR antagonist/5-HT2CR agonist treatment occurs independent of general behavioral disruption indicating that the combination of ultralow doses may be better tolerated than either drug alone. The reported side effects of M100907 in clinical studies in schizophrenic patients include headaches and constipation,34 while preclinical studies demonstrate efficacy of M100907 to increase sleep and decrease waking, observations that have resulted in clinical trials to assess the efficacy and safety of M100907 (volinanserin) in chronic primary insomnia.35 In preclinical studies, WAY163909 evokes hypomotility at doses > 3 mg/kg17 and at 10-fold higher doses has been noted to induce catalepsy.27 Clinical studies with lorcaserin, the only selective 5-HT2CR agonist administered to humans to date, reported no neurocognitive or perceptual effects36 and the side effects in clinical trials were limited to headache, nausea, and dizziness.37−39 While there is room to optimize doses of ligands, routes of administration, and pretreatment times to further explore this synergism, the chemical biology literature suggests that the targeted design of a single chemical entity which modulates two biologically relevant targets simultaneously is likely to result in therapeutic effects at lower doses, greater compliance, and minimized side effects relative to the two drugs alone.40−43 Thus, although studies could be conducted to optimize administration of the two ligands simultaneously, the success and superior efficacy of the ligand combinations at ultralow doses of the substituent ligands provides a solid scientific rationale for designing a bifunctional 5-HT2AR antagonist/5-HT2CR agonist ligand.
Based upon our present identification of synergism between a selective 5-HT2AR antagonist and 5-HT2CR agonist to attenuate impulsivity and cocaine-seeking, we hypothesize that a bifunctional 5-HT2AR antagonist/5-HT2CR agonist strategy would include efficacy to suppress impulsivity, cocaine-seeking, and potentially cocaine-taking. Selective 5-HT2CR agonists, including WAY163909,17,20,30,32,33 dose-dependently block cocaine-taking in self-administration assays; however, the synergistic efficacy of 5-HT2AR antagonist and 5-HT2CR agonist over cocaine intake has yet to be established. Nonetheless, the present studies set the stage to develop new ligands with such dual properties to improve efficacy, potency, selectivity, and in vivo profiles over the individual molecules. Such a compound might be found in recent drug discovery initiatives within the same molecule.24,44,45 New chemical molecules with such a profile will be extremely useful to further delineate the pharmacological interactions and biological substrates involved in these relapse vulnerability phenotypes to cocaine addiction.
The basis for the synergism between the 5-HT2AR antagonist and 5-HT2CR agonist is unknown at present. The 5-HT2AR and 5-HT2CR share a high degree of homology, overlapping pharmacological profiles, and utilize richly diverse, second messenger signaling systems. The synergistic effects of M100907 and WAY163909 described here may be transduced through 5-HT2AR and 5-HT2CR signaling in the same neurons or interacting neurons and/or separate nodes of the neural circuitry underlying these behavioral phenotypes. The recent demonstration of multiple examples of GPCR heteromers and the synthesis of multivalent ligands to selectively target such heteromers46−49 suggests that heteromers of 5-HT2AR and 5-HT2CR might assemble in neurons to serve as a binding scaffold for multivalent ligands. The formation of 5-HT2AR:5-HT2CR heteromeric receptors may have profound implications for cellular and behavioral phenotypes, as receptor clusters can endow cells with additional mechanisms through which to control signal transduction.46−50 Thus, the potential for formation of 5-HT2AR:5-HT2CR heteromeric receptors and the need to selectively target such protein complexes provides further rationale for the development of bivalent ligands that target these two receptors.
In summary, the synergy between M100907 and WAY163909 tantalizingly suggests the potential for bifunctional ligands with therapeutic efficacy and limited side effects in cocaine dependence. In addition, such novel ligands will provide a rich toolbox to probe the neurobiology of 5-HT2AR and 5-HT2CR systems in phenotypic vulnerability to cocaine dependence and relapse.
Methods
Animals
A total of 136 male Sprague–Dawley rats (Harlan, Inc., Indianapolis, IN) weighing 225–325 g at the start of the experiments were used. Rats were allowed to acclimate for 5–7 days in a colony room at a constant temperature (21–23 °C) and humidity (45–50%) on a 12 h light-dark cycle (lights on 0700–1900 h). Rats were housed either two (1-CSRT task, self-administration/reinstatement assay) or four rats per cage (locomotor activity assay). Food and water was available ad libitum throughout all phases of the locomotor and self-administration studies. For 1-CSRT studies, rats were food restricted to 90% free-feeding weight for the duration of experiments; water was available ad libitum except during daily operant sessions. All experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (2011) and with the approval of the UTMB Institutional Animal Care and Use Committee. All efforts were made to minimize animal suffering, to reduce the number of animals used, and to utilize alternatives to in vivo techniques, when available.
Drugs
M100907 [R-(1)-(2,3-dimethoxyphenyl)-1-[2-(4-fluorophenylethyl)]-4-piperidine-methanol] was synthesized by Kenner Rice (National Institute on Drug Abuse, Bethesda, MD) and dissolved in 1% Tween 80 in 0.9% NaCl (vehicle employed for comparison to M100907). WAY163909 [(7b-R,10a-R)-1,2,3,4,8,9,10,10a-octahydro-7bH-cyclopenta[b][1,4] diazepino [6,7,1hi] indole] was a gift from Pfizer Inc. (New York, NY) and dissolved in 0.9% NaCl (vehicle employed for comparison to WAY163909). (−)-Cocaine (National Institute on Drug Abuse, Research Triangle Park, NC) was dissolved in 0.9% NaCl (vehicle employed for comparison to cocaine).
Locomotor Activity
The effects of M100907, WAY163909, or the combination on motor activity were monitored and quantified under low light conditions using a modified open field activity system (San Diego Instruments, San Diego, CA) according to previous publications with minor modifications.17,28 Clear Plexiglas chambers (40 × 40 × 40 cm3) were surrounded by a 4 × 4 photobeam matrix positioned 4 cm from chamber floor. Horizontal ambulations were quantified as the sum of consecutive photobeam breaks that occurred within the inner central 16 × 16 cm2 perimeter and in the surrounding outer peripheral 16 × 16 cm2 perimeter of the activity monitor every 5 min. For all activity experiments, rats (n = 76) were acclimated to the colony room and, following one week of handling, rats were habituated to the activity monitors for one hour/day for three consecutive days prior to initiation of experiments, which commenced 24 h later with a one hour habituation prior to the start of the test session. Measurement of spontaneous motor activity began immediately following the last injection in each of the following studies and was assessed for 60 min.
-
(i)
The effects of M100907, WAY163909, or their combination on spontaneous motor activity were assessed in a between-subjects design. Rats were injected (i.p.) with M100907 (0.03 mg/kg) or vehicle (1% Tween) 30 min prior, plus WAY163909 (0.3 mg/kg) or vehicle (saline, 1 mL/kg) 15 min prior to the start of the test session.
-
(ii)
The effects of WAY163909 on cocaine-evoked motor activity were established in a within-subjects design. To control for order effects, drug doses and vehicles were administered in random sequence to individual rats across sessions such that all rats received all treatment combinations; test days were separated by one week during which rats were handled daily. On test day, rats received an i.p. injection of WAY163909 (0.1, 0.3, or 1 mg/kg) or vehicle (saline, 1 mL/kg) 15 min prior to i.p. injection of cocaine (15 mg/kg) or vehicle (saline, 1 mL/kg).
-
(iii)
The effects of M100907, WAY163909, or the combination on cocaine-evoked motor activity were assessed using a between-subjects design. Rats received a treatment injection (i.p.) of M100907 (0.03 mg/kg) or vehicle (1% Tween) at 30 min prior, and WAY163909 (0.3 mg/kg) or vehicle (saline, 1 mL/kg) 15 min prior to receiving an i.p. injection of cocaine (15 mg/kg) or vehicle (saline, 1 mL/kg).
One-Choice Serial Reaction Time (1-CSRT) Task
Impulsive action was assessed in the 1-CSRT task, as previously described.12,14 The 1-CSRT task procedures employed standard five-hole nosepoke operant chambers (Med Associates, Roanoke, VA) contained within a ventilated and sound-attenuating chamber. Each chamber was fitted with an array of five evenly spaced response apertures (2.5 × 2.5 × 2.2 cm3) positioned 2 cm above a bar floor, and each aperture contained a stimulus light. Nosepoke responses into these apertures were detected by a horizontally positioned infrared beam located 1 cm from the entrance to each hole. The chambers were also fitted on the opposite wall with a houselight, food tray, and an external pellet dispenser capable of delivering 45 mg pellets (Dustless Precision pellets, Bio-Serv, Frenchtown, NJ) to the food tray. The chambers were connected via an interface (Med Associates) to a PC computer running Med PC for Windows software (Med Associates).
1-CSRT Task Training
Rats (n = 16) were allowed 1 week to acclimate to the colony after which food restriction began. Rats were weighed daily to ensure that body weights were maintained at 90% of free-feeding levels throughout 1-CSRT task training. Training began with a pretraining stage in which the rat was habituated to the test chamber and introduced to nosepoke responses for food pellets. During this stage, all responses made in the correctly lit (center) hole resulted in the illumination of the magazine light and presentation of a single food pellet. The training stages thereafter were each composed of daily sessions of 100 trials to be completed in a maximum of 30 min; each training stage involved incrementally lowering the stimulus duration with a 5 s limited hold and an ITI of 5 s. Thus, a maximum of 100 correct responses in a session would result in a maximum of 100 reinforcers delivered; incorrect or premature responses or omissions resulted in a 5 s time out period and a reduction in potential reinforcers obtained. Rats were required to meet an acquisition criteria of a minimum of 50 correct responses, >80% accuracy [correct responses/(correct + incorrect) × 100] and <20% omissions (omitted responses/trials completed × 100) to move from one training stage to the next.12,14 Pharmacological test sessions commenced after animals met the stable training criteria of >80% accuracy and <20% omissions for five consecutive training sessions on the final training stage (0.5 s stimulus duration, 5 s limited hold, 5 s ITI) with less than 15% variability across sessions. Pharmacological evaluations in the 1-CSRT were conducted using a within-subjects design. To control for order effects, drug doses and vehicles were administered in random sequence to individual rats across reinstatement test sessions; a minimum of 2 days of stable training sessions intervened between test sessions.
-
(i)
The effects of M100907, WAY163909, or the combination on inherent impulsive action were established in rats after an injection (i.p.) of M100907 (0.01 mg/kg) or vehicle (1% Tween) at 30 min prior, plus WAY163909 (0.1 mg/kg) or vehicle (saline, 1 mL/kg) 15 min prior to the start of the 1-CSRT task session.
-
(ii)
The effects of WAY163909 on cocaine-evoked impulsive action were established in rats after an injection (i.p.) of WAY163909 (0.5, 1, 1.5 mg/kg) or vehicle (saline, 1 mL/kg) 15 min prior to an injection with cocaine (10 mg/kg, i.p) or vehicle (saline, 1 mL/kg, i.p.) which occurred immediately before the start of the 1-CSRT task session.
-
(iii)
The effects of M100907, WAY163909 or the combination on cocaine-evoked impulsive action were established in rats after an injection (i.p.) of M100907 (0.1 mg/kg) or vehicle (1% Tween) at 30 min prior, plus WAY163909 (0.5 mg/kg) or vehicle (saline, 1 mL/kg) 15 min prior to receiving an injection of cocaine (10 mg/kg) or vehicle (saline, 1 mL/kg), which occurred immediately before the start of the 1-CSRT task session.
Self-Administration Surgery
Implantations of intravenous catheters with back mounts were performed under anesthesia with a cocktail containing 8.6 mg/kg of xylazine, 1.5 mg/kg of acepromazine, and 43 mg/kg of ketamine in bacteriostatic saline as previously described.17,19 Daily flushes with a solution of 0.1 mL of bacteriostatic saline containing heparin sodium (10 U/mL; American Pharmaceutical Partners, East Schaumburg, IL), streptokinase (0.67 mg/mL; Sigma Chemical), and ticarcillin disodium (66.67 mg/mL; Research Products International, Mt. Prospect, IL) were performed after each cocaine self-administration session to maintain catheter patency. Proper catheter function was verified periodically throughout the experiment by intravenous administration of 10 mg/kg of methohexital sodium (Monarch Pharmaceuticals Inc., Bristol, TN), a dose sufficient to briefly anesthetize the animal only when administered intravenously. All rats were allowed at least 5 days of recovery after surgery before initiation of self-administration training.
Cocaine Self-Administration Training
Standard operant conditioning chambers (Med-Associates, Inc., St. Albans, VT) housed in ventilated sound-attenuating cubicles with fans (Med-Associates, Inc.) were utilized for cocaine self-administration studies. Each chamber was equipped with two retractable response levers, a stimulus light above each response lever, and a houselight opposite the levers. Cocaine infusions were delivered by a syringe attached to an infusion pump (Med Associates, Inc.) located outside the cubicle. The infusion pumps were connected to liquid swivels (Instech, Plymouth Meeting, PA) that were fastened to the catheters via polyethylene 20 tubing encased inside a metal spring leash. Cocaine self-administration training consisted of daily 180 min sessions during which rats (n = 44) were trained to press the active lever to obtain a cocaine infusion (0.75 mg/kg/0.1 mL infusion) on an FR 1 schedule of reinforcement before progressing to an FR 5 schedule. Animals were not food restricted or trained on an operant task prior to commencement of self-administration and no cocaine priming infusions were given during the experiment.
Rats that met the criterion for stable self-administration (<10% variation in the number of infusions received for three consecutive sessions) were subjected to daily 60-min extinction sessions, during which active and inactive lever presses were recorded but had no scheduled consequences (i.e., did not activate the infusion pump or result in presentation of the stimulus light). Once rats achieved the extinction criterion of response rates <15 total responses/h for three consecutive sessions, the pharmacological test sessions on cue-evoked or cocaine-primed reinstatement were examined. The criterion of <15 responses/h as indicative of extinction is commonly employed in self-administration studies and corresponds to approximately 15% of responses during active cocaine self-administration.17,19,51
Cue-Evoked Reinstatement
A single nonresponse contingent presentation of the cocaine-paired cues (pump and stimulus light) initiated cue-evoked reinstatement sessions. During the 60 min cue-evoked reinstatement test session, responses on the active lever were reinforced by presentation of the cocaine-paired cues on an FR 1 schedule. Rats received i.p. injections of M100907 (0.001 mg/kg) or vehicle (1% Tween) at 30 min prior, plus WAY163909 (0.1 mg/kg) or vehicle (saline, 1 mL/kg) 15 min prior to the start of the reinstatement session in a between-subjects design in which each animal was tested under one treatment condition.
Cocaine-Primed Reinstatement
Cocaine (15 mg/kg, i.p.) was administered immediately before placement into the chambers for evaluation of cocaine-primed reinstatement; cocaine-paired cues were not delivered during the 60 min session, and responses had no consequences. Each rat was required to meet the reinstatement criterion of more than double their extinction baseline rate to be included in the final data analyses.20
-
(i)
The effects of WAY163909 on cocaine-primed reinstatement were established using a between-subjects design. Rats received treatment injections (i.p.) of WAY163909 (0.5, 1, 1.5 mg/kg) or vehicle (saline, 1 mL/kg) 15 min prior to injection with cocaine (15 mg/kg).
-
(ii)
The effects of the combination of M100907 plus WAY163909 on cocaine-primed reinstatement were established using a within-subjects design. To control for order effects, drug doses and vehicles were administered in random sequence to individual rats across reinstatement test sessions. Rats received i.p. injections of M100907 (0.1 mg/kg) or vehicle (1% Tween) at 30 min prior, and WAY163909 (0.3 mg/kg) or vehicle (saline, 1 mL/kg) 15 min prior to cocaine (15 mg/kg).
Statistical Analyses
Locomotor activity data are presented as mean (±SEM) total horizontal ambulations within the 15, 30, 45, and 60 min time bins or totaled over the 60 min session. A two-way (treatment, time) analysis of variance (ANOVA) of horizontal ambulations/time bins was conducted using the general linear model (GLM) procedure (SAS for Windows, version 9.3). Specifically defined group comparisons of horizontal ambulations at each time bin and for total horizontal ambulations summed across the 60 min session were conducted by a one-way ANOVA and Dunnett’s procedure.52 All statistical analyses were conducted with an experimentwise error rate of a α = 0.05.
During the 1-CSRT task, the total number of responses (correct, incorrect, omissions, and premature) as well as the latency to start the task (s), time to finish (s), and time to retrieve the reinforcer (s) were recorded. The measures of latency to start, time to finish and time to retrieve the reinforcer provide indices of motility in the 1-CSRT. Incorrect responses consistently accounted for less than 5% of total responses during 1-CSRT task test sessions as rats were well-trained upon achieving the task criterion. Percent accuracy [(correct responses/correct + incorrect) × 100] was used as an indication of the attentional capacity of each rat. Percent omissions (omitted responses/trials completed × 100) and time to retrieve the reinforcer indicated the motivation level of each rat to perform the task. Responses during the ITI (premature responses) and the number of reinforcers earned during the 1-CSRT task were used to measure impulsive action.12,14 Responses on pharmacological test sessions are presented as the percentage of values observed on the vehicle test session that occurred one day prior to the pharmacological test session. Specifically defined group comparisons of premature responses, reinforcers earned, percent accuracy, percent omissions, time to start, time to finish, and time to retrieve the reinforcer were conducted by a one-way ANOVA followed by Dunnett’s procedure.52 All statistical analyses were conducted with an experimentwise error rate of a α = 0.05.
The data from the self-administration, extinction and reinstatement phases were analyzed separately. A one-way ANOVA (SAS for Windows, V9.3) for repeated measures was used to analyze the dependent measures of the total number of (1) active lever responses/session (mean ± SEM) and (2) inactive lever presses/session (mean ± SEM) during the self-administration phase and the total number of responses/session on the (3) previously active, or (4) -inactive lever (mean ± SEM) during the extinction phase. A one-way ANOVA was employed to compare responses on the previously active lever over the last three sessions of extinction with responses observed following vehicle treatment during reinstatement sessions and to analyze the effects of M100907, WAY163909, or the combination on the total number of (1) responses/session on the previously active, (2) -inactive lever, and (3) the latency to respond on the previously active lever during the reinstatement phase. Specifically defined group comparisons of responses were conducted by a one-way ANOVA and Dunnett’s procedure.52 All statistical analyses were conducted with an experimentwise error rate of α = 0.05.
Glossary
Abbreviations
- 1-CSRT
one-choice serial reaction time task
- 5-HT
serotonin (5-hydroxytryptamine)
- 5-HTXR
5-HTX receptor
- GPCR
G-protein coupled receptor
- FR
fixed ratio
- SA
self-administration
Author Contributions
K.A.C. conceptualized the experiments, designed and directed the pharmacological research, and wrote the manuscript. N.C.A. oversaw research activities, experimental design and conducted pharmacological and statistical analyses and interpretation and edited the manuscript. R.G.F. and S.J.S. carried out pharmacological evaluations; S.E.S. created figures and edited the manuscript. S.R.G., M.J.B., C.S.W., S.R.L., and F.G.M. were involved in conception of the project, design of the experiments, and edited the manuscript. K.C.R. synthesized M100907 which was provided under a Material Transfer Agreement.
This work was supported by NIDA grants P20 DA024157, K05 DA020087 (K.A.C.), and K02 DA000403 (F.G.M.) and by the Center for Addiction Research at the University of Texas Medical Branch. A portion of this work was also supported by the Intramural Research Programs of the National Institute on Drug Abuse and the National Institute on Alcohol Abuse and Alcoholism (K.C.R.).
The authors declare the following competing financial interest(s): Dr. Cunningham is an editor of Neuropsychopharmacology Reviews and receives compensation for this role from the American College of Neuropsychopharmacology.
Funding Statement
National Institutes of Health, United States
References
- Koob G. F.; Volkow N. D. (2010) Neurocircuitry of addiction. Neuropsychopharmacology 35(1), 217–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N. D.; Fowler J. S.; Wang G. J.; Baler R; Telang F. (2009) Imaging dopamine’s role in drug abuse and addiction. Neuropharmacology 56(Suppl 1), 3–8PMCID:PMC2696819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller F. G.; Dougherty D. M.; Barratt E. S.; Schmitz J. M.; Swann A. C.; Grabowski J. (2001) The impact of impulsivity on cocaine use and retention in treatment. J. Subst. Abuse Treat. 21(4), 193–198. [DOI] [PubMed] [Google Scholar]
- Lu L; Hope B. T.; Dempsey J; Liu S. Y.; Bossert J. M.; Shaham Y. (2005) Central amygdala ERK signaling pathway is critical to incubation of cocaine craving. Nat. Neurosci. 8(2), 212–219. [DOI] [PubMed] [Google Scholar]
- O’Brien C. P.; Childress A. R.; Ehrman R; Robbins S. J. (1998) Conditioning factors in drug abuse: can they explain compulsion?. J. Psychopharmacol. 12(1), 15–22. [DOI] [PubMed] [Google Scholar]
- Liu S; Lane S. D.; Schmitz J. M.; Waters A. J.; Cunningham K. A.; Moeller F. G. (2011) Relationship between attentional bias to cocaine-related stimuli and impulsivity in cocaine-dependent subjects. Am. J. Drug Alcohol Abuse 37(2), 117–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan M. J.; Marin P; Bockaert J; la Cour C. M. (2008) Signaling at G-protein-coupled serotonin receptors: recent advances and future research directions. Trends Pharmacol. Sci. 29(9), 454–464. [DOI] [PubMed] [Google Scholar]
- Hoyer D; Hannon J. P.; Martin G. R. (2002) Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol., Biochem. Behav. 71(4), 533–554. [DOI] [PubMed] [Google Scholar]
- Bockaert J; Claeysen S; Becamel C; Dumuis A; Marin P. (2006) Neuronal 5-HT metabotropic receptors: fine-tuning of their structure, signaling, and roles in synaptic modulation. Cell Tissue Res. 326(2), 553–572. [DOI] [PubMed] [Google Scholar]
- Fletcher P. J.; Tampakeras M; Sinyard J; Higgins G. A. (2007) Opposing effects of 5-HT(2A) and 5-HT(2C) receptor antagonists in the rat and mouse on premature responding in the five-choice serial reaction time test. Psychopharmacology (Berlin, Ger.) 195(2), 223–234. [DOI] [PubMed] [Google Scholar]
- Winstanley C. A.; Chudasama Y; Dalley J. W.; Theobald D. E.; Glennon J. C.; Robbins T. W. (2003) Intra-prefrontal 8-OH-DPAT and M100907 improve visuospatial attention and decrease impulsivity on the five-choice serial reaction time task in rats. Psychopharmacology (Berlin, Ger.) 167(3), 304–314. [DOI] [PubMed] [Google Scholar]
- Anastasio N. C.; Stoffel E. C.; Fox R. G.; Bubar M. J.; Rice K. C.; Moeller F. G.; Cunningham K. A. (2011) Serotonin (5-hydroxytryptamine) 5-HT2A receptor: Association with inherent and cocaine-evoked behavioral disinhibition in rats. Behav. Pharmacol. 22(3), 248–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarra R; Comery T. A.; Graf R; Rosenzweig-Lipson S; Day M. (2008) The 5-HT(2C) receptor agonist WAY-163909 decreases impulsivity in the 5-choice serial reaction time test. Behav. Brain Res. 188(2), 412–415. [DOI] [PubMed] [Google Scholar]
- Anastasio N. C., Gilbertson S. R., Bubar M. J., Seitz P. K., Agarkov A, Jeng Y. J., Bremer N. M., Smith T. D., Stutz S. J., Fox R. G. (2012) Dissociation of protein complex allosterically modulates serotonin (5-HT) 5-HT2C receptor (5-HT2CR) signaling. Unpublished observations.
- Robbins T. W. (2002) The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology (Berlin, Ger.) 163(3–4), 362–380. [DOI] [PubMed] [Google Scholar]
- Moeller F. G.; Barratt E. S.; Dougherty D. M.; Schmitz J. M.; Swann A. C. (2001) Psychiatric aspects of impulsivity. Am. J. Psychiatry 158(11), 1783–1793. [DOI] [PubMed] [Google Scholar]
- Cunningham K. A.; Fox R. G.; Anastasio N. C.; Bubar M. J.; Stutz S. J.; Moeller F. G.; Gilbertson S. R.; Rosenzweig-Lipson S. (2011) Selective serotonin 5-HT2C receptor activation suppresses the reinforcing efficacy of cocaine and sucrose but differentially affects the incentive-salience value of cocaine- vs. sucrose-associated cues. Neuropharmacology 61(3), 513–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher P. J.; Rizos Z; Sinyard J; Tampakeras M; Higgins G. A. (2008) The 5-HT(2C) receptor agonist RO 60–0175 reduces cocaine self-administration and reinstatement induced by the stressor yohimbine and contextual cues. Neuropsychopharmacology 33(6), 1402–1412. [DOI] [PubMed] [Google Scholar]
- Nic Dhonnchadha B. A.; Fox R. G.; Stutz S. J.; Rice K. C.; Cunningham K. A. (2009) Blockade of the serotonin 5-HT2A receptor suppresses cue-evoked reinstatement of cocaine-seeking behavior in a rat self-administration model. Behav. Neurosci. 123(2), 382–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neisewander J. L.; Acosta J. I. (2007) Stimulation of 5-HT2C receptors attenuates cue and cocaine-primed reinstatement of cocaine-seeking behavior in rats. Behav. Pharmacol. 18(8), 791–800. [DOI] [PubMed] [Google Scholar]
- Ullrich T; Rice K. C. (2000) A practical synthesis of the serotonin 5-HT2A receptor antagonist MDL 100907, its enantiomer and their 3-phenolic derivatives as precursors for [11C]labeled PET ligands. Bioorg. Med. Chem. 8(10), 2427–2432. [DOI] [PubMed] [Google Scholar]
- Dunlop J; Sabb A. L.; Mazandarani H; Zhang J; Kalgaonker S; Shukhina E; Sukoff S; Vogel R. L.; Stack G; Schechter L (2005) WAY-163909 ((7bR,10aR)-1,2,3,4,8,9,10,10a-octahydro-7bH-cyclopenta-[b][1,4]diazepino[ 6,7,1hi]indole): A novel 5-HT2C receptor selective agonist with anorectic activity. J. Pharmacol. Exp. Ther. 313(2), 862–869. [DOI] [PubMed] [Google Scholar]
- Herth M. M.; Kramer V; Piel M; Palner M; Riss P. J.; Knudsen G. M.; Rosch F. (2009) Synthesis and in vitro affinities of various MDL 100907 derivatives as potential 18F-radioligands for 5-HT2A receptor imaging with PET. Bioorg. Med. Chem. 17(8), 2989–3002. [DOI] [PubMed] [Google Scholar]
- Shashack M. J.; Cunningham K. A.; Seitz P. K.; McGinnis A; Smith T; Watson C. S.; Gilbertson S. R. (2011) Synthesis and evaluation of dimeric derivatives of 5-HT(2A) receptor (5-HT(2A)R) antagonist M-100907. ACS Chem. Neurosci. 2(11), 640–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pockros L. A.; Pentkowski N. S.; Swinford S. E.; Neisewander J. L. (2011) Blockade of 5-HT2A receptors in the medial prefrontal cortex attenuates reinstatement of cue-elicited cocaine-seeking behavior in rats. Psychopharmacology (Berlin, Ger.) 213(2–3), 307–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitz P. K.; Bremer N. M.; McGinnis A. G.; Cunningham K. A.; Watson C. S. (2012) Quantitative changes in intracellular calcium and extracellular-regulated kinase activition in CHO cells stably expressing serotonin (5-HT) 5-HT2A or 5-HT2C receptors. BMC Neurosci. 13(1), 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop J; Marquis K. L.; Lim H. K.; Leung L; Kao J; Cheesman C; Rosenzweig-Lipson S. (2006) Pharmacological profile of the 5-HT(2C) receptor agonist WAY-163909; therapeutic potential in multiple indications. CNS Drug Rev. 12(3–4), 167–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon L. R.; Cunningham K. A. (2001) Antagonism of 5-hydroxytryptamine(2a) receptors attenuates the behavioral effects of cocaine in rats. J. Pharmacol. Exp. Ther. 297(1), 357–363. [PubMed] [Google Scholar]
- Filip M; Bubar M. J.; Cunningham K. A. (2004) Contribution of serotonin (5-hydroxytryptamine; 5-HT) 5-HT2 receptor subtypes to the hyperlocomotor effects of cocaine: Acute and chronic pharmacological analyses. J. Pharmacol. Exp. Ther. 310(3), 1246–1254. [DOI] [PubMed] [Google Scholar]
- Grottick A. J.; Fletcher P. J.; Higgins G. A. (2000) Studies to investigate the role of 5-HT(2C) receptors on cocaine- and food-maintained behavior. J. Pharmacol. Exp. Ther. 295(3), 1183–1191. [PubMed] [Google Scholar]
- Chudasama Y; Passetti F; Rhodes S. E.; Lopian D; Desai A; Robbins T. W. (2003) Dissociable aspects of performance on the 5-choice serial reaction time task following lesions of the dorsal anterior cingulate, infralimbic and orbitofrontal cortex in the rat: differential effects on selectivity, impulsivity and compulsivity. Behav. Brain Res. 146(1–2), 105–119. [DOI] [PubMed] [Google Scholar]
- Fletcher P. J.; Grottick A. J.; Higgins G. A. (2002) Differential effects of the 5-HT2A receptor antagonist M100,907 and the 5-HT2C receptor antagonist SB242,084 on cocaine-induced locomotor activity, cocaine self-administration and cocaine-induced reinstatement of responding. Neuropsychopharmacology 27(4), 576–586. [DOI] [PubMed] [Google Scholar]
- Burbassi S; Cervo L. (2008) Stimulation of serotonin(2C) receptors influences cocaine-seeking behavior in response to drug-associated stimuli in rats. Psychopharmacology (Berlin, Ger.) 196(1), 15–27. [DOI] [PubMed] [Google Scholar]
- de P. T. (2001) M-100907 (Aventis). Curr. Opin. Invest. Drugs 2(1), 123–132. [PubMed] [Google Scholar]
- Monti J. M. (2011) Serotonin control of sleep-wake behavior. Sleep Med. Rev. 15(4), 269–281. [DOI] [PubMed] [Google Scholar]
- Shram M. J.; Schoedel K. A.; Bartlett C; Shazer R. L.; Anderson C. M.; Sellers E. M. (2011) Evaluation of the abuse potential of lorcaserin, a serotonin 2C (5-HT2C) receptor agonist, in recreational polydrug users. Clin. Pharmacol. Ther. 89(5), 683–692. [DOI] [PubMed] [Google Scholar]
- Smith B. M.; Smith J. M.; Tsai J. H.; Schultz J. A.; Gilson C. A.; Estrada S. A.; Chen R. R.; Park D. M.; Prieto E. B.; Gallardo C. S. (2008) Discovery and structure-activity relationship of (1R)-8-chloro-2,3,4,5-tetrahydro-1-methyl-1H-3-benzazepine (Lorcaserin), a selective serotonin 5-HT2C receptor agonist for the treatment of obesity. J. Med. Chem. 51(2), 305–313. [DOI] [PubMed] [Google Scholar]
- Smith S. R.; Weissman N. J.; Anderson C. M.; Sanchez M; Chuang E; Stubbe S; Bays H; Shanahan W. R. (2010) Multicenter, placebo-controlled trial of lorcaserin for weight management. N. Engl. J. Med. 363(3), 245–256. [DOI] [PubMed] [Google Scholar]
- Fidler M. C.; Sanchez M; Raether B; Weissman N. J.; Smith S. R.; Shanahan W. R.; Anderson C. M. (2011) A one-year randomized trial of lorcaserin for weight loss in obese and overweight adults: the BLOSSOM trial. J. Clin. Endocrinol. Metab. 96(10), 3067–3077. [DOI] [PubMed] [Google Scholar]
- Kiessling L. L.; Gestwicki J. E.; Strong L. E. (2006) Synthetic multivalent ligands as probes of signal transduction. Angew. Chem., Int. Ed. 45(15), 2348–2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morphy R; Kay C; Rankovic Z. (2004) From magic bullets to designed multiple ligands. Drug Discovery Today 9(15), 641–651. [DOI] [PubMed] [Google Scholar]
- Takeuchi K; Kohn T. J.; Honigschmidt N. A.; Rocco V. P.; Spinazze P. G.; Hemrick-Luecke S. K.; Thompson L. K.; Evans D. C.; Rasmussen K; Koger D (2006) Advances toward new antidepressants beyond SSRIs: 1-aryloxy-3-piperidinylpropan-2-ols with dual 5-HT1A receptor antagonism/SSRI activities. Part 5. Bioorg. Med. Chem. Lett. 16, 2347–2351. [DOI] [PubMed] [Google Scholar]
- Zhou D; Harrison B. L.; Shah U; Andree T. H.; Hornby G. A.; Scerni R; Schechter L. E.; Smith D. L.; Sullivan K. M.; Mewshaw R. E. (2006) Studies toward the discovery of the next generation of antidepressants. Part 5: 3,4-Dihydro-2H-benzo[1,4]oxazine derivatives with dual 5-HT1A receptor and serotonin transporter affinity. Bioorg. Med. Chem. Lett. 16(5), 1338–1341. [DOI] [PubMed] [Google Scholar]
- Booth R. G.; Fang L; Huang Y; Wilczynski A; Sivendran S. (2009) (1R, 3S)-(-)-trans-PAT: a novel full-efficacy serotonin 5-HT2C receptor agonist with 5-HT2A and 5-HT2B receptor inverse agonist/antagonist activity. Eur. J. Pharmacol. 615(1–3), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland N. E.; Crump E. M.; Nguyen N; Robertson K; Sun Z; Booth R. G. (2008) Effect of (-)-trans-PAT, a novel 5-HT2C receptor agonist, on intake of palatable food in mice. Pharmacol., Biochem. Behav. 91(1), 176–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yekkirala A. S.; Lunzer M. M.; McCurdy C. R.; Powers M. D.; Kalyuzhny A. E.; Roerig S. C.; Portoghese P. S. (2011) N-naphthoyl-beta-naltrexamine (NNTA), a highly selective and potent activator of mu/kappa-opioid heteromers. Proc. Natl. Acad. Sci. U.S.A. 108(12), 5098–5103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harikumar K. G.; Akgun E; Portoghese P. S.; Miller L. J. (2010) Modulation of cell surface expression of nonactivated cholecystokinin receptors using bivalent ligand-induced internalization. J. Med. Chem. 53(7), 2836–2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S; Yekkirala A; Tang Y; Portoghese P. S. (2009) A bivalent ligand (KMN-21) antagonist for mu/kappa heterodimeric opioid receptors. Bioorg. Med. Chem. Lett. 19(24), 6978–6980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldhoer M; Fong J; Jones R. M.; Lunzer M. M.; Sharma S. K.; Kostenis E; Portoghese P. S.; histler J. L. (2005) A heterodimer-selective agonist shows in vivo relevance of G protein-coupled receptor dimers. Proc. Natl. Acad. Sci. U.S.A. 102(25), 9050–9055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreault M. L.; Hasbi A; O’Dowd B. F.; George S. R. (2011) The dopamine d1-d2 receptor heteromer in striatal medium spiny neurons: evidence for a third distinct neuronal pathway in Basal Ganglia. Front. Neuroanat. 5, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leri F; Flores J; Rodaros D; Stewart J. (2002) Blockade of stress-induced but not cocaine-induced reinstatement by infusion of noradrenergic antagonists into the bed nucleus of the stria terminalis or the central nucleus of the amygdala. J. Neurosci. 22(13), 5713–5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keppel G. (1973) Design and Analysis: A Researcher’s Handbook, Prentice-Hall, Inc, Englewood Cliffs, NJ. [Google Scholar]