Abstract
Background & Aims
Ambulatory reflux testing is used to evaluate symptoms of gastroesophageal reflux disease (GERD) refractory to protein pump inhibitors (PPIs). We investigated the prevalence of PPI use in patients with negative results from Bravo™ pH or multichannel intraluminal impedance-pH (MII-pH) tests and factors that might predict the use of PPIs.
Methods
We analyzed data from patients who had undergone Bravo™ pH monitoring or MII-pH testing, without evidence of reflux disease, at Northwestern University. Demographics, endoscopy findings, pathology results, and provider recommendations were obtained via chart review. Eligible patients (n=90) were contacted by telephone, and a cross-sectional survey was administered with questions about symptom severity, demographics, medication use, and health behaviors. Patients were compared by current PPI use and statistical analyses were performed using SAS version 9.2.
Results
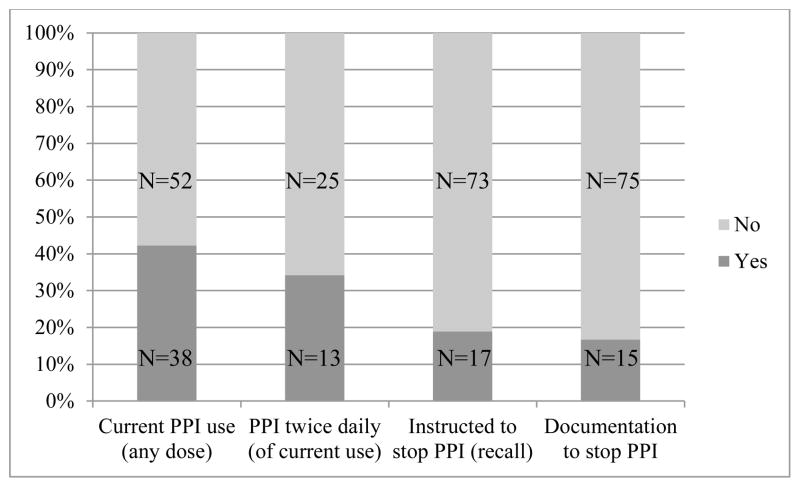
Thirty-eight patients (42.2%) reported current PPI use despite a negative result from a pH study. Only 17 patients (18.9%) recalled being instructed to stop taking PPIs; chart review showed documented instructions to stop PPI therapy for 15 patients (16.7%). There were no significant differences in demographic or clinical characteristics among patients compared by current PPI use. Patients taking a PPI were more likely than those not taking a PPI to report troublesome symptoms that affected their daily life, as measured by a questionnaire for the diagnosis of GERD (the GerdQ).
Conclusions
More than 42% of patients with negative results from pH monitoring studies continue PPI therapy despite physiologic data that they do not have GERD.
Keywords: esophagus, treatment, acid-suppressive medication, drug
INTRODUCTION
Gastroesophageal reflux disease (GERD) is a common, chronic disease that affects up to 20% of adults in the United States.1 GERD causes considerable morbidity and contributes substantial cost to the US health care system2 -- in excess of $10 billion in annual direct health care costs, with the majority of cost attributed to the use of acid-suppressive medications known as proton pump inhibitors (PPIs).3,4 GERD is the most frequent digestive system diagnosis in ambulatory care and the most common digestive disease diagnosed when patients are discharged from the hospital.4 Patients often report that GERD symptoms reduce their health-related quality of life by interfering with their ability to perform daily activities and to sleep.5 The symptoms of GERD are a major burden for many patients, disrupting their physical and emotional well-being.6
Despite the effectiveness of PPI therapy for most GERD patients, up to 25% still experience unresolved symptoms.7 If empiric PPI therapy fails to adequately relieve symptoms, current professional guidelines recommend pursuing endoscopy and ambulatory reflux testing, although evidence for this recommendation is weak.8 Negative results from endoscopy and reflux testing suggest an alternative diagnosis, and patients with such results may not require acid suppression. Presumably, further management of these patients should include a trial period of withdrawing PPI therapy and pursuing alternative diagnoses and treatments, but whether or not this strategy is applied in practice is unknown. The aims of this study were twofold: to determine the prevalence of continued (current) PPI use in patients whose endoscopic and Bravo™ pH9 or multichannel intraluminal impedance-pH (MII-pH) testing10,11 yielded negative results; and to determine any predictors of continued PPI use.
METHODS
Inclusion/Exclusion criteria
We investigated patients referred for ambulatory pH testing at a single tertiary referral academic center (Division of Gastroenterology, Northwestern University and Northwestern Memorial Hospital, Chicago, IL) whose follow-up endoscopic and pH-testing gave negative results. Patients who had undergone 48-hour Bravo™ pH monitoring from Jan 2006 through Jan 2010 while withholding PPI therapy were included in the study if they had: 1) total acid exposure (pH<4) less than 5%, and 2) a negative Symptom Index (SI) (<50%) on both days.12 Patients who had undergone MII-pH testing from 2006 to 2010 were included if they met three criteria: total acid exposure time <5%, total number of reflux events <73, and negative SI.12 Patients receiving PPI therapy during MII-pH testing, or those who had a history of anti-reflux surgery, Barrett’s esophagus, or eosinophilic esophagitis were excluded from the final analysis.
Study Design
We conducted a cross sectional observational study with manual chart review and scripted telephone survey for data collection. Demographic data (age, sex), endoscopy findings, pathology results, and provider recommendations were obtained via manual chart review. Eligible patients were contacted by telephone; if a patient was not available, a message was left if voicemail was available and 2 to 3 more attempts were made to obtain contact. If contacted, patients were told the purpose of the telephone call and study. We obtained consent from interested patients and administered a cross sectional survey to collect data about demographics (marital status, race, income, education attained), medication use, health behaviors, symptom severity (GerdQ)13, and health outcomes (EQ-5D, validated telephone version).14
The GerdQ is a validated questionnaire that quantifies a patient’s experience with GERD symptoms during the previous week and notes their impact on daily life.13 It was shown to have similar accuracy in diagnosing GERD as that of a gastroenterologist, is responsive to change, and can assess the relative impact of disease. The questionnaire contains 6 items asking individuals to rate the frequency of their symptoms and the impact of their disease on a 4-point scale corresponding to days of symptoms (0, 1, 2–3, and 4–7 days) and can be used to assess disease impact and treatment response. A sum score of ≥8 for all the questions was deemed in the initial validation study to have the greatest sensitivity and specificity for GERD.13 The sum score of the two questions about sleep disturbances and use of over-the-counter medications are accurate indicators of how GERD affects daily life (impact score). In the initial validation, patients with an impact score of ≥3 were most likely to be affected by their disease.
The EQ-5D is a validated, widely used measure of health outcomes.14 It has been applied to a wide range of health conditions and provides a simple descriptive profile across 5 domains and a single index value (0–100) for health status, known as the visual analogue scale score (VAS). We entered all data into a central database using Assessment Center™ (Chicago, IL).15,16 The study was approved by the Northwestern University Institutional Review Board.
Statistical Analyses
Patients were compared by current PPI use (off vs. on) and statistical analyses were performed using SAS version 9.2 (Cary, NC). The primary outcome and descriptive statistics were reported as proportions. Comparisons between groups were made using t-tests and Wilcoxon signed-rank tests (where appropriate) for continuous variables or Chi-square and Fisher exact tests (for counts ≤5) for categorical variables.
RESULTS
Patient Sample
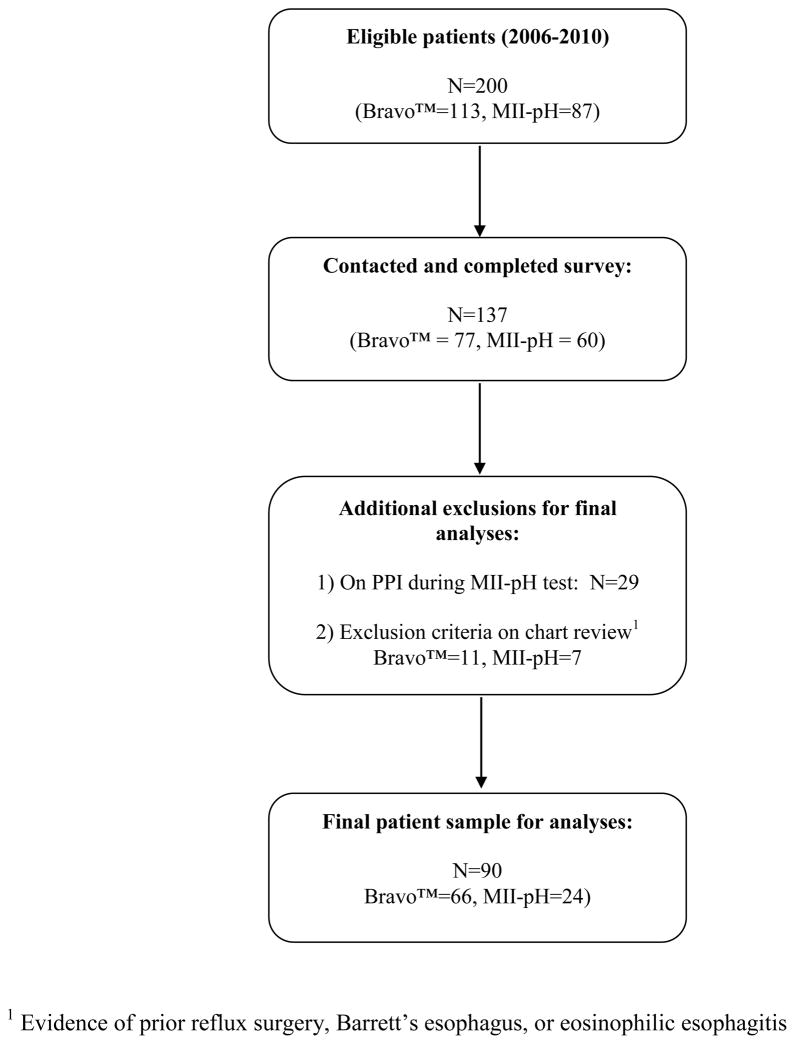
We identified a total of 200 patients who met physiologic inclusion criteria for the study (Figure 1). Of these patients, 113 had Bravo™ pH testing and 87 had MII-pH testing. A total of 137 (68.5%) patients were successfully contacted by telephone (77 Bravo, 60 MII-pH). Of the patients who had MII-pH testing, 29 had been tested while on PPI therapy and were excluded from the final analyses. The manual chart review done in parallel with the phone survey revealed that an additional 18 patients had evidence of another indication for PPI use not previously identified, including prior surgery, Barrett’s esophagus, or eosinophilic esophagitis, and these were also excluded from the final analysis. The final patient sample consisted of 90 patients (66 Bravo, 24 MII-pH). Comparing patients in our sample with those who were excluded or not contacted revealed no significant differences in age (48.4 years vs. 49.4 years, P=0.6) orsex (75.6% female vs. 70.9%, P=0.5). The average time from pH testing to study start date for the entire patient sample was 25.0 months (SD=15.4). There were no significant differences in terms of sex, race, marital status, income, or education level attained between patients undergoing Bravo™ testing and those undergoing MII-pH testing in the final patient sample.
Figure 1.
Patient sample and enrollment
Primary Outcome Measures
A total of 38 out of 90 (42.2%) patients reported current PPI use despite negative results from a pH study (Figure 2). For the entire study sample, 17 patients (18.9%) recalled being instructed to stop taking their PPI; chart review data showed documented instructions for 15 patients (16.7%) to stop PPI therapy. Of those patients taking a PPI, 13 out of 38 (34%) reported taking their PPI twice daily. Esomeprazole (n=13) and omeprazole (n=11) were the most common PPIs taken. Of note, out of the 90 total patients, 12 patients reported taking aspirin, and 8 patients reported taking NSAIDs (dosages not specified). When compared by current PPI use there was no difference in aspirin use (6 patients in each group, P=0.6) or NSAID use (4 patients in each group, P=0.7).
Figure 2.
PPI use after negative results from physiologic reflux testing
The mean age of patients currently taking a PPI was 50.3 years (SD=13.9) compared with 46.8 years (SD=15.4) for those not taking a PPI (P=0.3). Body mass index obtained from chart review data was also similar between patients on or off PPI therapy. Table 1 illustrates selected characteristics of patients by current PPI use (off vs. on). There was no difference in current PPI when stratified by type of diagnostic test (Bravo™ vs. MII-pH, P=0.6). There were also no significant differences between patients in terms of sex, race, marital status, income, or education level attained (Table 1, all P values >0.05) by current PPI use. A greater proportion of patients not taking a PPI reported alcohol use (90.0% vs. 62.9%, P<0.01). We noted a similar trend for tobacco use, but it did not reach statistical significance (34.0% vs. 17.1%, P=0.09).
Table 1.
Selected characteristics of patients with negative reflux testing stratified by current PPI use1
| Current PPI use | |||
|---|---|---|---|
|
| |||
| Off PPI (N=52) | On PPI (N=38) | P value | |
| Type of test | |||
| Bravo™ | 37 (71.2%) | 29 (76.3%) | |
| MII-pH | 15 (28.8%) | 9 (23.7%) | 0.62 |
|
| |||
| Sex | |||
| Male | 11 (21.1%) | 11 (28.9%) | |
| Female | 41 (78.9%) | 27 (71.1%) | 0.42 |
|
| |||
| Race | |||
| White | 42 (82.4%) | 28 (73.7%) | |
| Not White | 9 (17.7%) | 10 (26.3%) | 0.32 |
|
| |||
| Marital status | |||
| Married | 26 (53.1%) | 20 (57.1%) | |
| Previously Married | 14 (28.6%) | 6 (17.1%) | |
| Never Married | 9 (18.4%) | 9 (25.7%) | 0.42 |
|
| |||
| Income | |||
| < or = 100 K / yr | 24 (57.1%) | 18 (56.3%) | |
| >100 K / yr | 18 (42.9%) | 14 (43.8%) | 0.92 |
|
| |||
| Education | |||
| Less than college degree | 17 (34.0%) | 13 (37.1%) | |
| College degree | 13 (26.0%) | 11 (31.4%) | |
| Above college degree | 20 (40.0%) | 11 (31.4%) | 0.72 |
|
| |||
| Tobacco Use | |||
| Never (<100 lifetime) | 33 (66.0%) | 29 (82.9%) | |
| Ever (>100 lifetime) | 17 (34.0%) | 6 (17.1%) | 0.092 |
|
| |||
| Alcohol use | |||
| No | 5 (10.0%) | 13 (37.1%) | |
| Yes | 45 (90.0%) | 22 (62.9%) | <0.013 |
Total number in each category may not equal total sample size due to refusal by patients to answer certain questions
Chi-square test
Fisher’s exact test
Secondary Outcome Measures
The proportion of patients with a positive GerdQ score (sum ≥ 8) was greater in patients with continued PPI use versus patients who were not taking a PPI (55.6% vs. 21.1%, P<0.01). The proportion of patients with troublesome daily symptoms (as measured by a GerdQ impact score ≥ 3) was also greater among those currently taking a PPI (27.8% vs. 9.6%, P=0.04). General health outcomes were measured using the EQ-5D, a general measure of health states. As shown in Table 2, there were no differences between the two groups in all domains, which included anxiety/depression, mobility, self-care, pain/discomfort, and ability to participate in daily activities (all P values >0.05). The mean EQ-5D VAS was also similar between patients currently off and on a PPI (Table 1, P=0.5)
Table 2.
Health outcomes by PPI use as measured by the EQ-5D
| Current PPI use | |||
|---|---|---|---|
|
| |||
| EQ-5D Domain | Off PPI (N=52) | On PPI (N=38) | P value |
| Anxiety/Depression | |||
| No problems | 38 (74.5%) | 23 (65.7%) | |
| Problems | 13 (25.5%) | 12 (34.3%) | 0.41 |
|
| |||
| Mobility | |||
| No problems | 44 (86.3%) | 32 (91.4%) | |
| Problems | 7 (13.7%) | 3 (8.6%) | 0.52 |
|
| |||
| Pain/Discomfort | |||
| No problems | 29 (56.9%) | 18 (50.0%) | |
| Problems | 22 (43.1%) | 18 (50.0%) | 0.51 |
|
| |||
| Self Care | |||
| No problems | 49 (96.1%) | 34 (97.1%) | |
| Problems | 2 (3.9%) | 1 (2.9%) | 0.92 |
|
| |||
| Usual Activities | |||
| No problems | 45 (88.2%) | 30 (83.3%) | |
| Problems | 6 (11.8%) | 6 (16.7%) | 0.51 |
| Visual Analogue Score | |||
| Median (range) | 85.0 (5.0–100.0) | 85.0 (50.0–95.0) | 0.53 |
Chi-square test
Fisher’s exact test
Wilcoxon rank sum test
Other outcomes of interest by current PPI use are presented in Table 3. There were no differences in outpatient visits to primary care or gastroenterology physicians, history of depressive or anxiety disorders, or medication use for depression or anxiety. In the entire study sample, regardless of PPI use, very few patients (n=5) reported use of alternative therapies (either cognitive behavioral therapy, biofeedback, hypnotherapy, or massage therapy). Use of health behaviors shown to impact GERD-related symptoms (head of bed elevation, etc.) were reported by a slightly greater proportion of patients currently taking a PPI (82.9% vs. 66.0%, P=0.09).
Table 3.
Other health outcomes stratified by current PPI use1
| Current PPI use | |||
|---|---|---|---|
|
| |||
| Off PPI (N=52) | On PPI (N=38) | P value | |
| Seen by PCP in last 3 mo for GI symptoms | |||
| No | 48 (96.0%) | 31 (88.6%) | |
| Yes | 2 (4.0%) | 4 (11.4%) | 0.23 |
|
| |||
| Seen by GI in last 3 mo for GI symptoms | |||
| No | 45 (90.0%) | 27 (79.4%) | |
| Yes | 5 (10.0%) | 7 (20.6%) | 0.23 |
|
| |||
| History of depressive disorder | |||
| No | 43 (86.0%) | 29 (82.9%) | |
| Yes | 7 (14.0%) | 6 (17.1%) | 0.72 |
|
| |||
| History of anxiety disorder | |||
| No | 41 (82.0%) | 27 (77.1%) | |
| Yes | 9 (18.0%) | 8 (22.9%) | 0.62 |
|
| |||
| Ever taken medication for depression or anxiety | |||
| No | 33 (66.0%) | 25 (71.4%) | |
| Yes | 17 (34.0%) | 10 (28.6%) | 0.62 |
|
| |||
| Currently taking medication for depression or anxiety | |||
| No | 44 (88.0%) | 26 (74.3%) | |
| Yes | 6 (12.0%) | 9 (25.7%) | 0.12 |
|
| |||
| Any use of alternative therapy4 | |||
| No | 47 (94.0%) | 33 (94.3%) | |
| Yes | 3 (6.0%) | 2 (5.7%) | 0.93 |
|
| |||
| Any use of health behaviors for symptoms5 | |||
| No | 17 (34.0%) | 6 (17.1%) | |
| Yes | 33 (66.0%) | 29 (82.9%) | 0.092 |
Total number in each category may differ due to refusal to answer certain questions
Chi-square test
Fisher’s exact test
Cognitive behavior therapy, biofeedback, hypnotherapy, or massage therapy
Elevation of head of bed, avoidance of food that exacerbates symptoms, avoidance of food 3 hrs prior to bed, or sleeping on left side
DISCUSSION
To our knowledge, our study is the first of its kind to show continued PPI use despite physiologic evidence contraindicating the presence of acid reflux. Our findings suggest that a large proportion of patients with negative results from pH monitoring studies continue PPI therapy despite evidence contradicting the presence of GERD. In addition, most patients did not recall being counseled to stop their PPI, and such counsel was not documented in the majority of patients’ medical records. While PPIs are often prescribed for gastroprotection with concurrent ASA and NSAID use, this did not appear to be a major contributing factor for PPI use in our patient sample.
Our results may not be surprising, considering that PPI overuse has been documented in numerous studies, both for inpatient and outpatient settings.17–21 Possible factors contributing to this overuse have been postulated, including practice setting, physician type, formulary status, and direct-to-consumer advertising.18,22–24 It is likely that similar factors contributed to our results. Interestingly, there were no potential demographic or clinical predictors of continued PPI use, other than the use of alcohol. This suggests that those patients still taking a PPI may be avoiding substances or health behaviors that could exacerbate symptoms. This possibility is also suggested by our finding that the vast majority of patients (regardless of PPI use) have used specific health behaviors to address their symptoms.
Despite negative results from pH testing, a significant proportion of patients had GerdQ scores that would be considered diagnostic of GERD based on the questionnaire scoring parameters.13 These patients were also more likely to be taking a PPI. The GerdQ was primarily developed as a diagnostic questionnaire and initially revealed comparable sensitivity and specificity at diagnosing GERD as that of a clinician.13 More recently, Lacy et al. compared the GerdQ with 48-hr ambulatory pH monitoring and found more limited sensitivity and specificity than originally published for diagnostic purposes.25 Our study was not designed to compare or determine characteristics of the GerdQ, but, similar to Lacy’s findings, it does reveal that a significant proportion of patients have high scores (considered diagnostic of GERD) in the absence of physiologic data.
The GerdQ data also revealed that patients currently taking a PPI continue to have symptoms that affect their daily life. There are likely multiple reasons for this. In a recent systematic review by El-Serag et al. of patients with GERD who were taking a PPI, persistent GERD symptoms ranged from 17–32% (depending on symptom) across 19 studies included in the review.26 They found that persistent GERD symptoms on PPI therapy were associated with studies performed in the US and those studies with a higher proportion (>60%) of female participants. They also found that persistent symptoms were associated with decreased psychological and physical “well-being.” Indeed, multiple studies have shown associations between stress and anxiety and persistent GERD symptoms despite PPI therapy.27–30 Our patient sample was predominantly female (Table 1) and many patients in our study, regardless of PPI use, reported a history of anxiety and/or depression and medication use for these disorders. Thus, our results may be partially attributable to somatization, which has been shown to be associated with esophageal hypersensitivity and increased perceived symptoms.27, 30, 31 Many patients in our study could be classified as having “functional heartburn” simply based on the negative pH testing results with continued symptoms.
In a recent US community-based survey of 1,347 GERD patients, Chey et al. found that the majority of patients were satisfied with their treatment; however, 42% of them were using supplemental medication and ~27% were dissatisfied with their PPI therapy.32 They also found that 40% of patients were not taking their medication correctly (i.e., before meals), highlighting opportunities for cost-effective intervention strategies and patient counseling measures.32 Our study revealed potential opportunities for clinicians to improve patient counseling and perhaps take a more systematic approach to their patients whose reflux testing yields negative results.
Our findings suggest that clinicians should routinely recommend a trial of stopping PPI therapy for those patients who lack evidence of disease. Patients also reported very little use of alternative or complementary therapies for their symptoms, for which there is some evidence of benefit in treating functional GI disorders.33–35 This may indicate another potential area for clinicians to explore with their patients who seek relief from GERD symptoms.
Although this is the first study to determine PPI use after reflux testing with negative results, there are multiple limitations. The generalizability of our results may be limited because the study was conducted at a single academic tertiary medical center staffed by clinicians with expertise in treating esophageal disorders. In addition, the patient sample was predominantly white and of a higher socioeconomic status compared with the general population. This was a retrospective study, and the findings from the phone survey may have been subject to recall bias. However, the primary outcome of current PPI use should not have been affected by this. Our chart review data were limited as the referral system at our institution is open access for pH testing and is utilized by an assortment of physicians. We have access to testing results but do not manage therapy for all the patients. Physician recommendations to stop PPI therapy may have been discussed but not documented. Also, of the patients identified in our records as eligible for the study, 63 (31.5%) were not successfully contacted after multiple attempts.
Another potential limitation is the possible role of non-acid reflux disease in contributing to GERD symptoms that has been highlighted in recent years.36–38 In this study, it is possible that some of the patients who had Bravo™ pH testing could have non-acid reflux contributing to their symptoms. However, even if the prevalence of non-acid reflux was similar as has been identified in previous studies (~18%)37, there would still be a substantial proportion of patients on PPI therapy without reflux disease (acid or non-acid).
Overall, these results highlight the need for improved strategies to identify alternative diagnoses and treatment strategies for patients whose symptoms are inadequately relieved by PPIs. Professional society guidelines advocate diagnostic testing (endoscopy and reflux testing) for patients whose symptoms are not fully responsive to a limited trial of PPI therapy.1,12,39 A recent cost effectiveness analysis showed that early and increased use of pH monitoring could lead to less PPI use without a significant increase in cost to managed health care plans.40 However, testing alone is not sufficient if practitioners fail to communicate results to patients adequately or act upon them.
In summary, a large proportion of patients with negative results from pH monitoring studies continue PPI therapy despite physiologic evidence contradicting the presence of GERD. Although this study was not designed to compare testing modalities, prospective studies are needed to determine how different testing modalities affect subsequent medication use, prescription patterns, and patient outcomes. Future research should include efforts to better stratify patients who respond poorly to PPIs based on physiologic parameters, evaluate strategies that expedite diagnostic testing and improve follow up, and collect prospective outcome data to guide therapy.
Acknowledgments
Financial Support: This work was supported by R01 DK56033 (P.J.K.) and R01 DK079902 (J.E.P.) from the Public Health Service. Dr. Gawron is a National Research Service Award postdoctoral fellow at the Institute for Healthcare studies under an institutional award from the Agency for Healthcare Research and Quality, T-32 HS 000078 (PI: Jane L. Holl, MD MPH).
The authors would like to acknowledge Gina P. Vozenilek, MA (Institute for Healthcare Studies, Northwestern University) for her assistance in preparing this manuscript.
Abbreviations
- GERD
gastroesophageal reflux disease
- PPI
proton pump inhibitor
- MII-pH
multichannel intraluminal impedance-pH
- VAS
visual analogue scale
Footnotes
Disclosures:
PJ Kahrilas serves as a consultant for AstraZeneca, Eisai, EndoGastric Solutions, Ironwood, Torax, and Reckitt Benckiser
J Pandolfino serves as a consultant for Given, Sandhill, and Shire and on the advisory board for Crospon
Author contributions:
AJG: Study concept and design, statistical analysis and interpretation of data, drafting of manuscript
JR: Study concept and design, acquisition of data, interpretation of data
AJF: Statistical analysis
AF: Acquisition of data
ET: Acquisition of data
LB: Acquisition of data
PJK: Analysis and interpretation of data, drafting of manuscript
JEP: Study concept and design, analysis and interpretation of data, drafting of manuscript, study supervision
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kahrilas P. Clinical practice. Gastroesophageal reflux disease. N Engl J Med. 2008;359:1700–1707. doi: 10.1056/NEJMcp0804684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Everhart JE, Ruhl CE. Burden of digestive diseases in the United States part I: overall and upper gastrointestinal diseases. Gastroenterology. 2009;136:376–86. doi: 10.1053/j.gastro.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 3.Sandler R, Everhart J, Donowitz M, Adams E, Cronin K, Goodman C, Gemmen E, Shah S, Avdic A, Rubin R. The burden of selected digestive diseases in the United States. Gastroenterology. 2002;122:1500–1511. doi: 10.1053/gast.2002.32978. [DOI] [PubMed] [Google Scholar]
- 4.Shaheen N, Hansen R, Morgan D, Gangarosa L, Ringel Y, Thiny M, Russo M, Sandler R. The burden of gastrointestinal and liver diseases, 2006. Am J Gastroenterol. 2006;101:2128–2138. doi: 10.1111/j.1572-0241.2006.00723.x. [DOI] [PubMed] [Google Scholar]
- 5.Liker HR, Ducrotte P, Malfertheiner P. Unmet medical needs among patients with gastroesophageal reflux disease: a foundation for improving management in primary care. Dig Dis. 2009;27:62–7. doi: 10.1159/000210106. [DOI] [PubMed] [Google Scholar]
- 6.Flook NW, Wiklund I. Accounting for the effect of GERD symptoms on patients' health-related quality of life: supporting optimal disease management by primary care physicians. Int J Clin Pract. 2007;61:2071–8. doi: 10.1111/j.1742-1241.2007.01586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones R, Patrikios T. The effectiveness of esomeprazole 40 mg in patients with persistent symptoms of gastro-oesophageal reflux disease following treatment with a full dose proton pump inhibitor. Int J Clin Pract. 2008;62:1844–50. doi: 10.1111/j.1742-1241.2008.01923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kahrilas P, Shaheen N, Vaezi M. American Gastroenterological Association Institute Technical Review on the Management of Gastroesophageal Reflux Disease. Gastroenterology. 2008;135:1392–1413. doi: 10.1053/j.gastro.2008.08.044. [DOI] [PubMed] [Google Scholar]
- 9.Pandolfino JE, Richter JE, Ours T, Guardino JM, Chapman J, Kahrilas PJ. Ambulatory esophageal pH monitoring using a wireless system. Am J Gastroenterol. 2003;98:740–749. doi: 10.1111/j.1572-0241.2003.07398.x. [DOI] [PubMed] [Google Scholar]
- 10.Srinivasan R, Vela M, Katz P, Tutuian R, Castell J, Castell D. Esophageal function testing using multichannel intraluminal impedance. Am J Physiol Gastrointest Liver Physiol. 2001;280:G457–62. doi: 10.1152/ajpgi.2001.280.3.G457. [DOI] [PubMed] [Google Scholar]
- 11.Vela M, Camacho-Lobato L, Srinivasan R, Tutuian R, Katz P, Castell D. Simultaneous intraesophageal impedance and pH measurement of acid and nonacid gastroesophageal reflux: effect of omeprazole. Gastroenterology. 2001;120:1599–606. doi: 10.1053/gast.2001.24840. [DOI] [PubMed] [Google Scholar]
- 12.Pandolfino JE, Vela MF. Esophageal-reflux monitoring. Gastrointest Endosc. 2009;69:917–30. 930, e1. doi: 10.1016/j.gie.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 13.Jones R, Junghard O, Dent J, Vakil N, Halling K, Wernersson B, Lind T. Development of the GerdQ, a tool for the diagnosis and management of gastro-oesophageal reflux disease in primary care. Aliment Pharmacol Ther. 2009;30:1030–1038. doi: 10.1111/j.1365-2036.2009.04142.x. [DOI] [PubMed] [Google Scholar]
- 14.EuroQol--a new facility for the measurement of health-related quality of life. The EuroQol Group. Health Policy. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 15.Gershon R, Rothrock NE, Hanrahan RT, Jansky LJ, Harniss M, Riley W. The development of a clinical outcomes survey research application: Assessment Center. Qual Life Res. 2010;19:677–85. doi: 10.1007/s11136-010-9634-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cella D, Yount S, Rothrock N, Gershon R, Cook K, Reeve B, Ader D, Fries JF, Bruce B, Rose M. The Patient-Reported Outcomes Measurement Information System (PROMIS): progress of an NIH Roadmap cooperative group during its first two years. Med Care. 2007;45:S3–S11. doi: 10.1097/01.mlr.0000258615.42478.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naunton M, Peterson GM, Bleasel MD. Overuse of proton pump inhibitors. J Clin Pharm Ther. 2000;25:333–40. doi: 10.1046/j.1365-2710.2000.00312.x. [DOI] [PubMed] [Google Scholar]
- 18.Heidelbaugh JJ, Goldberg KL, Inadomi JM. Overutilization of proton pump inhibitors: a review of cost-effectiveness and risk [corrected] Am J Gastroenterol. 2009;104 (Suppl 2):S27–32. doi: 10.1038/ajg.2009.49. [DOI] [PubMed] [Google Scholar]
- 19.Hollingworth S, Duncan EL, Martin JH. Marked increase in proton pump inhibitors use in Australia. Pharmacoepidemiol Drug Saf. 2010;19:1019–24. doi: 10.1002/pds.1969. [DOI] [PubMed] [Google Scholar]
- 20.Heidelbaugh JJ, Goldberg KL, Inadomi JM. Magnitude and economic effect of overuse of antisecretory therapy in the ambulatory care setting. Am J Manag Care. 2010;16:e228–34. [PubMed] [Google Scholar]
- 21.Heidelbaugh JJ, Inadomi JM. Magnitude and economic impact of inappropriate use of stress ulcer prophylaxis in non-ICU hospitalized patients. Am J Gastroenterol. 2006;101:2200–5. doi: 10.1111/j.1572-0241.2006.00839.x. [DOI] [PubMed] [Google Scholar]
- 22.George CJ, Korc B, Ross JS. Appropriate proton pump inhibitor use among older adults: a retrospective chart review. Am J Geriatr Pharmacother. 2008;6:249–54. doi: 10.1016/j.amjopharm.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 23.Friedenberg FK, Hanlon A, Vanar V, Nehemia D, Mekapati J, Nelson DB, Richter JE. Trends in gastroesophageal reflux disease as measured by the National Ambulatory Medical Care Survey. Dig Dis Sci. 2010;55:1911–7. doi: 10.1007/s10620-009-1004-0. [DOI] [PubMed] [Google Scholar]
- 24.van Boxel OS, Hagenaars MP, Smout AJ, Siersema PD. Socio-demographic factors influence chronic proton pump inhibitor use by a large population in the Netherlands. Aliment Pharmacol Ther. 2009;29:571–9. doi: 10.1111/j.1365-2036.2008.03900.x. [DOI] [PubMed] [Google Scholar]
- 25.Lacy BE, Chehade R, Crowell MD. A Prospective Study to Compare a Symptom-Based Reflux Disease Questionnaire to 48-h Wireless pH Monitoring for the Identification of Gastroesophageal Reflux (revised 2–26–11) Am J Gastroenterol. 2011;106:1604–11. doi: 10.1038/ajg.2011.180. [DOI] [PubMed] [Google Scholar]
- 26.El-Serag H, Becher A, Jones R. Systematic review: persistent reflux symptoms on proton pump inhibitor therapy in primary care and community studies. Aliment Pharmacol Ther. 2010;32:720–37. doi: 10.1111/j.1365-2036.2010.04406.x. [DOI] [PubMed] [Google Scholar]
- 27.Rubenstein JH, Nojkov B, Korsnes S, Adlis SA, Shaw MJ, Weinman B, Inadomi JM, Saad R, Chey WD. Oesophageal hypersensitivity is associated with features of psychiatric disorders and the irritable bowel syndrome. Aliment Pharmacol Ther. 2007;26:443–52. doi: 10.1111/j.1365-2036.2007.03393.x. [DOI] [PubMed] [Google Scholar]
- 28.van der Velden AW, de Wit NJ, Quartero AO, Grobbee DE, Numans ME. Maintenance treatment for GERD: residual symptoms are associated with psychological distress. Digestion. 2008;77:207–13. doi: 10.1159/000143796. [DOI] [PubMed] [Google Scholar]
- 29.Wiklund I, Carlsson R, Carlsson J, Glise H. Psychological factors as a predictor of treatment response in patients with heartburn: a pooled analysis of clinical trials. Scand J Gastroenterol. 2006;41:288–93. doi: 10.1080/00365520500292970. [DOI] [PubMed] [Google Scholar]
- 30.Wright CE, Ebrecht M, Mitchell R, Anggiansah A, Weinman J. The effect of psychological stress on symptom severity and perception in patients with gastro-oesophageal reflux. J Psychosom Res. 2005;59:415–24. doi: 10.1016/j.jpsychores.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 31.Johnston BT, Lewis SA, Collins JS, McFarland RJ, Love AH. Acid perception in gastro-oesophageal reflux disease is dependent on psychosocial factors. Scand J Gastroenterol. 1995;30:1–5. doi: 10.3109/00365529509093228. [DOI] [PubMed] [Google Scholar]
- 32.Chey W, Mody R, Wu E, Chen L, Kothari S, Persson B, Beaulieu N, Lu M. Treatment patterns and symptom control in patients with GERD: US community-based survey. Curr Med Res Opin. 2009;25:1869–1878. doi: 10.1185/03007990903035745. [DOI] [PubMed] [Google Scholar]
- 33.Haug TT, Wilhelmsen I, Svebak S, Berstad A, Ursin H. Psychotherapy in functional dyspepsia. J Psychosom Res. 1994;38:735–44. doi: 10.1016/0022-3999(94)90026-4. [DOI] [PubMed] [Google Scholar]
- 34.Delgado-Aros S, Cremonini F, Talley NJ. Treatment of Functional Dyspepsia. Curr Treat Options Gastroenterol. 2004;7:121–131. doi: 10.1007/s11938-004-0033-1. [DOI] [PubMed] [Google Scholar]
- 35.Kiebles JL, Kwiatek MA, Pandolfino JE, Kahrilas PJ, Keefer L. Do patients with globus sensation respond to hypnotically assisted relaxation therapy? A case series report. Dis Esophagus. 2010;23:545–53. doi: 10.1111/j.1442-2050.2010.01064.x. [DOI] [PubMed] [Google Scholar]
- 36.Iwakiri K, Kawami N, Sano H, Tanaka Y, Umezawa M, Kotoyori M, Hoshihara Y, Sakamoto C. Acid and non-acid reflux in Japanese patients with non-erosive reflux disease with persistent reflux symptoms, despite taking a double-dose of proton pump inhibitor: a study using combined pH-impedance monitoring. J Gastroenterol. 2009;44:708–12. doi: 10.1007/s00535-009-0070-6. [DOI] [PubMed] [Google Scholar]
- 37.Savarino E, Zentilin P, Tutuian R, Pohl D, Casa DD, Frazzoni M, Cestari R, Savarino V. The role of nonacid reflux in NERD: lessons learned from impedance-pH monitoring in 150 patients off therapy. Am J Gastroenterol. 2008;103:2685–93. doi: 10.1111/j.1572-0241.2008.02119.x. [DOI] [PubMed] [Google Scholar]
- 38.Sifrim D, Castell D, Dent J, Kahrilas PJ. Gastro-oesophageal reflux monitoring: review and consensus report on detection and definitions of acid, non-acid, and gas reflux. Gut. 2004;53:1024–31. doi: 10.1136/gut.2003.033290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hirano I, Richter JE. ACG practice guidelines: esophageal reflux testing. Am J Gastroenterol. 2007;102:668–85. doi: 10.1111/j.1572-0241.2006.00936.x. [DOI] [PubMed] [Google Scholar]
- 40.Lee WC, Yeh YC, Lacy BE, Pandolfino JE, Brill JV, Weinstein ML, Carlson AM, Williams MJ, Wittek MR, Pashos CL. Timely confirmation of gastro-esophageal reflux disease via pH monitoring: estimating budget impact on managed care organizations. Curr Med Res Opin. 2008;24:1317–27. doi: 10.1185/030079908x280680. [DOI] [PubMed] [Google Scholar]