Abstract
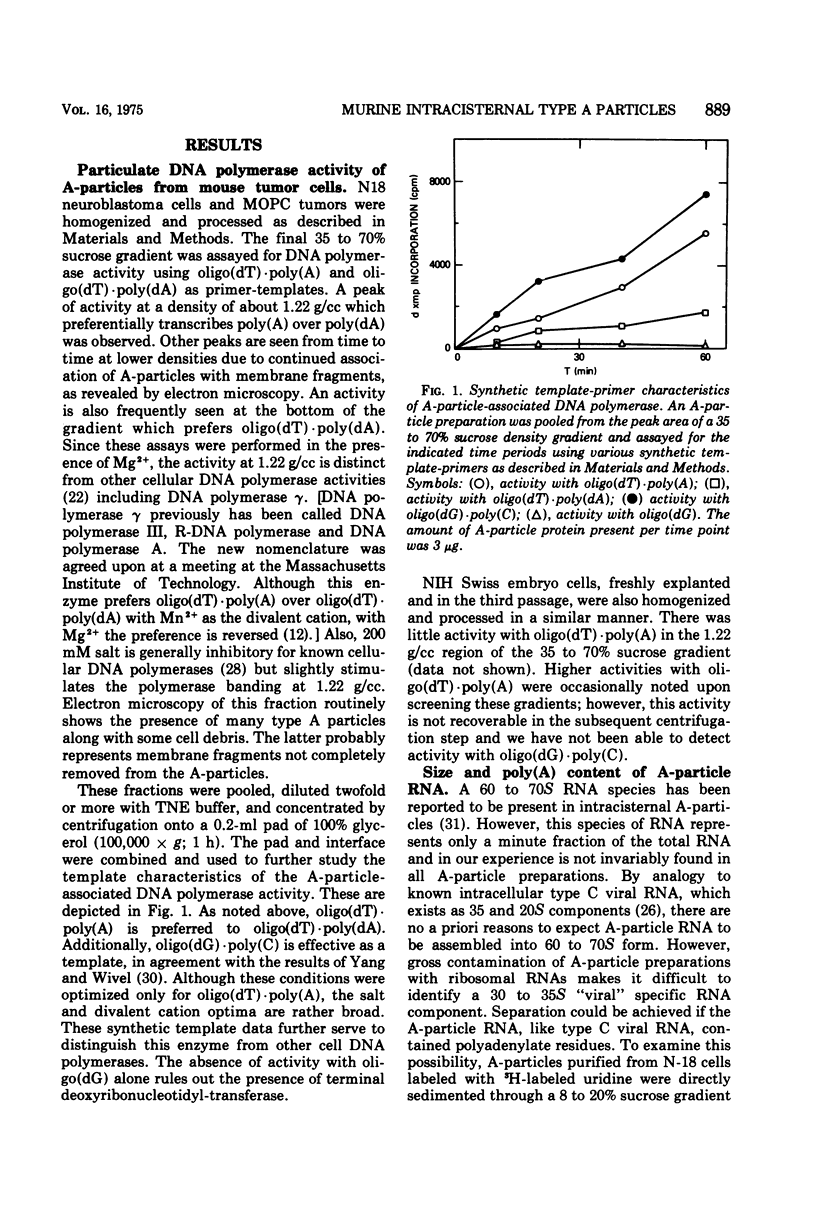
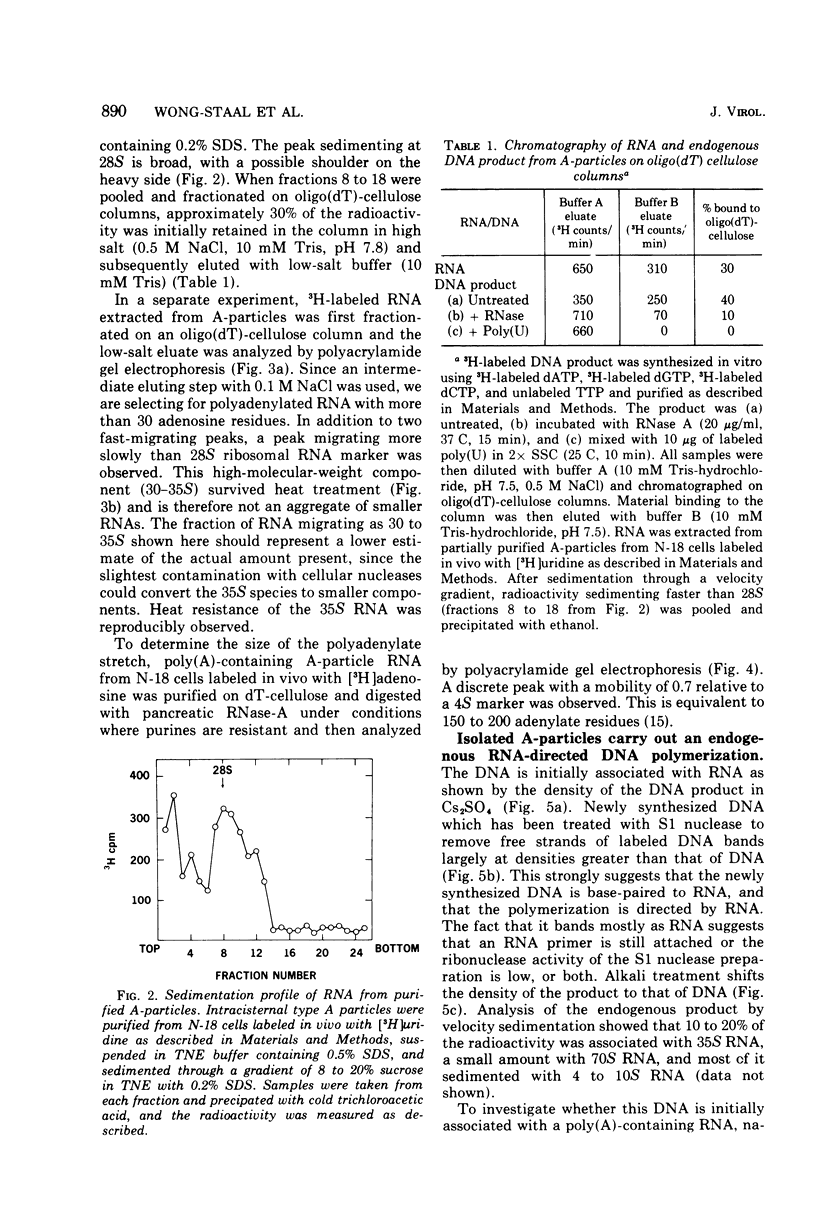
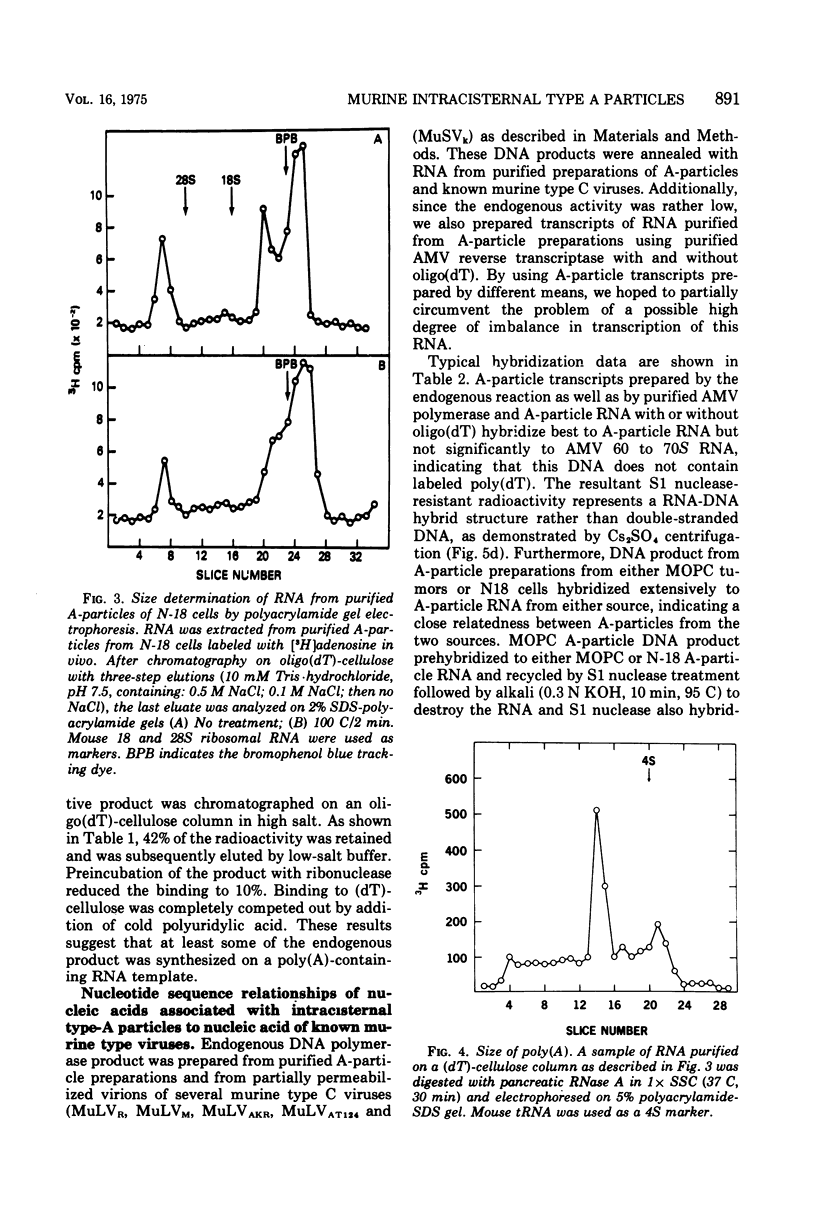
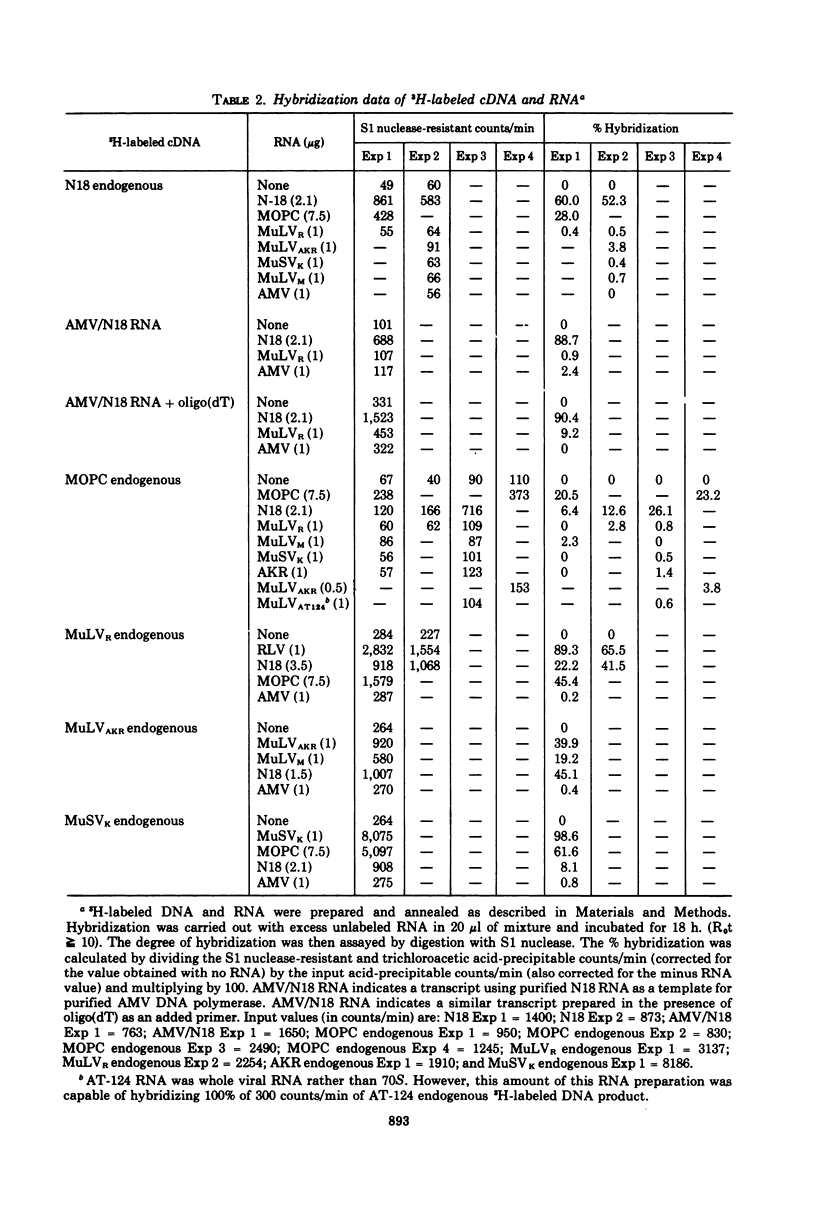
Intracisternal A particle preparations from a murine neuroblastoma cell line (N18) and from a mineral oil-induced murine plasmacytoma (MOPC-104E) contain both an endogenous RNA-dependent DNA polymerase activity and high molecular-weight polyadenylic acid (poly[A])-containing RNA. The DNA polymerase activity is stimulated by oligo(dG)-poly(C) and oligo(dT)-poly(A) and to a lesser extent by oligo(dT)-poly(dA), in agreement with previous reports. The high-molecular-weight RNA is predominantly 35S and contains a poly(A) tract of approximately 220 nucleotides as judged by polyacrylamide gel electrophoresis. Small amounts of 70S RNA are also present. This RNA preparation contains RNA homologous to RNA from type-C particles, as judged by molecular hybridization experiments. However, since this RNA derives only in part from A-particles and in part from other cellular RNA, hybridization of A-particle endogenously synthesized DNA or reverse transcripts of A-particle RNA to purified type C viral 70S RNA may more accurately reflect the relationship of A-particle RNA to RNA from C-particles. None of these DNA transcripts hybridizes significantly to C-particle 70S RNA, although MOPC and N18 DNA transcripts share significant homology. Our interpretation of these results is that murine intracisternal A particles are not closely related genetically to the tested murine type C viruses, although an alternate possibility is that all the A-particle DNA transcripts are copied from only a small part of the genome, which is unrelated to C-particle RNA.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrell J. W., Gallo R. C. Purification, characterization, and comparison of the DNA polymerases from two primate RNA tumor viruses. J Virol. 1973 Sep;12(3):431–439. doi: 10.1128/jvi.12.3.431-439.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERNHARD W. The detection and study of tumor viruses with the electron microscope. Cancer Res. 1960 Jun;20:712–727. [PubMed] [Google Scholar]
- Benveniste R. E., Scolnick E. M. RNA in mammalian sarcoma virus transformed nonproducer cells homologous to murine leukemia virus RNA. Virology. 1973 Feb;51(2):370–382. doi: 10.1016/0042-6822(73)90436-4. [DOI] [PubMed] [Google Scholar]
- Benveniste R. E., Todaro G. J. Homology between type-C viruses of various species as determined by molecular hybridization. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3316–3320. doi: 10.1073/pnas.70.12.3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya J., Xuma M., Reitz M., Sarin P. S., Gallo R. C. Utilization of mammalian 70S RNA by a purified reverse transcriptase from human myelocytic leukemic cells. Biochem Biophys Res Commun. 1973 Sep 5;54(1):324–334. doi: 10.1016/0006-291x(73)90926-1. [DOI] [PubMed] [Google Scholar]
- Bishop D. H., Claybrook J. R., Spiegelman S. Electrophoretic separation of viral nucleic acids on polyacrylamide gels. J Mol Biol. 1967 Jun 28;26(3):373–387. doi: 10.1016/0022-2836(67)90310-5. [DOI] [PubMed] [Google Scholar]
- Calarco P. G., Szollosi D. Intracisternal A particles in ova and preimplantation stages of the mouse. Nat New Biol. 1973 May 16;243(124):91–93. [PubMed] [Google Scholar]
- DALTON A. J., POTTER M., MERWIN R. M. Some ultrastructural characteristics of a series of primary and transplanted plasma-cell tumors of the mouse. J Natl Cancer Inst. 1961 May;26:1221–1267. [PubMed] [Google Scholar]
- Dalton A. J. RNA tumor viruses. Terminology and ultrastructural aspects of virion morphology and replication. J Natl Cancer Inst. 1972 Aug;49(2):323–327. [PubMed] [Google Scholar]
- Dion A. S., Vaidya B., Fout G. S., Moore D. H. Isolation and characterization of RNA-directed DNA polymerase from a B-type RNA tumor virus. J Virol. 1974 Jul;14(1):40–46. doi: 10.1128/jvi.14.1.40-46.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garapin A. C., Varmus H. E., Faras A. J., Levinson W. E., Bishop J. M. RNA-directed DNA synthesis by virions of Rous sarcoma virus: further characterization of the templates and the extent of their transcription. Virology. 1973 Mar;52(1):264–274. doi: 10.1016/0042-6822(73)90414-5. [DOI] [PubMed] [Google Scholar]
- Gardner M. B., Officer J. E., Rongey R. W., Estes J. D., Turner H. C., Huebner R. J. C-type RNA tumour virus genome expression in wild house mice. Nature. 1971 Aug 27;232(5313):617–620. doi: 10.1038/232617a0. [DOI] [PubMed] [Google Scholar]
- Hall W. T., Hartley J. W., Sanford K. K. Characteristics of and relationship between C particles and intracisternal A particles in cloned cell strains. J Virol. 1968 Mar;2(3):238–247. doi: 10.1128/jvi.2.3.238-247.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihle J. N., Lee K. L., Kennedy F. T. Fractionation of 34 S ribonucleic acid subunits from oncornaviruses on polyuridylate-sepharose columns. J Biol Chem. 1974 Jan 10;249(1):38–42. [PubMed] [Google Scholar]
- Kuff E. L., Leuders K. K., Ozer H. L., Wivel N. A. Some structural and antigenic properties of intracisternal A particles occurring in mouse tumors (complement fixation-immunodiffusion-neuroblastoma-plasma-cell tumor). Proc Natl Acad Sci U S A. 1972 Jan;69(1):218–222. doi: 10.1073/pnas.69.1.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuff E. L., Wivel N. A., Lueders K. K. The extraction of intracisternal A-particles from a mouse plasma-cell tumor. Cancer Res. 1968 Oct;28(10):2137–2148. [PubMed] [Google Scholar]
- Miller N. R., Saxinger W. C., Reitz M. S., Gallagher R. E., Wu A. M., Gallo R. C., Gillespie D. Systematics of RNA tumor viruses and virus-like particles of human origin. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3177–3181. doi: 10.1073/pnas.71.8.3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitz M. S., Jr, Abrell J. W., Trainor C. D., Gallo R. C. Precipitation of nucleic acids with cetyltrimethylammonium bromide: a method for preparing viral and cellular DNA polymerase products for cesium sulfate density gradient analysis. Biochem Biophys Res Commun. 1972 Oct 6;49(1):30–38. doi: 10.1016/0006-291x(72)90005-8. [DOI] [PubMed] [Google Scholar]
- Robert M. S., Smith R. G., Gallo R. C., Sarin P. S., Abrell J. W. Viral and cellular DNA polymerase: comparison of activities with synthetic and natural RNA templates. Science. 1972 May 19;176(4036):798–800. doi: 10.1126/science.176.4036.798. [DOI] [PubMed] [Google Scholar]
- Robertson D. L., Baenziger N. L., Dobbertin D. C., Thach R. E. Characterization of DNA polymerase and RNA associated with A-type particles from murine myeloma cells. J Virol. 1975 Feb;15(2):407–415. doi: 10.1128/jvi.15.2.407-415.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan D., Aviv H., Leder P. Purification and properties of biologically active messenger RNA for a myeloma light chain. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1967–1971. doi: 10.1073/pnas.69.7.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavitian A., Hamelin R., Tchen P., Olofsson B., Boiron M. Extent of transcription of mouse sarcoma-leukemia virus by RNA-directed DNA polymerase. Proc Natl Acad Sci U S A. 1974 Mar;71(3):755–759. doi: 10.1073/pnas.71.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchida N., Robin M. S., Green M. Viral RNA subunits in cells transformed by RNA tumor viruses. Science. 1972 Jun 30;176(4042):1418–1420. doi: 10.1126/science.176.4042.1418. [DOI] [PubMed] [Google Scholar]
- Wilson S. H., Bohn E. W., Matsukage A., Lueders K. K., Kuff E. L. Studies on the relationship between deoxyribonucleic acid polymerase activity and intracisternal A-type particles in mouse myeloma. Biochemistry. 1974 Mar 12;13(6):1087–1094. doi: 10.1021/bi00703a005. [DOI] [PubMed] [Google Scholar]
- Wilson S. H., Kuff E. L. A novel DNA polymerase activity found in association with intracisternal A-type particles. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1531–1536. doi: 10.1073/pnas.69.6.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu A. M., Reitz M. S., Paran M., Gallo R. C. Mechanism of stimulation of murine type-C RNA tumor virus production by glucocorticoids: post-transcriptional effects. J Virol. 1974 Oct;14(4):802–812. doi: 10.1128/jvi.14.4.802-812.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S. S., Wivel N. A. Analysis of high-molecular-weight ribonucleic acid associated with intracisternal A particles. J Virol. 1973 Feb;11(2):287–298. doi: 10.1128/jvi.11.2.287-298.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S. S., Wivel N. A. Characterization of an endogenous RNA-dependent DNA polymerase associated with murine intracisternal A particles. J Virol. 1974 Mar;13(3):712–720. doi: 10.1128/jvi.13.3.712-720.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yotsuyanagi Y., Ephrussi B. Behavior of three types of ribovirus-like particles in segregating hamster times mouse somatic hybrids. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4575–4578. doi: 10.1073/pnas.71.11.4575. [DOI] [PMC free article] [PubMed] [Google Scholar]



