Abstract
Neurotrophins can directly modulate the function of diverse types of central nervous system synapses. Brain-derived neurotrophic factor (BDNF) might be released by nociceptors onto spinal neurons and mediate central sensitization associated with chronic pain. We have studied the role of BDNF and neurotrophin-4 (NT-4), both ligands of the trkB tyrosine kinase receptor, in synaptic transmission and reflex plasticity in the mouse spinal cord. We used an in vitro spinal cord preparation to measure monosynaptic and polysynaptic reflexes evoked by primary afferents in BDNF- and NT-4-deficient mice. In situ hybridization studies show that both these neurotrophins are synthesized by sensory neurons, and NT-4, but not BDNF, also is expressed by spinal neurons. BDNF null mutants display selective deficits in the ventral root potential (VRP) evoked by stimulating nociceptive primary afferents whereas the non-nociceptive portion of the VRP remained unaltered. In addition, activity-dependent plasticity of the VRP evoked by repetitive (1 Hz) stimulation of nociceptive primary afferents (termed wind-up) was substantially reduced in BDNF-deficient mice. This plasticity also was reduced in a reversible manner by the protein kinase inhibitor K252a. Although the trkB ligand NT-4 is normally present, reflex properties in NT-4 null mutant mice were normal. Pharmacological studies also indicated that spinal N-methyl-d-aspartate receptor function was unaltered in BDNF-deficient mice. Using immunocytochemistry for markers of nociceptive neurons we found no evidence that their number or connectivity was substantially altered in BDNF-deficient mice. Our data therefore are consistent with a direct role for presynaptic BDNF release from sensory neurons in the modulation of pain-related neurotransmission.
Brain-derived neurotrophic factor (BDNF) and neurotrophin-4 (NT-4) are structurally related neurotrophins that act through a common tyrosine kinase trkB receptor and regulate neuronal survival and differentiation (1). In addition, it is now known that neurotrophins are powerful modulators of both peripheral and central nervous system synapses (2). Thus both basal synaptic transmission and long-term synaptic plasticity can be regulated by neurotrophins (3).
In the spinal cord, a form of synaptic plasticity termed central sensitization underlies many forms of hyperalgesia, and neurotrophins play an important role in this process (4, 5). For example, the hyperalgesia and pain associated with peripheral inflammation depends on up-regulation of nerve growth factor (NGF) in inflamed tissues (6–8). NGF can directly sensitize primary afferent nociceptors expressing the trkA receptor and also indirectly increase primary afferent connectivity in the spinal cord (6, 9, 10, 11). BDNF is synthesized by trkA-positive sensory neurons, and NGF treatment dramatically increases levels of BDNF in these neurons (12). Furthermore, BDNF is anterogradely transported to the spinal terminals of nociceptive neurons where it is localized in dense core vesicles and presumably is released onto first-order spinal neurons (13).
Pharmacological studies recently have addressed the role of BDNF in spinal cord sensitization (14, 15). They report that inflammation-evoked changes in spinal sensory processing can be blocked by intrathecal administration of trkB-IgG fusion proteins that sequester BDNF. However, such methods have no selectivity for NT-4 or BDNF and may not be sensitive enough to reveal changes in basal nociceptive transmission. Because BDNF is constitutively expressed by small-diameter sensory neurons and has been demonstrated to rapidly modify synaptic efficacy, we have investigated the role of the trkB ligands in acute spinal nociception.
We examined spinal reflexes in mice deficient in BDNF and NT-4 by using an in vitro hemisected spinal cord preparation. We report that nociceptive, but not non-nociceptive, reflexes were substantially reduced in BDNF-deficient mice but not NT-4-deficient mice. Moreover, wind-up, the activity-dependent facilitation of this reflex that follows repetitive stimulation, also was attenuated in mice lacking BDNF. The plasticity of the reflex also could be reversibly reduced in the presence of a trk receptor blocker, K252a (16, 17). We further evaluated the relative contribution of presynaptic and postsynaptic mechanisms in BDNF null mutant mice and suggest that endogenous BDNF may act rapidly and directly to modify nociceptive reflexes and reflex plasticity in the spinal cord.
Methods
Knockout Mice.
We examined spinal reflexes by using age-matched littermates of BDNF+/+, BDNF+/−, and BDNF−/− mice. The generation of these mice originally was described by Korte et al. (18). NT-4 mice were obtained from The Jackson Laboratory (002497) with an appropriate control strain (129S3, catalogue 002448). Experiments were carried out blindly as to the genotype and then mice were genotyped by using standard PCR protocols (19).
Immunocytochemistry and Isolectin B4 (IB4) Staining.
We used a rabbit polyclonal anti-calcitonin gene-related peptide (CGRP) antibody (Sigma) to detect CGRP in peptidergic sensory neurons and FITC-labeled IB4 to identify nonpeptidergic nociceptors. Tissue from P4-P7 neonatal mice was fixed in 4% paraformaldehyde, and frozen spinal columns were sectioned (10 μm) and prepared as described (20). Sections were incubated with CGRP antibody and CY3-conjugated goat anti-rabbit IgG followed by FITC-labeled IB4.
To assess the size of the nociceptive sensory neuron population, the number of positively stained CGRP and IB4 neurons per section from the L4 and L5 dorsal root ganglion (DRG) were counted. Sections from three BDNF+/+ mice and three BDNF−/− mice were analyzed in parallel.
The termination pattern of peptidergic nociceptors in the lumbar spinal cord was examined by using openlab software (Improvision, Coventry, U.K.). We measured the depth of the CGRP staining in the medial dorsal horn and quantified the density of this staining in two 400-μm2 grids placed over the labeled regions of the medial superficial dorsal horn. These measurements were made from three BDNF+/+ mice and three BDNF−/− mice analyzed in parallel.
In Situ Hybridization.
In situ hybridization was performed with 10-μm cryosections of lumbar DRG and spinal cord taken from P4-P7 neonatal mice as described (20). Sections were hybridized with digoxygenin-labeled cRNA probes of 600 bp complementary to NT-4 and 600 bp complementary to BDNF. The same sequences were used to synthesize sense probes.
Electrophysiology.
An in vitro hemisected spinal cord preparation was used to record spinal cord reflexes as described (21). Spinal cords were removed from neonatal mice (P5-P9) and were hemisected down the midline, placed in a Perspex recording chamber, and superfused with oxygenated modified Krebs solution (21). This preparation does not appear to be viable in older animals probably due to increasing myelin content of the cord. Only one hemisected cord was used per mouse. Direct current recordings were made after a 2-h recovery period with a close-fitting glass suction electrode attached to the L5 ventral root. The L5 dorsal root was stimulated via a glass suction electrode at current sufficient to activate C fibers (500 μA, 500 μs), and wind-up was evoked by repetitive stimulation at 1 Hz for 20 s. The A fiber-mediated component of the ventral root potential (VRP) was measured as the maximal response to dorsal root stimulation, and C-fiber responses were measured as the response 2 s after stimulation and as the integrated area of the VRP. Wind-up was determined by normalizing the increase in size of the VRP to the initial magnitude and expressing it as a percentage increase. Traces were acquired with the Powerlab 4.0 system using superscope software.
Drugs were applied by perfusing the whole spinal cord. 6-Cyano-7-nitroquinoxaline-2,3-dione (20 μM), dl-2-amino-5-phosphovaleric acid (APV, 20 μM), K252a (150 nM), and K252b (200 nM) were dissolved in Krebs solution and recirculated for 20 min. Recordings were made before antagonist application, immediately after application, and at 20-min intervals during an additional 1-h washout with Krebs solution.
Unless otherwise stated t tests (paired or unpaired as appropriate) were carried out to determine statistical significance. All values are given as mean ± SEM.
Results
Properties of the Mouse VRP.
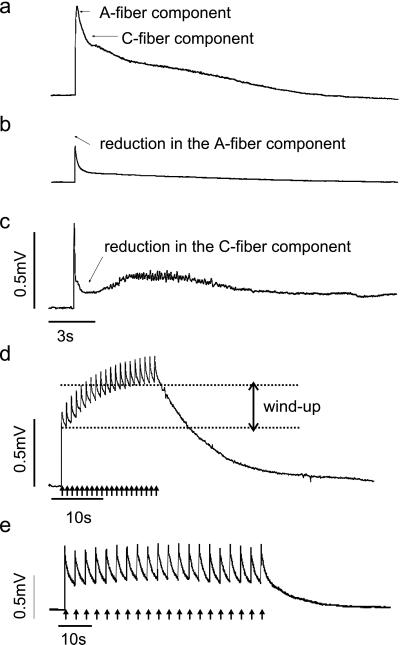
We used an in vitro neonatal mouse hemisected spinal cord preparation to examine the role of NT-4 and BDNF in spinal sensory processing. This preparation has been well characterized in the rat (15). Ventral root reflexes were evoked by electrically stimulating primary afferents in the dorsal root at high intensity (500 μA, 500 μs). This stimulus activates C fibers, which evoke a long-lasting VRP recorded as a direct current potential from the segmental ventral root via a suction electrode. As in the rat, the VRP has an initial monosynaptic short latency component evoked by rapidly conducting Aβ afferents and a late polysynaptic component that is only activated by C-fiber strength stimuli (22) (Fig. 1a). These two components are also pharmacologically distinct; α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor antagonists (20 min perfusion of 20 μM 6-cyano-7-nitroquinoxaline-2,3-dione) block the A-fiber component and N-methyl-d-aspartate (NMDA) receptor antagonists (20 min of 20 μM APV) reduce the later C fiber-evoked response (22) (Fig. 1c).
Figure 1.
Properties of the neonatal mouse VRP. (a) High-intensity stimulation (500 μA, 500 μs) of dorsal roots evokes a long-lasting VRP that can be recorded from the ventral roots. The neonatal mouse VRP consists of an initial short latency component evoked by A fibers and a slower long duration component activated by C fibers. (b) Perfusion of the spinal cord with AMPA receptor antagonist 6-cyano-7-nitroquinoxaline-2,3-dione (20 μM) for 20 min reduces the A-fiber component of the VRP. (c) A 20-min perfusion of 20 μM APV (NMDA antagonist) reduces the C-fiber component of the VRP. The increased noise in the record between 3 and 10 s after the stimulus is biological and probably represents late asynchronous firing of motoneurons. (d) Repetitive stimulation (500 μA, 500 μs) at 1 Hz for 20 s brings about a frequency-dependent facilitation of the VRP that we termed wind-up. (e) Stimulation at 0.3 Hz does not produce wind-up
Repetitive electrical stimulation of afferent fibers at C-fiber strength results in a progressive, frequency-dependent facilitation of spinal neuronal responses termed wind-up (23). In the hemisected spinal cord preparation, wind-up is evoked by repetitive stimulation (500 μA, 500 μs) at 1 Hz for 20 s and is measured as the increase in amplitude from the initial to the final response (Fig. 1d). As in the rat, wind-up can be partially blocked by application of an NMDA receptor antagonist and is activity-dependent such that stimuli given at 0.3 Hz do not produce wind-up (Fig. 1e).
BDNF and NT-4 mRNA Expression in the Spinal Cord and DRG of Neonatal Mice.
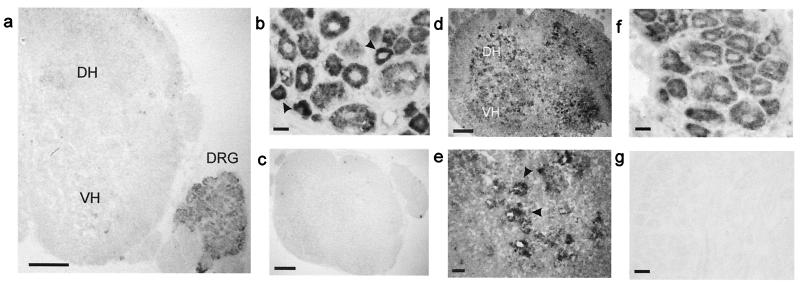
We used nonradioactive in situ hybridization to detect BDNF and NT-4 mRNA expression in the DRG and spinal cord of P7 mice (Fig. 2). BDNF mRNA expression was detected in most neurons of the DRG, and as in the adult, it was most prominent in smaller neurons (12, 13). BDNF mRNA was not detectable in the spinal cord of animals at this age (Fig. 2a). In contrast NT-4 was abundantly expressed by both DRG neurons and spinal cord neurons (Fig. 2 d–f). In the spinal cord NT-4 expression was very prominent in motoneuron pools of the ventral horn (Fig. 2 d and e).
Figure 2.
BDNF and NT-4 expression in neonatal mouse spinal cord and DRG. (a) Image of a neonatal spinal cord with DRG attached showing no BDNF expression in the spinal cord (DH, dorsal horn and VH, ventral horn). (b) BDNF mRNA is expressed by DRG neurons with more prominent expression in smaller cells (arrows). (c) BDNF sense probe does not hybridize. (d) NT-4 is expressed in the spinal cord of the neonatal mouse, (e) predominantly around ventral horn motor neurons (arrows). (f) Most neurons in the DRG express NT-4 mRNA. (g) NT-4 sense probe control. (Scale bars: a, c, and d, 200 μm; b and e–g, 20 μm.)
NT-4 and BDNF Differentially Modulate Spinal Cord Neuronal Excitability.
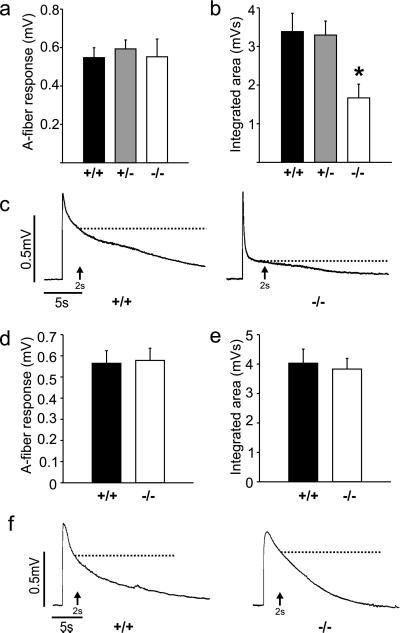
We investigated the functions of BDNF and NT-4 in the spinal cord by analyzing the VRP recorded from heterozygote BDNF+/− mice (n = 37), BDNF−/− null mice (n = 7), NT-4−/− null mutant mice (n = 16), and wild-type littermates (BDNF+/+, n = 20; NT-4+/+, n = 18). By examining the magnitude of the VRP at different latencies we could assess the contribution of trkB ligands to non-nociceptive A fiber-evoked and nociceptive C fiber-evoked reflexes (Fig. 3). The A fiber-mediated component, measured as the amplitude of the initial peak of the VRP, was unchanged in both BDNF and NT-4 mutants. However, the prolonged C-fiber response was significantly (P < 0.05) reduced in BDNF−/− but not in NT-4−/− mice. This late component can be measured as the amplitude (at 2 s) or integrated area of the VRP (the majority of the VRP is composed of C fiber-evoked activity). By both measures, spinal cords taken from BDNF−/− mice displayed a significantly reduced C fiber-evoked response (57 ± 9% of control amplitude, 49 ± 10% of control integrated area), suggesting that BDNF plays an important role in basal nociceptive transmission in the spinal cord.
Figure 3.
Quantification of the VRP in BDNF and NT-4 knockout mice. (a) A fiber-evoked responses of the VRP are not different between BDNF+/+, BDNF+/−, or BDNF−/− mice. (b) The integrated area of the VRP mainly reflects C fiber-evoked activity, which is significantly less in BDNF−/− mice (P < 0.05). (c) Representative examples of VRPs from BDNF+/+ and BDNF−/− mice. (d) A fiber-evoked responses in NT-4−/− mice are not different from control. (e) C fiber-evoked responses measured as the integrated area of the VRP are unchanged in NT-4−/− mice. (f) Examples of the VRP in NT-4+/+ and NT-4−/− mice. Dotted lines in the physiological traces indicate where the amplitude of the potential was measured for quantification.
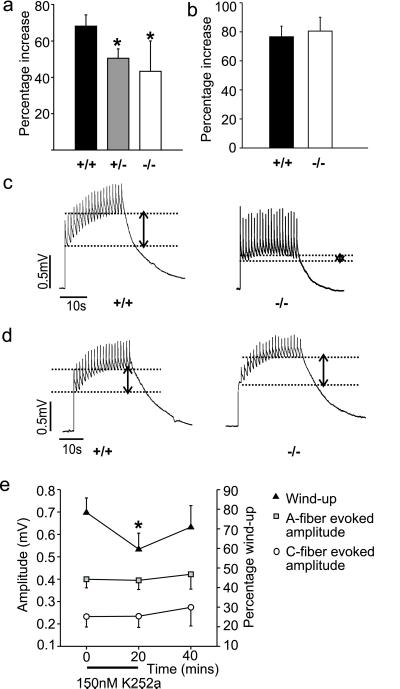
Wind-up of the VRP also was measured in spinal cords taken from mutant and wild-type animals. There was a significant attenuation of wind-up in BDNF+/− and BDNF−/− mice but no change was observed in NT-4−/− mice (Fig. 4). Note that repetitive stimulation of afferent fibers from control mice brought about an equivalent increase in amplitude in both control strains (69 ± 6% in BDNF+/+ animals and 76 ± 7% in NT-4+/+ animals). Thus BDNF, but not NT-4, is necessary for normal wind-up of the nociceptive ventral root reflex.
Figure 4.
Wind-up in BDNF and NT-4 knockout mice (a) Wind-up is significantly reduced in both BDNF+/− and BDNF−/− mice (P < 0.05). (b) NT-4−/− mice display normal wind-up. (c) Examples of wind-up in BDNF+/+ and BDNF−/− mice. (d) Examples of wind-up in NT-4+/+ and NT-4−/− mice. Dotted lines on the physiological records indicate the magnitude of facilitation measured in each case. (e) The protein tyrosine kinase blocker K252a reversibly reduced the magnitude of the wind-up in control spinal cords. No effect was observed on the magnitude of the A and C fiber-evoked potentials.
In wild-type spinal cord in the presence of 150 nM K252a, a protein kinase blocker that is selective for neurotrophin trk receptors at this concentration (16, 17), wind-up was reversibly and significantly inhibited (30% reversal, P < 0.05) (Fig. 4e). The magnitude of the A fiber- and C fiber-evoked potential was not changed by the same dose of K252a (Fig. 4e). Application of the structurally related protein kinase inhibitor K252b (200 nM), which is less effective at trk receptors than K252a (16), did not alter spinal reflexes or wind-up (data not shown).
The Nociceptor Population Is Unaltered in BDNF−/− Mice.
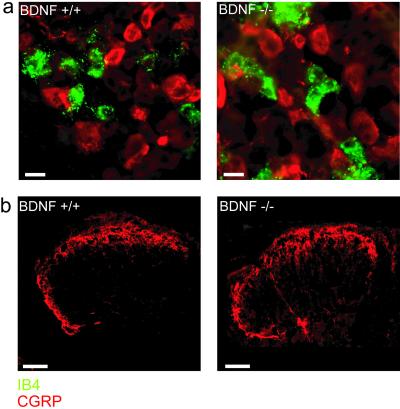
Our data indicate that BDNF is required to maintain normal basal nociceptive transmission in the spinal cord (Fig. 3). The reduction in the size of C fiber-evoked potential might, however, reflect a reduction in the number or connectivity of nociceptive sensory neurons. Small-diameter sensory neurons, which are probably nociceptors (24), can be divided into two major neurochemical subtypes: one contains neuropeptides such as CGRP, the other binds IB4 (25). We therefore counted the number of neurons positive for IB4 or CGRP in cryostat sections of the lumbar DRGs taken from neonatal BDNF+/+ and BDNF−/− mice. We observed no difference in the number of IB4-positive and CGRP-positive neurons in the DRG between wild-type and mutant mice (Fig. 5). More relevant for the nociceptive reflex is probably the presence of normal nociceptor synaptic terminals within the superficial dorsal horn in mice lacking BDNF. Thus we also examined the termination pattern of CGRP-positive sensory terminals in the superficial dorsal horn (Fig. 5). We measured the density of immunostained terminals in the lateral and medial superficial dorsal horn and the dorsal/ventral depth of the immunostained fibers. In spinal cord sections from both wild-type and mutant mice, CGRP-positive sensory fibers in the dorsal horn appeared normal, the density of immunostained terminals was not different (BDNF+/+ 220 ± 1, BDNF−/− 195 ± 8; P = 0.12, Mann–Whitney u test), and the dorso-ventral depth of the termination zone also was not changed in BDNF−/− mice compared with wild-type controls (BDNF+/+ 33 ± 2 μm, BDNF−/− 25 ± 1 μm, P = 0.17, Mann–Whitney u test). Taken together these data suggest that nociceptors in BDNF-deficient mice are present in normal numbers and make normal anatomical connections with first-order spinal neurons.
Figure 5.
CGRP and IB4 staining in spinal cord and DRG from BDNF+/+ and BDNF−/− mice. (a) CGRP (red) and IB4 (green) expression are not significantly different between BDNF+/+ (n = 3) and BDNF−/− mice (n = 3). We counted cells expressing these markers to investigate the size of the nociceptor population in mutant and wild-type animals. CGRP was expressed by 18 ± 0.7 neurons per section in BDNF+/+ mice and 15 ± 1 neurons per section in BDNF−/− mice (P = 0.25). IB4 stained 19 ± 1 cells per section in BDNF+/+ animals and 15 ± 8 per section in −/− animals (P = 0.78). Little or no overlap between IB4-positive and CGRP-positive sensory neurons was observed at this age. (Scale bars: 20 μm.) (b) Sensory neurons positive for CGRP have a similar termination pattern in the dorsal horn of the spinal cord in both BDNF+/+ and BDNF−/− mice. (Scale bars: 100 μm.)
NMDA Receptors Do Not Contribute to Reduced Reflex Excitability in BDNF-Deficient Mice.
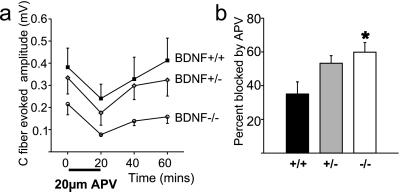
The data above are consistent with rapid release of BDNF from nociceptive primary afferents having direct fast actions on postsynaptic spinal cord neurons. Modulation of NMDA receptors by trkB activation has been described (26), and BDNF can potentiate NMDA-evoked responses in the spinal cord (15). Accordingly, we have investigated the role of spinal NMDA receptors in BDNF+/+ and BDNF−/− mice by comparing the size of the NMDA receptor-dependent component of the VRP and wind-up in these animals. We perfused spinal cords from BDNF+/+ and BDNF−/− animals with an NMDA receptor antagonist (20 μM APV for 20 min) and measured the reduction in the VRP and wind-up. We found that APV reduced the C fiber-evoked component of the VRP equally in both experimental groups (BDNF+/+ 0.14 ± 0.04 mV, BDNF−/− 0.13 ± 0.04 mV) (Fig. 6a). Thus, in BDNF-deficient mice, NMDA receptors appear unaltered, but loss of an NMDA-independent component of the VRP appears to increase the relative contribution made by NMDA receptors to the nociceptive reflex (Fig. 6b). Wind-up also was inhibited by 20 μM APV to an equal extent in BDNF+/+ animals (reduction by 19 ± 16%) as it was in BDNF−/− animals (reduction by 20 ± 20%). Therefore the contribution of BDNF to the synaptic plasticity associated with reflex facilitation does not appear to involve modulation of NMDA receptor activity in the spinal cord.
Figure 6.
NMDA receptor function is not altered in the spinal cord of BDNF−/− mice. (a) Spinal cords from BDNF+/+ (n = 6), BDNF+/− (n = 8), and BDNF−/− (n = 5) mice display similar reduction and recovery of the C-fiber component of the VRP after perfusion of 20 μM APV for 20 min. (b) A significantly higher proportion of the nociceptive reflex is NMDA-dependent in BDNF−/− mice (P < 0.05).
Discussion
These results indicate that BDNF, but not NT-4, is an essential component of nociceptive spinal sensory processing in vivo. We demonstrate that although both of these trkB ligands are expressed by sensory neurons of the DRG, only BDNF and not NT-4 null mutant animals display deficits in nociceptive reflex properties and reflex plasticity. We have further analyzed these deficits and find no substantial loss of nociceptive sensory neurons in BDNF null mutant mice, implying that the reduced nociceptive reflex is not caused by a quantitative reduction in presynaptic nociceptive input or loss of connectivity. Acute pharmacological blockade of kinase receptor signaling with K252a reversibly reduced the magnitude of the flexion reflex facilitation associated with repetitive C-fiber stimulation, thus demonstrating that fast actions of the BDNF release in the spinal cord account in part for deficits seen in BDNF mutants. Although BDNF application to isolated spinal cord can potentiate NMDA-evoked VRPs (15) we found no evidence that NMDA receptor function is negatively modulated in BDNF null mice. Our data are consistent with a role for BDNF release from presynaptic nociceptive sensory neurons in modulating or even mediating reflex excitability.
We detected BDNF mRNA expression in DRG neurons but not in spinal cord neurons. This finding is in broad agreement with previous reports on neurotrophin localization in adult animals, specifically, prominent expression of BDNF by small-diameter sensory neurons (12, 13). It is therefore likely that, as in the adult animal, BDNF is anterogradely transported to the central terminals of these (presumably nociceptive) neurons and, after a sufficient stimulus, is released into the spinal cord. NT-4 mRNA was found by us and by others (27, 28), to be present both in DRG and spinal neurons.
The role of trkB ligands in spinal sensory processing recently has been investigated in the context of sustained nociception and peripheral inflammation (14, 15). Because NGF brings about a large increase in the expression of BDNF in sensory neurons (12, 13), it was proposed that BDNF might be a central mediator of the pro-nociceptive action of NGF. In two separate studies, baseline nociceptive responses were not reduced by intrathecal application of trkB-IgG, but increases in behavioral and spinal reflexes evoked by inflammation (15) or the progressive hypersensitivity elicited by low-intensity tactile stimulation of inflamed tissue were attenuated (14). By examining spinal reflexes in mice deficient in BDNF and NT-4 we could determine the role of endogenous BDNF or NT-4 without pharmacological complications, such as impaired penetration and nonselective blockade of both BDNF and NT-4, associated with BDNF-sequestering proteins. Despite the fact that NT-4 is abundantly expressed in DRG and spinal cord we found that NT-4-deficient mice have a normal VRP and associated plasticity. In contrast, mice deficient in BDNF displayed a selective reduction in ventral root reflex to nociceptive strength stimulation. Thus BDNF appears to be necessary for normal baseline nociceptive responses in the spinal cord. We also show that BDNF is required for the activity-dependent increase (wind-up) in reflex excitability that follows repetitive nociceptive stimulation. Wind-up is an experimental correlate of the increased neuronal excitability that follows sustained noxious input such as that produced by acute injury (23). These data therefore further support a role for BDNF in sensitization of spinal cord circuits after an inflammatory stimulus (14, 15). We found no significant correlation in normal mice between the magnitude of the VRP and the degree of reflex facilitation (no significant correlation, r2 = 0.08, n = 54). Thus the decrease in wind-up observed in BDNF null mutant mice may be unrelated to the decreased C fiber-evoked VRP. In agreement with this interpretation wind-up was also significantly attenuated in BDNF+/− animals and in cords treated with the protein kinase inhibitor K252a. In both cases no significant change in the baseline nociceptive reflex magnitude was observed (see also ref. 21).
NT-4 is also a ligand for trkB receptors and is expressed by sensory neurons; however, we found no deficit in spinal cord reflex properties in NT-4−/− mice. In the hippocampus both NT-4 and BDNF mutant mice display reduced plasticity in hippocampal slices (18, 29, 30). Using mice with a mutated trkB receptor that cannot recruit Shc adaptor proteins it has recently been shown that NT-4 and BDNF might use different signal transduction pathways through the same receptor (31). Thus distinct roles for NT-4 and BDNF in spinal synaptic transmission might conceivably be mediated by the same receptor.
Published cell counts from the DRG of BDNF null mutant mice reveal a decrease of about 30% in the total sensory neuron population (32). In a previous study we showed that cutaneous myelinated sensory neurons are not reduced in number in BDNF mutant mice (19), which is consistent with the present study where the A fiber-evoked VRP was unchanged in BDNF null mutants (Fig. 3). Using immunocytochemical markers that identify nociceptors with projections to the superficial dorsal horn we found no evidence in the present study of a substantial loss of nociceptive sensory neurons. The termination pattern of CGRP-positive sensory neurons, which also express the NGF receptor trkA and also probably release BDNF (12, 13), was grossly normal in the spinal cord. It is still conceivable that the efficacy of synaptic transmission is reduced in BDNF mutants because of a developmental delay in these animals, resulting in for example, slower sensory terminal maturation or reduced numbers or availability of synaptic vesicles (33).
BDNF has been shown to have rapid modulatory effects at mammalian synapses in the hippocampus and cortex (2, 3). In primary sensory neurons, BDNF is localized in dense core vesicles at their central terminals (13), and recent evidence indicates that in visceral sensory neurons it can be released after patterned electrical stimulation (34). This finding suggests that BDNF might be released from presynaptic terminals in a similar manner to conventional peptide neurotransmitters such as substance P (35).
A number of studies have addressed the mechanisms of action of synaptically released BDNF. In the hippocampus, BDNF has been shown to act on presynaptic neurons and enhance vesicle release (3). In the spinal cord, BDNF is not produced postsynaptically (Fig. 2) and therefore a presynaptic mechanism would require that trkB receptors are present on the central terminals of nociceptive C fibers. However, trkB receptors are expressed predominantly by large- and medium-diameter sensory neurons (36). Furthermore, functional deficits have so far only been described in non-nociceptive sensory neurons in BDNF-deficient mice (19). We have therefore primarily considered postsynaptic effects. Two potential targets for trkB-mediated modulation are AMPA and NMDA receptors. BDNF has previously been demonstrated to inhibit AMPA-mediated currents in postsynaptic sensory relay neurons (37). However, AMPA receptors mediate the initial A fiber-sensitive component of the VRP and this component was unchanged in BDNF−/− mice.
NMDA receptors are strongly recruited by nociceptive stimuli and mediate much of the polysynaptic C fiber-evoked discharge from the spinal cord (16). Furthermore, in hippocampal neurons, trkB receptor activation leads to phosphorylation of NMDA receptor subunits I and 2B (26, 38) and increases the open probability of these channels (39, 40). We compared the size of the NMDA receptor-dependent component of the VRP in BDNF+/+ and BDNF−/− animals. If BDNF is released by an activity-dependent process and acts as a neuromodulator via NMDA receptors, it follows that a deficiency in BDNF also should lead to a reduction in the NMDA receptor-dependent activity. Thus a reduced sensitivity to NMDA receptor antagonist would indicate that less synaptic activity is being driven by NMDA receptors. However, we found that APV blocked the nociceptive reflex equally in mutant and wild-type spinal cords, hence the NMDA receptor component appeared unaltered in the absence of BDNF. Therefore the reduction in C fiber-evoked potential in BDNF−/− mice appears to occur independently of modulation of NMDA receptor function and may involve some other element of the nociceptive reflex.
Recently, Kafitz et al. (41) demonstrated that neurotrophins can rapidly elicit action potentials in central nervous system neurons independently of AMPA and NMDA receptors. It is therefore possible that in the absence of changes to the presynaptic C-fiber population or to postsynaptic AMPA and NMDA receptor components of the VRP, BDNF−/− mice display a reduced nociceptive reflex because BDNF directly excites dorsal horn neurons in the spinal cord. However, our finding that K252a does not reduce the baseline reflex amplitude (Fig. 4e) argues against a role for BDNF in mediating the VRP at least via trkB activation.
The present experiments indicate that BDNF, but not NT-4, acts rapidly in the spinal cord to potentiate neuronal responses. This effect is restricted to polysynaptic nociceptive reflexes and is not seen in reflexes evoked by non-nociceptive A fibers. Thus BDNF is necessary for the maintenance of basal nociceptive responses and plasticity in the spinal cord and exhibits many of the physiological characteristics of a primary afferent neuropeptide transmitter in the pain pathway.
Acknowledgments
We thank Anke Kanehl and Heike Thränhardt for excellent technical assistance. This work was supported by a Deutsche Forschungsgemeinschaft grant (SPP 1026) to G.R.L. and a Marie Curie fellowship to P.A.H.
Abbreviations
- BDNF
brain-derived neurotrophic factor
- NT-4
neurotrophin-4
- VRP
ventral root potential
- NMDA
N-methyl-d-aspartate
- NGF
nerve growth factor
- CGRP
calcitonin gene-related peptide
- DRG
dorsal root ganglion
- APV
2-amino-5-phosphovaleric acid
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- IB4
isolectin B4
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Lewin G R, Barde Y A. Annu Rev Neurosci. 1996;19:289–317. doi: 10.1146/annurev.ne.19.030196.001445. [DOI] [PubMed] [Google Scholar]
- 2.Berninger B, Poo M. Curr Opin Neurobiol. 1996;6:324–330. doi: 10.1016/s0959-4388(96)80115-2. [DOI] [PubMed] [Google Scholar]
- 3.Lu B, Chow A. J Neurosci Res. 1999;58:76–87. [PubMed] [Google Scholar]
- 4.Lewin G R, Mendell L M. Trends Neurosci. 1993;16:353–359. doi: 10.1016/0166-2236(93)90092-z. [DOI] [PubMed] [Google Scholar]
- 5.Heppenstall P A, Lewin G R. Curr Opin Anaesthesiol. 2000;13:563–566. doi: 10.1097/00001503-200010000-00015. [DOI] [PubMed] [Google Scholar]
- 6.Lewin G R, Rueff A, Mendell L M. Eur J Neurosci. 1994;6:1903–1912. doi: 10.1111/j.1460-9568.1994.tb00581.x. [DOI] [PubMed] [Google Scholar]
- 7.Woolf C J, Safieh-Garabedian B, Ma Q P, Crilly P, Winter J. Neuroscience. 1994;62:327–331. doi: 10.1016/0306-4522(94)90366-2. [DOI] [PubMed] [Google Scholar]
- 8.McMahon S B, Bennett D L, Priestley J V, Shelton D L. Nat Med. 1995;1:774–780. doi: 10.1038/nm0895-774. [DOI] [PubMed] [Google Scholar]
- 9.Lewin G R, Winter J, McMahon S B. Eur J Neurosci. 1992;4:700–707. doi: 10.1111/j.1460-9568.1992.tb00179.x. [DOI] [PubMed] [Google Scholar]
- 10.Lewin G R, Ritter A M, Mendell L M. J Neurosci. 1993;13:2136–2148. doi: 10.1523/JNEUROSCI.13-05-02136.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shu X, Mendell L M. Neurosci Lett. 1999;274:159–162. doi: 10.1016/s0304-3940(99)00701-6. [DOI] [PubMed] [Google Scholar]
- 12.Apfel S C, Wright D E, Wiideman A M, Dormia C, Snider W D, Kessler J A. Mol Cell Neurosci. 1996;7:134–142. doi: 10.1006/mcne.1996.0010. [DOI] [PubMed] [Google Scholar]
- 13.Michael G J, Averill S, Nitkunan A, Rattray M, Bennett D L, Yan Q, Priestley J V. J Neurosci. 1997;17:8476–8490. doi: 10.1523/JNEUROSCI.17-21-08476.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mannion R J, Costigan M, Decosterd I, Amaya F, Ma Q P, Holstege J C, Ji R R, Acheson A, Lindsay R M, Wilkinson G A, Woolf C J. Proc Natl Acad Sci USA. 1999;96:9385–9390. doi: 10.1073/pnas.96.16.9385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kerr B J, Bradbury E J, Bennett D L, Trivedi P M, Dassan P, French J, Shelton D B, McMahon S B, Thompson S W. J Neurosci. 1999;19:5138–5148. doi: 10.1523/JNEUROSCI.19-12-05138.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knusel B, Hefti F. J Neurochem. 1992;59:1987–1996. doi: 10.1111/j.1471-4159.1992.tb10085.x. [DOI] [PubMed] [Google Scholar]
- 17.Arvanov V L, Seebach B S, Mendell L M. J Neurophysiol. 2000;84:752–758. doi: 10.1152/jn.2000.84.2.752. [DOI] [PubMed] [Google Scholar]
- 18.Korte M, Carroll P, Wolf E, Brem G, Thoenen H, Bonhoeffer T. Proc Natl Acad Sci USA. 1995;92:8856–8860. doi: 10.1073/pnas.92.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carroll P, Lewin G R, Koltzenburg M, Toyka K V, Thoenen H. Nat Neurosci. 1998;1:42–46. doi: 10.1038/242. [DOI] [PubMed] [Google Scholar]
- 20.Mannsfeldt A G, Carroll P, Stucky C L, Lewin G R. Mol Cell Neurosci. 1999;13:391–404. doi: 10.1006/mcne.1999.0761. [DOI] [PubMed] [Google Scholar]
- 21.Pesquero J B, Araujo R C, Heppenstall P A, Stucky C L, Silva J A, Jr, Walther T, Oliveira S M, Pesquero J L, Paiva A C, Calixto J B, et al. Proc Natl Acad Sci USA. 2000;97:8140–8145. doi: 10.1073/pnas.120035997. . (First Published June 20, 2000, 10.1073/pnas.120035997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thompson S W, Gerber G, Sivilotti L G, Woolf C J. Brain Res. 1992;595:87–97. doi: 10.1016/0006-8993(92)91456-o. [DOI] [PubMed] [Google Scholar]
- 23.Herrero J F, Laird J M, Lopez-Garcia J A. Prog Neurobiol. 2000;61:169–203. doi: 10.1016/s0301-0082(99)00051-9. [DOI] [PubMed] [Google Scholar]
- 24.Stucky C L, Lewin G R. J Neurosci. 1999;19:6497–6505. doi: 10.1523/JNEUROSCI.19-15-06497.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagy J I, Hunt S P. Neuroscience. 1982;7:89–97. doi: 10.1016/0306-4522(82)90155-5. [DOI] [PubMed] [Google Scholar]
- 26.Lin S Y, Wu K, Levine E S, Mount H T, Suen P C, Black I B. Brain Res Mol Brain Res. 1998;55:20–27. doi: 10.1016/s0169-328x(97)00349-5. [DOI] [PubMed] [Google Scholar]
- 27.Liebl D J, Klesse L J, Tessarollo L, Wohlman T, Parada L F. Proc Natl Acad Sci USA. 2000;97:2297–2302. doi: 10.1073/pnas.040562597. . (First Published February 18, 2000, 10.1073/pnas.040562597) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buck C R, Seburn K L, Cope T C. J Comp Neurol. 2000;416:309–318. doi: 10.1002/(sici)1096-9861(20000117)416:3<309::aid-cne3>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 29.Xie C W, Sayah D, Chen Q S, Wei W Z, Smith D, Liu X. Proc Natl Acad Sci USA. 2000;97:8116–8121. doi: 10.1073/pnas.140204597. . (First Published June 27, 2000, 10.1073/pnas.140204597) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patterson S L, Abel T, Deuel T A, Martin K C, Rose J C, Kandel E R. Neuron. 1996;16:1137–1145. doi: 10.1016/s0896-6273(00)80140-3. [DOI] [PubMed] [Google Scholar]
- 31.Minichiello L, Casagranda F, Tatche R S, Stucky C L, Postigo A, Lewin G R, Davies A M, Klein R. Neuron. 1998;21:335–345. doi: 10.1016/s0896-6273(00)80543-7. [DOI] [PubMed] [Google Scholar]
- 32.Ernfors P, Lee K F, Jaenisch R. Nature (London) 1994;368:147–150. doi: 10.1038/368147a0. [DOI] [PubMed] [Google Scholar]
- 33.Pozzo-Miller L D, Gottschalk W, Zhang L, McDermott K, Du J, Gopalakrishnan R, Oho C, Sheng Z H, Lu B. J Neurosci. 1999;19:4972–4983. doi: 10.1523/JNEUROSCI.19-12-04972.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Balkowiec A, Katz D M. J Neurosci. 2000;20:7417–7423. doi: 10.1523/JNEUROSCI.20-19-07417.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duggan A W, Riley R C, Mark M A, MacMillan S J, Schaible H G. Neuroscience. 1995;65:849–858. doi: 10.1016/0306-4522(94)00541-c. [DOI] [PubMed] [Google Scholar]
- 36.McMahon S B, Armanini M P, Ling L H, Phillips H S. Neuron. 1994;12:1161–1171. doi: 10.1016/0896-6273(94)90323-9. [DOI] [PubMed] [Google Scholar]
- 37.Balkowiec A, Kunze D L, Katz D M. J Neurosci. 2000;20:1904–1911. doi: 10.1523/JNEUROSCI.20-05-01904.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suen P C, Wu K, Xu J L, Lin S Y, Levine E S, Black I B. Brain Res Mol Brain Res. 1998;59:215–228. doi: 10.1016/s0169-328x(98)00157-0. [DOI] [PubMed] [Google Scholar]
- 39.Levine E S, Crozier R A, Black I B, Plummer M R. Proc Natl Acad Sci USA. 1998;95:10235–10239. doi: 10.1073/pnas.95.17.10235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jarvis C R, Xiong Z G, Plant J R, Churchill D, Lu W Y, MacVicar B A, MacDonald J F. J Neurophysiol. 1997;78:2363–2371. doi: 10.1152/jn.1997.78.5.2363. [DOI] [PubMed] [Google Scholar]
- 41.Kafitz K W, Rose C R, Thoenen H, Konnerth A. Nature (London) 1999;401:918–921. doi: 10.1038/44847. [DOI] [PubMed] [Google Scholar]