Summary
We aimed to verify that Micrus 3D microcoils can be used as first coils in the treatment of saccular wide-necked aneurysms to provide stable protection to the neck by keeping the coil mesh inside the aneurysm.
We selected 22 wide-necked aneurysms and calculated the ratio between sac diameter and neck width which was close to unity. The aneurysms were treated by endovascular approach using Micrus 3D (Spherical) microcoils as first coils. The coils proved stable during placement and detachment and compatible with both GDC and Micrus coils used for filling and packing. The endovascular procedure was suspended in only two out of 22 aneurysms selected for treatment.Aneurysm bleeding occurred in two endovascular procedures but was controlled. No complications were encountered in the remaining 18 patients treated. The percentage of technical failures has been reduced since the adoption of 3D Micrus microcoils in our practice.
Key words: brain aneurysm, endovascular treatment, coils, GDC
Introduction
The endovascular treatment of aneurysms has become an increasingly popular and widely applied method in the last decade1,2,3. The different anatomical situations encountered during endovascular procedures have led to the development of techniques tailored to overcome the drawbacks to treatment4,5,6,7,8. The most common problem in treating these lesions is the unfavourable ratio between the size of the aneurysm sac and its neck. Too wide a neck will fail to contain the coils within the aneurysm, but external “containment” methods based on balloon remodelling or stent placement across the aneurysm neck4,5 are technically difficult and carry some risk.
Because of their special features, Micrus microcoils have recently been proposed in the treatment of wide-necked aneurysms. These coils have a stable shape and remain within the aneurysm after deployment thereby offering adequate support to the other coils subsequently inserted to complete occlusion9.
We describe our experience using Micrus 3D microcoils to treat saccular intracranial aneurysms in twenty patients.
Materials and Methods
From May 2001 to October 2002, we treated 20 consecutive patients (six men, 14 women; average age 62 years) with saccular intracranial aneurysms. Eleven patients had one aneurysm, whereas nine presented multiple aneurysms: seven (cases 1, 2, 3, 9, 13, 15, 10) had two aneurysms and one (case 11) had three aneurysms. In the latter eight cases, only one aneurysm in each patient was treated with microcoils. One patient (case 18) had four aneurysms which were all treated by endovascular procedure.
A total of 32 aneurysms were detected of which 22 were treated by endovascular procedure (table 1). The remainder were treated surgically.
Table 1.
22 Aneurysms treated om 20 patients - May 2001-October 2002
| Cases | PTS. | Age | Sex | Location | First choiche coils |
Packing coils |
Complication |
|---|---|---|---|---|---|---|---|
| 1 | BP | 80 | F | Siphon | Micrus 3D | Micrus GDC | NO |
| 2 | BMR | 61 | F | Pica | Micrus 3D | GDC | NO |
| 3 | BM | 42 | M | Siphon | Procedure Suspended. |
||
| 4 | DS | 66 | F | P. com. | Micrus 3D | GDC | NO |
| 5 | GI | 68 | M | A1-A2 | Micrus 3D | GDC | NO |
| 6 | GL | 54 | F | Siphon | Micrus 3D | NO | NO |
| 7 | LM | 80 | F | Siphon | Micrus 3D | Micrus GDC | NO |
| 8 | MAP | 53 | F | A1-A2 | Micrus 3D | Micrus GDC | Controlled SAH |
| 9 | PC | 73 | F | Siphon | Micrus 3D | GDC | Controlled SAH |
| 10 | RD | 56 | F | Bas.Art. | Micrus 3D | Micrus GDC | NO |
| 11 | RC | 57 | F | Siphon | Procedure Suspended. |
||
| 12 | RN | 54 | F | Siphon | Micrus 3D | MICRUS | NO |
| 13 | RG | 30 | M | Ophthalcar. | Micrus 3D | MICRUS | NO |
| 14 | SP | 42 | F | Car bifur. | Micrus 3D | Micrus GDC | NO |
| 15 | TR | 42 | F | Pica | Micrus 3D | GDC | NO |
| 16 | ZA | 80 | M | Siphon | Micrus 3D | NO | NO |
| 17 | SAM | 49 | F | Art. Bas. | Micrus 3D | Micrus GDC | NO |
| 18 a | FG | 43 | M | Siphon | Micrus 3D | GDC | NO |
| 18 b | FG | 43 | M | M1 | Micrus 3D | Micrus GDC | NO |
| 18 c | FG | 43 | M | Art. Bas. | Micrus 3D | Micrus GDC | NO |
| 19 | LA | 62 | F | Art. Bas. | Micrus 3D | Micrus GDC | NO |
| 20 | MA | 71 | M | Com. A. | Micrus 3D | GDC | NO |
The 22 aneurysms subjected to Micrus coil embolization were located as follows: nine in the carotid siphon (cases 1, 3, 6, 7, 9, 11, 12, 16, 18a), four in the basilar artery (cases 10,17,18c, 19), two in the PICA (cases 2, 15), two aneurysms in A1-A2 (cases 5, 8), one in the carotid-ophthalmic region (case 13), one in the carotid bifurcation (case 14), one in the posterior communicating artery (case 4), one in the anterior communicating artery (case 20) and one in M1 (case 18b). All 22 aneurysms were wide-necked and hence were treated using Micrus 3D coils.
The aneurysm sac diameter and neck width were measured and the ratio between the two measurements calculated: the ratio was close to unity in all 22 cases (table 2).
Table 2.
Aneurysm sac diameter - mecl width ratio
| Cases | Sac Diam. (mm) |
Neck Width (mm) |
Sac/Neck Ratio |
|---|---|---|---|
| 1 | 7.63 | 5.48 | 1.39 |
| 2 | 8.78 | 5.58 | 1.57 |
| 3 | 3.26 | 3 | 1.26 |
| 4 | 5.49 | 3.58 | 1.53 |
| 5 | 8 | 5.25 | 1.52 |
| 6 | 3.17 | 2 | 1.58 |
| 7 | 5 | 3.59 | 1.39 |
| 8 | 2.07 | 1.96 | 1.05 |
| 9 | 3 | 1.82 | 1.64 |
| 10 | 19 | 12 | 1.58 |
| 11 | 3.26 | 2.71 | 1.2 |
| 12 | 10.51 | 7.52 | 1.39 |
| 13 | 4.38 | 3.25 | 1.34 |
| 14 | 8.78 | 5.65 | 1.55 |
| 15 | 3.49 | 2.25 | 1.55 |
| 16 | 2 | 1.88 | 1.06 |
| 17 | 8 | 6.74 | 1.18 |
| 18 a) | 2,5 | 1.7 | 1.4 |
| 18 b) | 3 | 2.3 | 1.3 |
| 18 c) | 5 | 3 | 1.5 |
| 19 | 12.2 | 10 | 1.22 |
| 20 | 10.96 | 9.67 | 1.13 |
Micrus 3D was used as the first choice coil in 21 aneurysms. In case 6, the only instance of redo embolization, a Micrus 3D coil was positioned in the recanalisation during the second endovascular procedure. Packing coils were not inserted in two patients (cases 6 and 16). In the remaining 18 cases packing coils were subsequently positioned using both Micrus 3D and GDC coils in nine patients (cases 1,7, 8,10,14, 17,18b, 18c, 19) only GDC coils in seven (cases 2, 4, 5, 9,18a, 15, 20) and only Micrus 3D coils in two patients (cases 12 and 13). Spherical 18 coils were used in three patients (cases 10, 12, 14) and Spherical 10 in the remaining 19.
Results
Of the 22 aneurysms treated by endovascular approach in this series, two procedures (cases 3 and 11) were suspended for technical reasons. Periprocedural SAH occurred in two patients (cases 8 and 9), but was controlled and in both cases the aneurysms were fully excluded from the circulation and the patients subsequently discharged without any deficit. No periprocedural complications were encountered in the remaining 18 patients.
Seven of these cases (4, 5,14, 16, 17, 18 and 19) are discussed in more detail as significant examples in terms of aneurysm features, endovascular procedure and outcome.
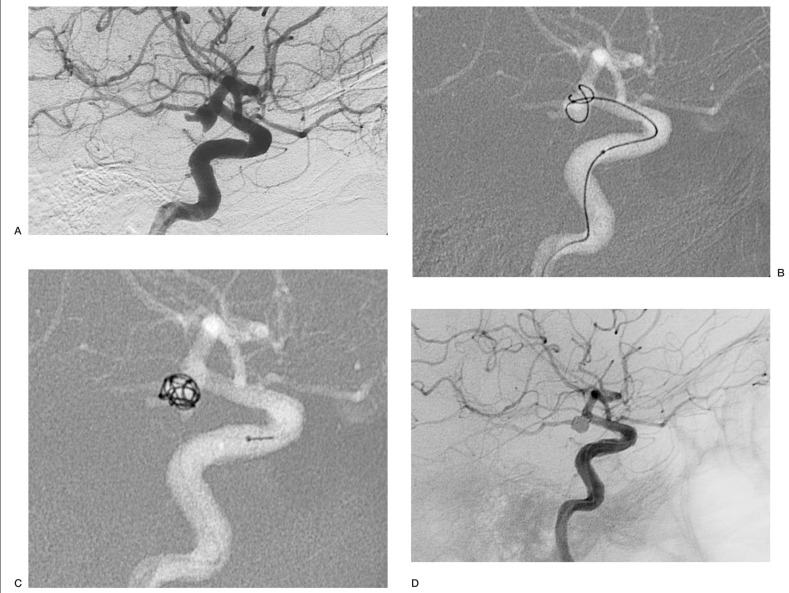
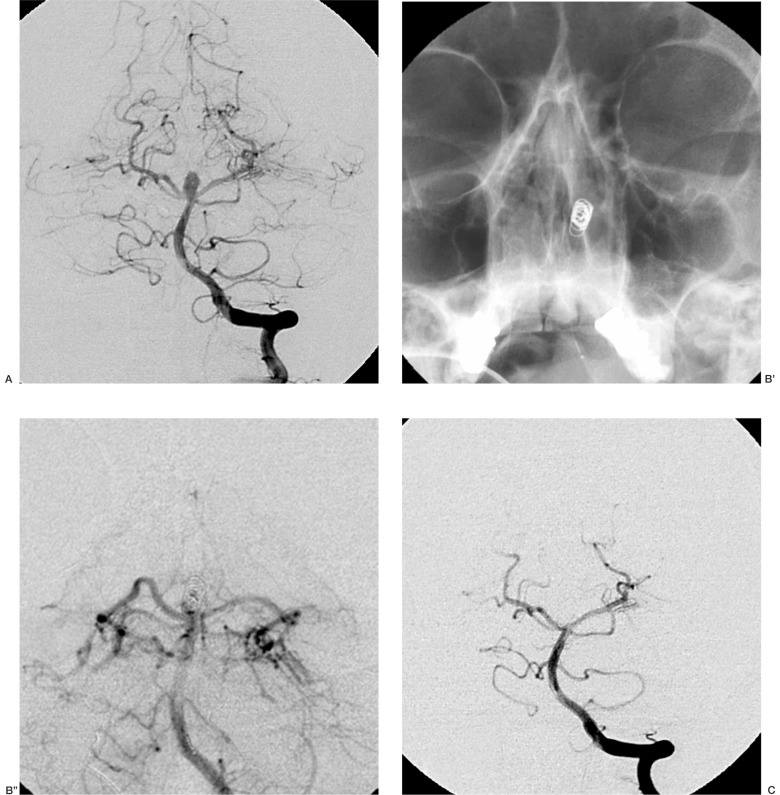
Case 4: Aneurysm of the posterior communicating artery. The Micrus coil initially migrated out of the siphon, but it resumed its spherical shape as it was expelled by the microcatheter and spontaneously re-entered the aneurysmal cavity; at the end of the procedure the sac was densely packed (figure 1).
Figure 1.
Case 4: aneurysm of the posterior communicating artery (A);the Micrus coil initially migrates out of the siphon (B), but resumes its shape as the coil is detached and re-enters the aneurysm (C);at the end of the procedure the sac is densely packed (D).
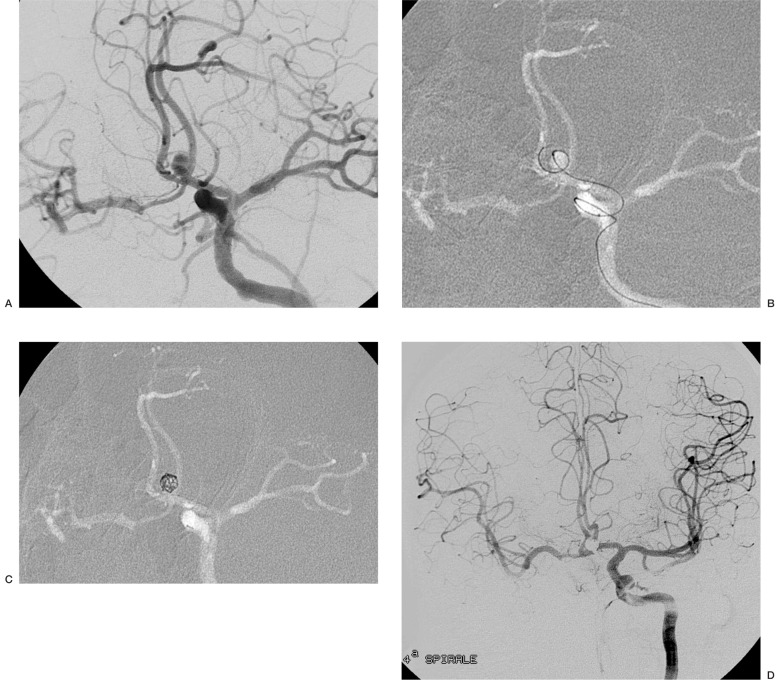
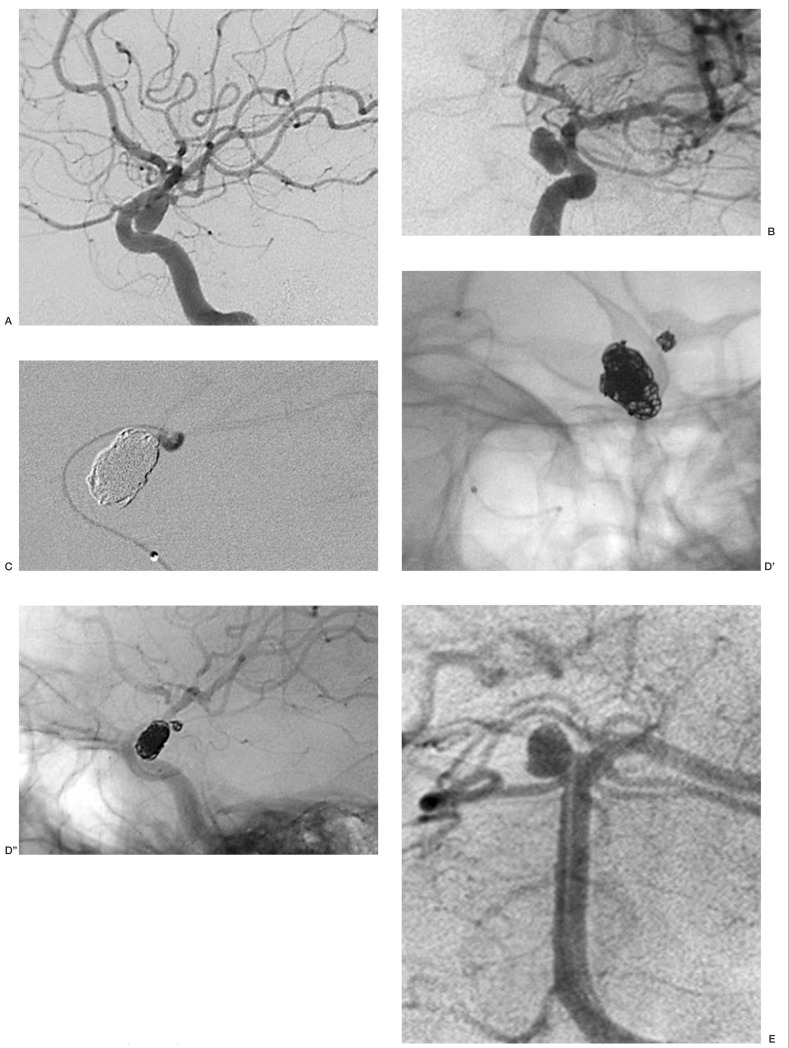
Case 5: Aneurysm of the left A1-A2 angle. The neck-sac ratio was 1.52: as it was deemed to have enough protection, a first coil 2D was chosen. The coil entered the aneursym and proceeded to the contralateral anterior cerebral artery. The use of a spherical coil maintained the coil mesh inside the aneurysmal sac (figure 2).
Figure 2.
Case 5: aneurysm of the A1-A2 angle (A). An initial 2D coil entered the aneurysm but then proceeded into the contralateral anterior cerebral artery and was replaced by a 3D Micrus coil (B).The spherical shape (C) facilitated the insertion of additional coils. At the end of the procedure, the aneurysm is excluded from the circulation (D).
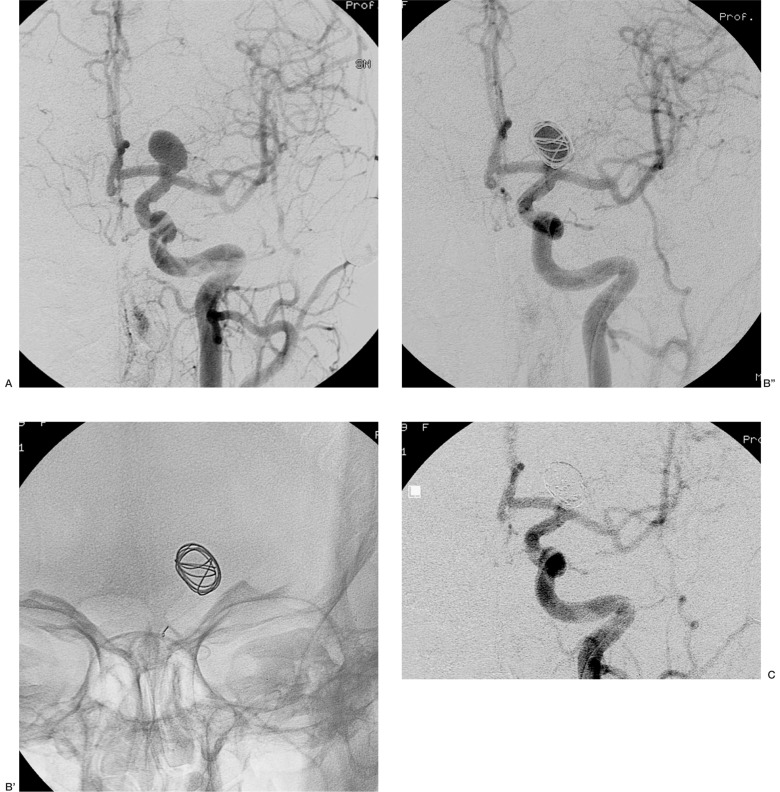
Case 14: Large aneurysm of the left carotid bifurcation. The first 3D Micrus coil bridged the aneurysmal neck allowing complete filling of the sac (figure 3). In this case, the use of a Trispan device was also possible. The advantage of choosing the 3D Micrus coil was the possibility to complete the procedure by only one arterial access and single microcatheterization instead of the double microcatheters needed to deploy a Trispan.
Figure 3.
Case 14: large aneurysm of the carotid bifurcation (A). Positioning the first Micrus coil (B', B''), allowed a good protection of the aneurysmal neck. The final control shows normal circulation in the ipsilateral carotid (C).
Case 16: Small aneurysm of the right carotid siphon (2 mm sac diameter, 1.8 mm neck width) in which total occlusion was achieved with a single Micrus 3D Spherical 10 coil. (figure 4).
Figure 4.
Case 16: A) small aneurysm of the right carotid siphon (2mm sac diameter, 1.8 mm neck width) in which total occlusion was achieved with a single Micrus 3D Spherical 10 coil (B).
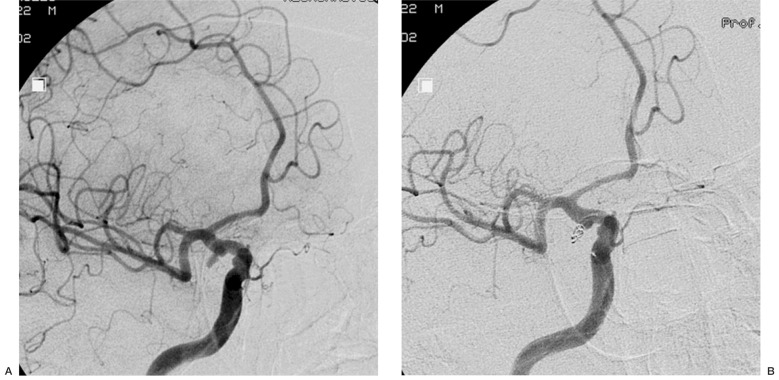
Case 17: Basilar tip aneurysm, sac/neck ratio 1.18. As usual in these cases the main problem is to occlude the aneurysm without interfering with the blood flow in both posterior cerebral arteries. Deployment of a first 3D Micrus coil allowed the complete filling of the sack without the need of a complex two microcatheters procedure and keeping the perfect flow in the posterior cerebral arteries (figure 5).
Figure 5.
Case 17: aneurysm of the basilar artery tip (A). The first Micrus 3D coil protects the origin of the posterior cerebral arteries (B', B''). At the end of the procedure the anatomy of the basilar tip region is normal (C).
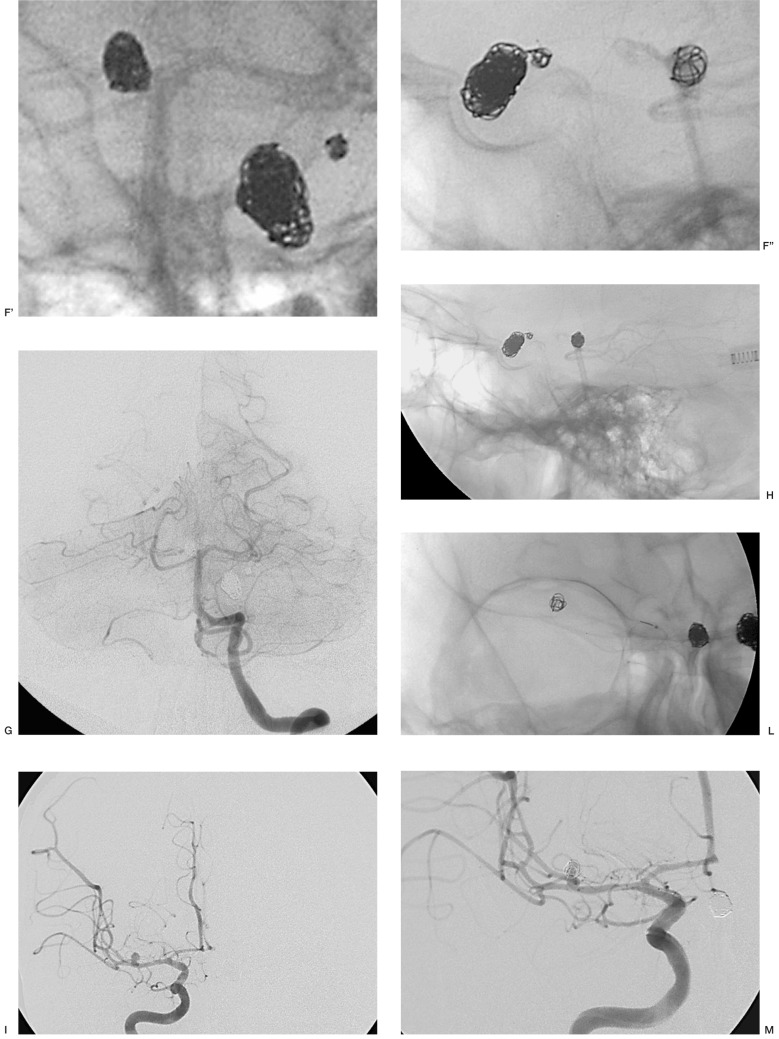
Case 18: was the only patient presenting four aneurysms, two in the siphon of which one larger lesion was medially inclined and the other smaller aneurysm located in the distal stretch of the siphon adjacent to the posterior communicating artery, one in the basilar artery and one in M1. Three of these (two in the siphon and one in the basilar artery) were treated in the same sitting and the M1 aneurysm embolized in a subsequent procedure. Micrus 3D Spherical 10 was used as the first coil except in the case of the larger aneurysm (10 mm in diameter) located in the carotid siphon and medially inclined, which had a small neck and hence amenable to GDC 3D coils. Packing was successful leading to occlusion of the aneurysms in the siphon and basilar artery with excellent visualization of the intracranial circulation, whereas occlusion was partial in the case of the M1 aneurysm (figure 6).
Figure 6.
Case 18: patient presenting four aneurysms. Two aneurysms are located in the right carotid siphon (A, B), one larger lesion medially inclined and a small aneurysm in the distal stretch adjacent to the posterior communicating artery. At the same sitting the larger aneurysm, with a small neck, in the siphon was packed (C), using 3D GDC coils (Boston Scientific) followed by the smaller lesion (C, D', D'') and then the basilar artery tip aneurysm (E-H). The aneurysm of the right M1 segment was occluded in a subsequent procedure (I-M);only a partial embolization was possible to preserve the origin of a small Sylvian artery visible close to the neck (I, arrow).
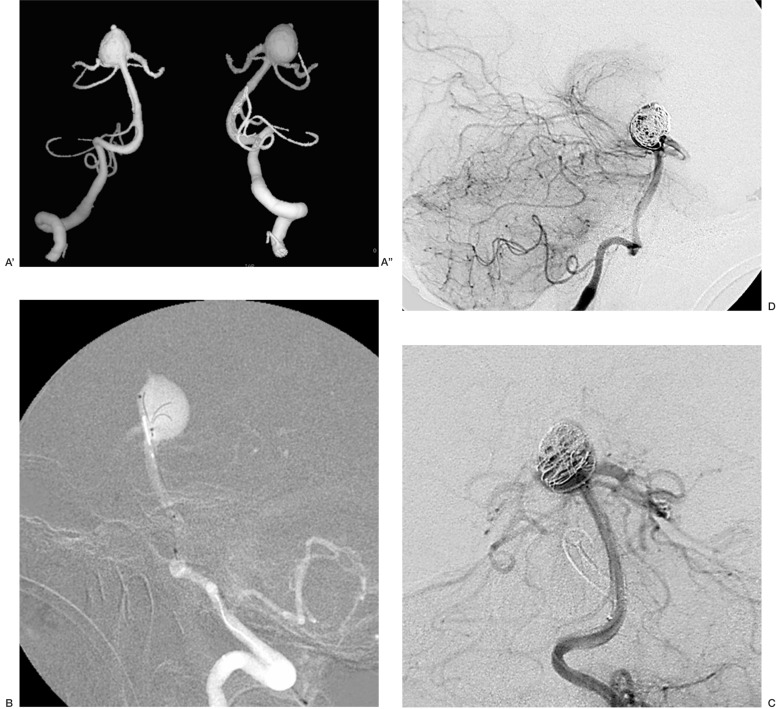
Case 19: was an aneurysm of the basilarartery tip without a neck and with a proximal portion comprising the origin of both posterior cerebral arteries and the right superior cerebellar artery. The diameter of the most proximal part of the lesion excluding the origin of the vessels was considered the virtual neck, the limit to coiling that could preserve the flow into both posterior cerebral arteries. A Trispan device was then positioned followed by a 3D spherical Micrus coil to enhance the stability of the coil mesh.. Both posterior cerebral and superior cerebellar arteries were patent on radiological control at the end of the procedure (figure 7).
Figure 7.
Case 19: aneurysm of the basilar artery tip without a neck and with a proximal portion comprising the origin of both posterior cerebral arteries and the right superior cerebellar artery (A',A''). The diameter of the most proximal part of the lesion excluding the origin of the vessels was considered the virtual neck, the limit to coiling that could preserve the flow into both posterior cerebral arteries. A Trispan device was then positioned (B) followed by a 3D spherical Micrus coil to enhance the stability of the coil mesh (C). Both posterior cerebral and superior cerebellar arteries were patent on radiological control at the end of the procedure (D).
Angiography was performed in all cases at the end of the endovascular procedure and follow-up magnetic resonance angiography recommended.
Six months follow-up with MRA 3D TOF with MIP reconstructions showed a perfect correspondence between MRA findings and digital subtraction angiography performed at the end of the treatment, demonstrating the stability of the embolization. Further follow-up at two years is planned.
Discussion
Micrus microcoils have been proposed as an alternative to established treatments for wide-necked aneurysms such as remodelling, insertion of an intracranial shunt or Trispan device, or 3D GDC coil placement. The procedure of choice is determined on a case by case basis and will depend on whether two microcatheters are needed instead of one. Plainly, interventions requiring two microcatheters are more complicated and are often hindered by different problems, namely anatomical drawbacks. The remodelling technique first devised by Moret5 is a valid procedure, but requires two microcatheters and occlusion of the parent artery sometimes for prolonged periods. The Trispan device (Boston Scientific), a nitinol trefoil which serves to contain coil packing, also requires two microcatheter approaches. In addition, this procedure is only advised when the access route is vertical rather than lateral which reduces treatment possibilities to aneurysms of the basilar tip or carotid-ophthalmic arteries or the anterior cerebral-middle cerebral bifurcation. In one case (n. 19), we used the Trispan device followed by a 3D Micrus coil to ensure the stability of coil packing (figure 7). Intracranial stent insertion is a single access procedure we have used in some unpublished cases, but which presented serious problems in placement of metallic stents. Nitinol stents (Boston Scientific) have recently been advocated as more flexible, but it is still too soon and experience too limited to draw any conclusions. We used Micrus microcoils to treat wide neck aneurysms with a sac/neck ratio of 1.5: hence wide but not very wide since the latter can only be tackled endovascularly by remodelling or stent placement. We used the Trispan device in treating basilar tip aneurysms which often entail protecting the origin of the posterior cerebral or even superior cerebellar arteries, as in our Case 19. A Trispan was positioned in this patient above the origin of the posterior cerebral arteries followed by placement of a 3D Micrus coil which stabilized the situation so that the aneurysm could be packed with ordinary coils. The optimal 3D GDC coil was reserved for aneurysms with narrower necks such as the fourth aneurysm in Case 18, included here because the first three aneurysms had been treated by 3D Micrus as first coil. An analysis of the additional coils used for filling and packing is beyond the scope of this paper.
Conclusions
Micrus 3D coils proved stable and suitable for accurate positioning even in the case of wide-necked aneurysms. This was particularly evident when the 3D coil migrated from the aneurysmal sac into the parent artery. In this situation, the Micrus coil promptly resumed its spherical shape as it was expelled by the microcatheter and spontaneously re-entered the aneurysmal cavity (figure 1). This never occurred using 3D GDC coils that have a lower shape memory. The containment of the packing coils required to complete aneurysm occlusion was excellent.
Micrus 3D coils proved very useful in the treatment of wide-necked, albeit not very wide-necked aneurysms. When the sac:neck ratio is 1:1 or less it is usually essential to deploy additional protective (Trispan) or external devices such as stent or balloon-assisted coil placement as in the “remodelling” technique.
It is difficult to evaluate the impact of this new material in our practice. We treated 34 brain aneurysms during the year 2000 with eight technical failures whereas the following year 41 aneurysms were treated with 11 failures.
During 2002, after full adoption of 3D Micrus microcoils, we treated 45 brain aneurysms with only two technical failures.
These data are of interest because the main reason for technical failure in the endovascular treatment of intracranial aneurysms is wide neck diameter.
Since the presentation of this article 20 more aneurysms have been embolized in our Department using 3D Micrus microcoils.
No funding for this study was received from Micrus and the opinions stated herein are those of the authors alone.
References
- 1.Cognard C, Weill A, et al. Long-term angiographic follow-up of 169 intracranial berry aneurysms occluded with detachable coils. Radiology. 1999;212:3448–356. doi: 10.1148/radiology.212.2.r99jl47348. [DOI] [PubMed] [Google Scholar]
- 2.Vinuela F, Duckwiler G, et al. Guglielmi detachable coil embolization of acute intracranial aneurysm: perioperative anatomical and clinical outcome in 403 patients. J Neurosurg. 1997;86:475–482. doi: 10.3171/jns.1997.86.3.0475. [DOI] [PubMed] [Google Scholar]
- 3.Molyneux AJ, Kerr RSC. The future management of subarachnoid haemorrhage. J de Neuroradiologie. 2002;29:74–75. [PubMed] [Google Scholar]
- 4.Wakhloo AK, Lanzino G, et al. Stents for Intracranial Aneurysms: The beginning of new endovascular era? Neurosurgery. 1998;43:377–379. doi: 10.1097/00006123-199808000-00126. [DOI] [PubMed] [Google Scholar]
- 5.Moret J, Cognard C, et al. The “remodelling technique” in the treatment of wide neck intracranial aneurysms. Interv Neuroradiol. 1997;3:21–35. doi: 10.1177/159101999700300103. [DOI] [PubMed] [Google Scholar]
- 6.Baxter B, Rosso D, et al. Double microcatheter technique for detachable coil treatment of large, widenecked intracranial aneurysms. Am J Neuroradiol. 1998 Jun;19:1176–1178. [PMC free article] [PubMed] [Google Scholar]
- 7.Levy DI, Ku A. Balloon-assisted coil placement in wide-necked aneurysms (technical note) J Neurosurg. 1997;86:724–727. doi: 10.3171/jns.1997.86.4.0724. [DOI] [PubMed] [Google Scholar]
- 8.Moret J, Cognard C, et al. La technique de reconstruction dans le traitment des anevrismes intracraniens a collet large. J Neuroradiol. 1997;24:30–44. [PubMed] [Google Scholar]
- 9.Pierot L, Flandroy L, et al. Selective endovascular treatment of intracranial aneurysms using Micrus microcoils: preliminary results in a series of 78 patients. J de Neuroradiologie. 2002;29:114–121. [PubMed] [Google Scholar]
- 10.Sekhon L, Morgan M, et al. Combined endovascular stent implantation and endosaccular coil placement for the treatment of a wide-necked vertebral artery aneurysm: technical case report. Neurosurgery. 1998;43:380–383. doi: 10.1097/00006123-199808000-00127. [DOI] [PubMed] [Google Scholar]
- 11.Lavine S, Larsen D, et al. Parent vessel Guglielmi detachable coil herniation during wide-necked aneurysm embolization: treatment with intracranial stent placement: two technical case reports. Neurosurgery. 2000;46:1013–1017. [PubMed] [Google Scholar]
- 12.Zubillaga A, Guglielmi G, et al. Endovascular occlusion of intracranial aneurysms with electrically detachable coils: correlation of aneurysms neck size and treatment results. Am J Neuroradiol. 1994;15:815–820. [PMC free article] [PubMed] [Google Scholar]