Abstract
Despite the widespread use of antidepressant medications that block serotonin (5-hydroxytryptamine; 5-HT) and/or norepinephrine (NE) transporters, such as SSRIs (selective serotonin reuptake inhibitors) or SNRIs (serotonin and norepinephrine reuptake inhibitors), the underlying neurobiological basis of action of these agents is poorly understood. Increases in serotonergic function are hypothesized to have beneficial effects on depressive symptoms. However, which of the 14 different neuronal receptors sensitive to 5-HT accounts for the therapeutic effects of SSRIs and SNRIs remains undetermined. The development of drugs that activate or block specific 5-HT receptors may help to circumvent the two main limitations of current antidepressants: low efficacy and delayed onset of therapeutic action. What follows is a short summary of the author’s views on this matter.
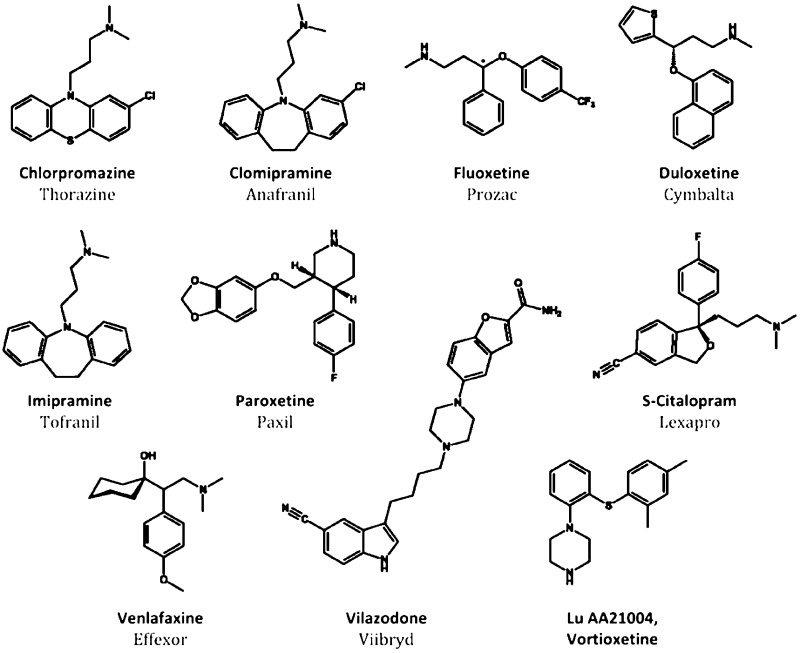
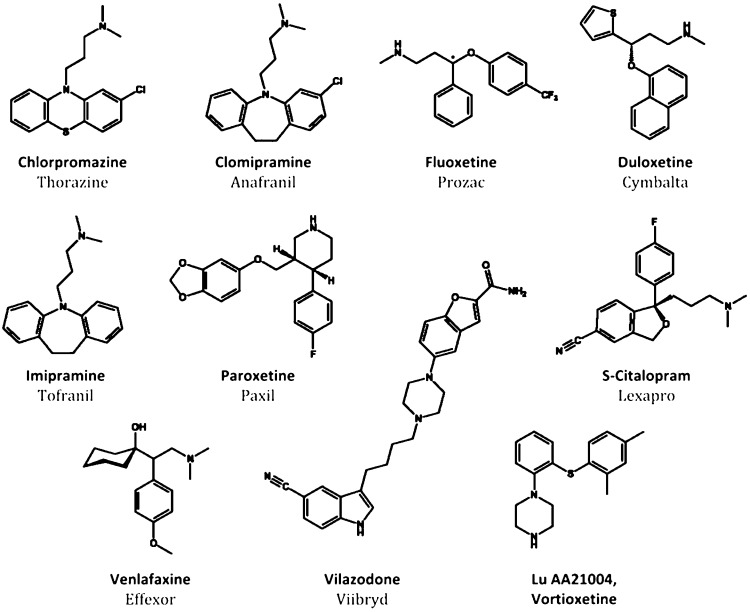
Major depression is a severe psychiatric syndrome with a lifetime prevalence of 10% and 20% for men and women, respectively, and high socioeconomic impact.1 Unfortunately, the increasing cost of depression and related affective disorders has not been paralleled by improvements in the efficacy of antidepressant treatment. SSRIs and SNRIs are pharmacological refinements of the first generation of antidepressant drugs – tricyclic drugs such as imipramine or chlorimipramine – discovered serendipitously while searching for new antipsychotic drugs with a chemical structure similar to that of chlorpromazine (Figure 1). In addition to blocking monoamine reuptake, tricyclic antidepressants exhibit high affinity for a number of neurotransmitter receptors (α1-adrenoceptors, histamine H1, muscarininc receptors, etc.). The interaction of tricyclic drugs with these receptors is responsible for their adverse side effects (postural hypotension, dry mouth, blurred vision, constipation, memory or cognitive impairment, etc.), which force many patients to abandon treatment with these drugs. The synthesis of new molecules inhibiting monoamine reuptake, devoid of these additional pharmacological activities, fostered the development of a new era in the treatment of major depression and other psychiatric diseases. The SSRIs (and later, the SNRIs) allowed patients to comply with therapeutic regimes, thereby increasing the overall numbers of patients experiencing antidepressant effects.
Figure 1.
Structures of psychoactive drugs. Chlorpromazine is an antipsychotic that served as the framework for the discoveries of imipramine and clomipramine, tricyclic monoamine reuptake inhibiting antidepressants having multiple off-target effects that limit usage. Based on the efficacy of the tricyclics, newer antidepressants have been developed that selectively inhibit serotonin reuptake (SSRIs; fluoxetine, paroxetine, S-citalopram) or are dual serotonin-norepinephrine uptake inhibitors (SNRIs; duloxetine, venlafaxine). New antidepressant drugs that inhibit serotonin reuptake and have added action as partial 5-HT1A agonists have recently been developed: vilazodone (EMD 68843) and vortioxetine (LuAA21004). The latter drug shows also affinity for other 5-HT receptors (see text).
However, despite increased compliance, the efficacy of SSRIs did not surpass that of some tricyclic drugs, such as clomipramine.2,3 Clinical trials with selected patient populations typically indicated response and remission rates of 60% and 40%, respectively, with SSRIs.4,5 However, naturalistic studies, such as the STAR*D (Sequenced Treatment Alternatives to Relieve Depression) trial revealed a less promising situation, with response and remission rates of ∼50% and ∼30%, respectively, after treatment with the SSRI S-citalopram (Figure 1). Augmentation strategies with drugs not targeting the serotonin transporter (SERT) in patients not responding to SSRIs yielded similar remission rates, around 30%.6 Thus, these “real world” figures indicate that a very large percentage of patients treated with SSRIs show partial responses, leaving much room for improvement in antidepressant treatment.
The last two decades have witnessed major advances in our knowledge of glutamatergic transmission. In addition to direct excitatory activity mediated by ionotropic receptors (AMPA, NMDA, KA), glutamate can exert modulatory actions, similar to those of monoamines, via the activation of three families of metabotropic receptors, with a total of eight different receptors identified (mGluR1-mGluR8), which offer potentially new avenues for neuropsychiatric drug development. For example, an mGluR2/3 agonist is being developed for the treatment of schizophrenia.7 This is the first antipsychotic drug devoid of affinity for dopamine receptors, illustrating the potential of mGluRs in drug development. Moreover, the noncompetitive NMDA antagonist ketamine can evoke rapid (∼2 h) and persistent (up to 1 week) antidepressant effects after a single dose in treatment-resistant depressed patients.8 Interestingly, the dose used in antidepressant clinical trials of ketamine is the same as that displaying psychotomimetic effects. Overall, this suggests that the glutamatergic system may offer excellent opportunities for the development of antidepressant drugs overcoming the limitations of SSRIs and SNRIs. Yet, despite these promising results, the development of glutamatergic antidepressants may be hampered or delayed for several reasons, described briefly below.
With the exception of the basal ganglia, where GABA is the principle neurotransmitter, the basic wiring of the brain comprises excitatory glutamatergic neurons. This raises the possibility that glutamatergic drugs will elicit new and unexpected side effects, different from those of SSRIs or SNRIs, given the distinct roles played by glutamate and the monoamines in brain function. In addition to efficacy, the safety of new glutamatergic drugs will be a major issue that will require testing in large numbers of patients for long periods of time in phase IV clinical trials. Further, at the time of writing, CNS drug development is being abandoned or reduced by several large pharmaceutical companies, thus reducing overall research and development aimed at identifying new targets and novel therapeutics. Thus, it is likely that antidepressant drug development in upcoming years will not broaden beyond monoaminergic systems. Monoaminergic drugs offer advantages associated with >50 years of clinical experience, with millions of patients treated, and a relatively good knowledge of their side effects.
As stated above, a major problem of SSRIs and SNRIs is their poor efficacy and slow clinical action. With an almost saturated antidepressant market, new drugs should be faster and/or more efficacious than SSRIs and SNRIs to achieve success. These new developments must be based on the knowledge of the role played by the different 5-HT receptors in depression, a highly complex field, given the existence of 14 different 5-HT receptors, different localizations in brain networks, and sometimes opposing actions on neuronal activity after stimulation by 5-HT. Presynaptic 5-HT1A and 5-HT1B autoreceptors are suspected of playing a major detrimental role in current antidepressant therapy due to activation of negative feedback mechanisms operating in 5-HT neurons.9,10 Conversely, activation of postsynaptic 5-HT1A receptors in corticolimbic networks has positive antidepressant action.11,12 This paradoxical situation may perhaps be solved by small-interferring RNA (siRNA) mechanisms selectively targeting pre- or postsynaptic receptors (13) or by the development of agonists selective for postsynaptic 5-HT1A receptors.14,15 On the other hand, blockade of 5-HT2A/2C receptors improves the actions of SSRIs, whereas 5-HT2B receptor activation enhances serotonergic activity and shows antidepressant-like activity in rodents.16 5-HT3 receptor blockade can also augment the antidepressant action of SERT inhibition, whereas 5-HT4 receptor activation has antidepressant effects on its own and augments SSRI effects.17 Finally, blockade of 5-HT6 and 5-HT7 receptors may also improve the antidepressant effects of SERT inhibition (very little is known about the role of 5-HT5 receptors).18,19 Given the poor capabilities to model depressive symptoms in animals, it is unclear whether these different serotonin receptor pharmacologies have selective actions on particular groups of symptoms (e.g., affective, cognitive, somatic, etc.) or affect a range of symptoms, similar to SSRIs and SNRIs.
These observations indicate that the activation of the various 5-HT receptors by SSRI and SNRI has opposing effects on the activity of brain networks underlying their therapeutic effects. Therefore, it would be advisible to design antidepressant drugs encompassing agonist and antagonist activities at most relevant 5-HT receptors, an objective likely unfeasible from a chemical point of view. On the other hand, it is uncertain whether the selective activation of a single 5-HT receptor subtype is able to elicit antidepressant effects superior to those of current drugs. Therefore, future antidepressant strategies might include combinations of SERT blockade plus some of the above activities, an approach initiated by recently developed antidepressant drugs (e.g., vilazodone or Lu AA21004, also known as vortioxetine; Figure 1). In addition to blocking SERT, both drugs incorporate partial agonist activity at 5-HT1A receptors. Also, vortioxetine shows antagonist activity at 5-HT3, 5-HT1B, and 5-HT7 receptors. Both drugs elevate extracellular 5-HT to a great extent compared to SSRIs, possibly by overcoming negative feed-back mechanisms that limit the full effect of SERT blockade.20,21 Future serotonergic drugs may also include other targets potentially useful for antidepressant activity, such as blockade of 5-HT2A/2C receptors or activation of 5-HT4 receptors. Likewise, further clarification of brain elements (neurotransmitters, receptors, cells, networks) responsible for the therapeutic effects of antidepressant drugs will guide the development of new generations of antidepressants overcoming the important limitations of current drugs.
Acknowledgments
The author thanks Mr. Sarawut Cheunkar for graphics assistance.
The author’s research is supported by the Innovative Medicine Initiative Joint Undertaking under Grant Agreement No. 115008, of which resources are composed of EFPIA in-kind contribution and financial contribution from the European Union’s Seventh Framework Programme (FP7/2007-2013). Support from the Generalitat de Catalunya (2009-SGR220) and the Centro de Investigación Biomédica en Red de Salud Mental (CIBERSAM) is also acknowledged.
The authors declare the following competing financial interest(s): F. Artigas has received lecture fees from Eli Lilly and Lunbeck A/S and consultancy fees from Lundbeck A/S on the mechanism of action of antidepressant drugs.
References
- Smith K. (2011) Trillion-dollar brain drain. Nature 478, 15. [DOI] [PubMed] [Google Scholar]
- (1986) Citalopram: Clinical effect profile in comparison with clomipramine. a controlled multicenter study. Psychopharmacology (Berlin, Ger.) 90, 131–138. [DOI] [PubMed] [Google Scholar]
- (1990) Paroxetine: A selective serotonin reuptake inhibitor showing better tolerance, but weaker antidepressant effect than clomipramine in a controlled multicenter study. J. Affective Disord. 18, 289–299. [DOI] [PubMed] [Google Scholar]
- Tollefson G. D.; Holman S. L. (1994) How long to onset of antidepressant action: A meta-analysis of patients treated with fluoxetine or placebo. Int. Clin. Psychopharmacol. 9, 245–250. [DOI] [PubMed] [Google Scholar]
- Thase M. E.; Entsuah A. R.; Rudolph R. L. (2001) Remission rates during treatment with venlafaxine or selective serotonin reuptake inhibitors. Br. J. Psychiatry 178, 234–241. [DOI] [PubMed] [Google Scholar]
- Trivedi M. H.; Fava M.; Wisniewski S. R.; Thase M. E.; Quitkin F.; Warden D.; Ritz L.; Nierenberg A. A.; Lebowitz B. D.; Biggs M. M.; Luther J. F.; Shores-Wilson K.; Rush A. J. (2006) Medication augmentation after the failure of SSRIs for depression. N. Engl. J. Med. 354, 1243–52. [DOI] [PubMed] [Google Scholar]
- Patil S. T.; Zhang L.; Martenyi F.; Lowe S. L.; Jackson K. A.; Andreev B. V.; Avedisova A. S.; Bardenstein L. M.; Gurovich I. Y.; Morozova M. A.; Mosolov S. N.; Neznanov N. G.; Reznik A. M.; Smulevich A. B.; Tochilov V. A.; Johnson B. G.; Monn J. A.; Schoepp D. D. (2007) Activation of mGlu2/3 receptors as a new approach to treat schizophrenia: A randomized phase II clinical trial. Nat. Med. 13, 1102–1107. [DOI] [PubMed] [Google Scholar]
- Zarate C. A. Jr.; Singh J. B.; Carlson P. J.; Brutsche N. E.; Ameli R.; Luckenbaugh D. A.; Charney D. S.; Manji H. K (2006) A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch. Gen. Psychiatry 63, 856–864. [DOI] [PubMed] [Google Scholar]
- Artigas F.; Romero L.; de Montignty C.; Blier P. (1996) Acceleration of the effect of selected antidepresant drugs by 5-HT1A antaognists. Trends Neurosci. 19, 378–383. [DOI] [PubMed] [Google Scholar]
- Artigas F.; Celada P.; Laruelle M.; Adell A. (2001) How does pindolol improve antidepressant action?. Trends Pharmacol. Sci. 22, 224–228. [DOI] [PubMed] [Google Scholar]
- Haddjeri N.; Blier P.; De Montigny C. (1998) Long-term antidepressant treatments result in a tonic activation of forebrain 5-HT1A receptors. J. Neurosci. 18, 10150–10156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scorza M. C., Llado-Pelfort L., Oller S., Cortes R., Puigdemont D., Portella M. J., Perez-Egea R., Alvarez E., Celada P., Perez V., Artigas F (2012). Preclinical and clinical characterization of the selective serotonin-1A receptor antagonist DU-125530 for antidepressant treatment. Br. J. Pharmacol. Epub. PMID: 22050051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolozzi A.; Castane A.; Semakova J.; Santana N.; Alvarado G.; Cortes R.; Ferres-Coy A.; Fernandez G.; Carmona M. C.; Toth M.; Perales J. C.; Montefeltro A.; Artigas F. (2012) New antidepressant strategy based on acute siRNA silencing of 5-HT1A autoreceptors. Mol. Psychiatry 17, 612–623. [DOI] [PubMed] [Google Scholar]
- Depoortere R.; Auclair A. L.; Bardin L.; Colpaert F. C.; Vacher B.; Newman-Tancredi A. (XXXX) F15599, a preferential post-synaptic 5-HT1A receptor agonist: Activity in models of cognition in comparison with reference 5-HT1A receptor agonists. Eur. Neuropsychopharmacol. 20, 641–654. [DOI] [PubMed] [Google Scholar]
- Lladó-Pelfort L.; Assié M. B.; Newman-Tancredi A.; Artigas F.; Celada P. (2010) Preferential in vivo action of F15599, a novel 5-HT(1A) receptor agonist, at postsynaptic 5-HT(1A) receptors. Br. J. Pharmacol. 160, 1929–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz S. L.; Doly S.; Narboux-Neme N.; Fernandez S.; Mazot P.; Banas S. M.; Boutourlinsky K.; Moutkine I.; Belmer A.; Roumier A.; Maroteaux L. (2012) 5-HT(2B) receptors are required for serotonin-selective antidepressant actions. Mol. Psychiatry 17, 154–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas G.; Rymar V. V.; Du J.; Mnie-Filali O.; Bisgaard C.; Manta S.; Lambas-Senas L.; Wiborg O.; Haddjeri N.; Pineyro G.; Sadikot A. F.; Debonnel G. (2007) Serotonin(4) (5-HT(4)) receptor agonists are putative antidepressants with a rapid onset of action. Neuron 55, 712–725. [DOI] [PubMed] [Google Scholar]
- Wesolowska A.; Nikiforuk A. (2007) Effects of the brain-penetrant and selective 5-HT6 receptor antagonist SB-399885 in animal models of anxiety and depression. Neuropharmacology 52, 1274–1283. [DOI] [PubMed] [Google Scholar]
- Bonaventure P.; Kelly L.; Aluisio L.; Shelton J.; Lord B.; Galici R.; Miller K.; Atack J.; Lovenberg T. W.; Dugovic C. (2007) Selective blockade of 5-hydroxytryptamine (5-HT)7 receptors enhances 5-HT transmission, antidepressant-like behavior, and rapid eye movement sleep suppression induced by citalopram in rodents. J. Pharmacol. Exp. Ther. 321, 690–698. [DOI] [PubMed] [Google Scholar]
- Page M. E.; Cryan J. F.; Sullivan A.; Dalvi A.; Saucy B.; Manning D. R.; Lucki I. (2002) Behavioral and neurochemical effects of 5-(4-[4-(5-cyano-3-indolyl)-butyl)-butyl]-1-piperazinyl)-benzofuran-2-carboxamide (EMD 68843): A combined selective inhibitor of serotonin reuptake and 5-hydroxytryptamine(1A) receptor partial agonist. J. Pharmacol. Exp. Ther. 302, 1220–1227. [DOI] [PubMed] [Google Scholar]
- Mork A.; Pehrson A.; Brennum L. T.; Nielsen S. M.; Zhong H.; Lassen A. B.; Miller S.; Westrich L.; Boyle N. J.; Sanchez C.; Fischer C. W.; Liebenberg N.; Wegener G.; Bundgaard C.; Hogg S.; Bang-Andersen B.; Stensbol T. B. (2012) Pharmacological effects of Lu AA21004: a novel multimodal compound for the treatment of major depressive disorder. J. Pharmacol. Exp. Ther. 340, 666–675. [DOI] [PubMed] [Google Scholar]